AbstractSilica nanocapsules (SiNC) have gained considerable interest in their application as adsorbents due to its excellent physical structure and surface chemistry. The SiNC were synthesised via emulsion technique, whereby the effect of different solvents and calcination process were investigated. Diethyl ether as a solvent produced SiNC with the biggest surface area, 644 m2/g, followed by toluene and ethanol, 575 and 533 m2/g, respectively. The calcined SiNC had bigger surface area and pore volume, but smaller pore size as compared to their non-calcined counterparts. Diethyl ether and toluene as solvents produced SiNC with capsules or hollowed-core morphological structure, whereas by using ethanol, the SiNC was of solid core. The spectral analysis showed that the SiNC were of similar characteristics, whereby the ethanol-based SiNC had more intense hydroxyl (−OH) peaks and diethyl ether and toluene had an extra −CH3 peak. The carbon dioxide (CO2) adsorption measurement study showed that toluene-based SiNC performed the highest CO2 adsorption capacity (Q = 2.59 mmol/g), followed by diethyl ether-based (1.45 mmol/g) and ethanol-based, (1.28 mmol/g). The sufficiently competent CO2 adsorption capacity of the SiNC combined with their excellent physicochemical characteristics indicated their promising prospects for application as an adsorbent in CO2 adsorption.
Graphical Abstract
1. IntroductionGlobalisation and urbanisation have become the driving forces behind rapid development and growth in populations. As a result, the utilisation of fossil fuels-based products, such as petroleum and natural gas for energy generation, transportation and other industrial activities has also increased significantly [1–4]. These anthropogenic activities release carbon dioxide (CO2) gas, one of the main by-products of fossil fuels utilisation [5]. In response to the increased emission of CO2 gas into the atmosphere, various carbon capture technologies such as adsorption, membrane separation, cryogenic distillation and absorption have been studied. Membrane separation, cryogenic distillation and absorption were disadvantageous as these processes require large capital cost [6], large process spaces [7, 8], high energy consumption [8, 9] as well as equipment corrosion problem [10, 11]. Adsorption process is deemed more feasible for CO2 capture application as the process is more practical economically for scale-up application [12], easy to operate [13, 14] and produce none to minimal secondary pollutants or by-products [10].
Adsorbents such as activated carbon [13, 15, 16], mesoporous silica [1, 12], zeolites [17], and metal-organic framework (MOF) [6, 18] have been developed and comprehensively studied for their efficacies and feasibilities in CO2 capture application. As the CO2 capture can be limited by the interaction of gas molecules and adsorbent [17, 19], the adsorbent utilised must be carefully selected based on their surface area and porosity characteristics, surface chemistry as well as their ability to be operated at a wide range of concentration. Mesoporous silica or silica-based adsorbent has attained the interest of researchers for their CO2 capture application as the adsorbent provides sufficient surface area [20] and porosity with adjustable pore size [14] and abundant presence hydroxyl-group functionalities on its surface [11, 20].
In recent years, silica nanocapsules (SiNC), a type of silica materials, had received considerable attention amongst researchers due to their applicability in corrosion protection [21, 22], fragrance [23], material transport [24] and biomedical [25–29]. SiNC materials are particularly interesting as the silica has a high surface area, high permeability, low density, size-adjustable inner cavity, higher colloidal stability as well as easily modified surface chemistry [25, 30–32]. Furthermore, the adjustable size of the inner cavity of the SiNC materials makes the SiNC extremely useful in encapsulation of various substances within their confined capsules cavity. The encapsulated substances will be entrapped and dangled on the silica framework structure, making it accessible and easier for controlled released applications such as in the medicinal field for material and drug transport [25].
Synthesis methods such as cavity generating method via hard template synthesis (e.g., solid nanoparticles) [33, 34], soft template method (e.g., emulsion method, gas bubble synthesis method, template- free method) [35], shell-forming method [36, 37] and supramolecular- template deposition have been explored for the synthesis of silica nanocapsules materials. The cavity-generating method used solid nanoparticles, droplets of emulsion or gas bubbles synthesis to form additives-loaded, multifunctional silica nanocapsules [38]. The hard-templating synthesis method suffers from drawback related to a complicated functionalisation process, harsh core removal condition, and the excessive cost of using inorganic nanoparticles [39, 40]. Soft-templating synthesis method is more desirable for synthesis of SiNC as it offers facile synthesis procedures, in-situ encapsulation techniques, and effective encapsulation of compounds and additives to its cavity.
The tuneable surface area and pore characteristics of the silica nanocapsules piqued the interest of researchers in the development of an efficient adsorbent for pollutant removal via adsorption process. High adsorption capacity and high pollutant removal require the adsorbents to have a high surface area and high porosity accompanied by an excellent number of pores. These characteristics can be achieved for the silica nanocapsules by turning the type of solvent used, the amount of catalyst used and conditions of the SiNC synthesis process. Amongst these synthesis parameters, the nature of solvent [41] and type of solvent [42] used during synthesis majorly influenced the final shapes and sizes of SiNC materials. Flood-Garibay and Méndez-Rojas [42] reported that the solvent controlled the hydrolysis rate of the silica source, in which slower rate produced bigger size silica and vice versa. Salabat et al. [43] and Themis and Erdogan [44] reported that solvents with high viscosity resulted in higher emulsion agglomeration, resulting in a bigger size end-product. Yoo and Stein [45] added that in regard to nature of the solvent, alcoholic solvent produced silica that was of solid and minimal porosity while, non-alcoholic ones produced silica with higher porosity.
In order to achieve the capsules-like structure or characterised by the hollowed core of the silica nanocapsules, calcination process can be conducted. Calcination refers to the process of exposing the silica precipitate to a high-temperature treatment, in which organic and volatile materials will be removed during the process. In the previous investigation reported in Thangarajoo et al. [46] and Abdul Rahim et al. [47], calcination process removed the template materials which were utilised during the synthesis process, resulting in hollowed-core characteristics. In addition to the hollowed-core characteristics, the calcination process helped to enhance the porosity and surface area of silica materials. In a study by Yang et al. [48], as the calcination pulverizes the organic materials on the surface of the silica, pores on the surface became more exposed, resulting in higher surface area and porosity measurement.
In respect to the type of solvent used, numerous studies attempted synthesis of silica through utilisation of common solvent such as ethanol and ethyl ether. With the objectives to developed hollowed internal structure, Yilmaz [49] observed that utilisation of ethanol produced mesoporous silica sphere with light centre and darker edges indicating the adsorbent hollow structure. Similar morphological finding was reported by Li et al. [50] in their studies for synthesis of silica nanoparticles for biomedical application. In addition to ethanol as solvent in hollowed-silica synthesis, several studies also report on the utilisation of diethyl ether. Ruggiero et al. reported a successful encapsulation of antifouling agent in their silica synthesis where through comparisons both empty and loaded-silica, the empty silica materials show an empty core-shell imaging [51]. However, in a study by Lu et al. [52], utilisation of diethyl ether as solvent in silica only produced small mesoporous silica materials. Their morphological imaging shows the silica inner core-shell of the silica was not hollow.
Therefore, to the best of the researchers’ knowledge, there is apparent scarcity on the availability of studies that reports on the synthesis process of the SiNC adsorbent materials as affected by calcination process and the type of solvent used, as well as their prospect for the application in CO2 removal process. On this account, this study reports on the finding through using characterisation analysis such as Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), transmission electron microscopy (TEM) and N2 adsorption/desorption. The adsorptive capability of the SiNC adsorbent towards carbon dioxide (CO2) gas will be preliminarily evaluated by using a lab-scale CO2 adsorption rig system.
2. Experimental Section2.1. Materials and ChemicalsHexadecyltrimethylammonium bromide (CTAB, 98.00%), ammonia solution (NH3OH, 25.00%), tetraethyl orthosilicate (TEOS, pure), toluene (C7H8, 99.99%) and diethyl ether ((C2H5)2O, 99.99%) were purchased from Merck, Germany. Ethanol absolute (C2H5OH, 99.40%) was purchased from Fisher, USA. Distilled water used in the synthesis was produced by the Divine Pure Water Drinking System in the laboratory. The carbon dioxide (CO2, 99.99%) gas and the nitrogen (N2, 99.99%) gas were purchased from Linde Sdn Bhd, Malaysia.
2.2. Synthesis of Silica Nanocapsules (SiNC) AdsorbentThe silica nanocapsules, SiNC adsorbents were synthesised according to a modified method that have been established by Maia et al. [21]. In the synthesis process, CTAB was used as particle templating, ammonia solution acted as the process catalyst, TEOS as the silica precursor, and ethanol, toluene, or ethyl diether as the solvent. Firstly, Solution 1 was prepared by dissolving CTAB in water and adding ammonia solution. Then, Solution 2, containing only solvent (i.e., toluene, ethanol, or ethyl diether), was mixed with Solution 1. The mixture of Solution 1 and Solution 2 was stirred on a 600 rpm-magnetic stirrer at room temperature. As the solution was homogenised, TEOS was added dropwise to the mixture solution and was left to stir continuously overnight. After being stirred overnight, a white precipitate was formed as a result of hydrolysis. The resultant precipitate was filtered by using a vacuum filtration apparatus. The filtered precipitate was collected, and oven dried overnight in the laboratory at 80°C. The calcination process was conducted at 550°C for 5 h by using a muffle furnace under atmospheric conditions. The resultant calcined samples were collected, labelled, and stored in a laboratory desiccator before being further utilised for characterisations and experiments. Tabulated in Table S1 are the SiNC adsorbent descriptions based on the synthesis method that was employed.
2.3. Characterisation of Silica Nanocapsules (SiNC) AdsorbentFourier transform infrared, FTIR spectroscopy (Pelkin Elmer Spectrum One, USA) was utilised to analyse the functional groups on the surface of the SiNC. FTIR analysis was conducted in the wavenumber region of 4000 cm−1 to 400 cm−1 by using the KBr disc method. The morphologies of the SiNC adsorbent particles were observed by using High-Resolution Transmission Electron Microscopy, HR-TEM (Zeiss Libra 200, Germany), under a few appropriate magnifications in the range of 15,000 × to 80,000 ×. The surface area and porosity of the SiNC adsorbents were measured by using the BET Surface Area and Pore Size Analyzer (Micromeritics ASAP 2000, USA). The X-ray diffraction (XRD) patterns of the SiNC adsorbents were obtained using Cu Kα radiation of X-ray diffractometer (Xpert3 Powder, Panalytical). The thermogravimetric analysis, TGA was carried out by using the thermogravimetric analyser under atmospheric conditions at a temperature range of 30°C to 600°C with a heating rate of 10°C/min
2.4. Carbon Dioxide (CO2) Adsorption Performance MeasurementThe synthesised SiNC adsorbents were employed for preliminary carbon dioxide (CO2) gas adsorption to measure their adsorptive capability towards removing CO2 gas. Fig. S1 exhibit the schematic of the setup for the study of CO2 adsorption by using the SiNC adsorbents. An appropriate amount of the SiNC adsorbent was weighed and packed into the 5-mm diameter stainless steel adsorption column, sandwiched between glass wool. The packed SiNC adsorbent was activated by using thermal activation at 110°C for 1 h under a nitrogen (N2) gas flow. After 1 h, the SiNC adsorbent was left to cool at desired experimental temperature before turning on the CO2 gas flow. After the setup reached the desired temperature, the outlet of the adsorption reactor was connected to the gas analyser, and the CO2 gas flow was turned on at a rate of 60 ml/min. The outlet concentration of CO2 gas was recorded until adsorption equilibrium was achieved, which was indicated by a static trend in the data recorder. The adsorption capacity of the SiNC adsorbent, Q (mmol/g), was calculated by using the Eq. (1) [53, 54]:
where F is inlet flow rate of CO2 gas (mmol/min), CCO2,0 and CCO2,t is CO2 gas concentration at inlet and at time t, respectively, and W is mass of packed SiNC adsorbent (g).
3. Results and Discussions3.1. Characteristics of SiNC Adsorbent
Fig. 1 displays the infrared (IR) spectra of the silica nanocapsules (SiNC) adsorbent as observed under the wavelength region of 4000 cm−1 to 400 cm−1. The red spectra represented the IR of non-calcined SiNC adsorbent, while the blue spectra represented the IR of calcined SiNC adsorbent. The spectra of the SiNC adsorbent were typical of silica materials. The broad and rounded absorption peaks in the wavelength region of ~3400 cm−1 was attributed to the presence of hydroxyl functional group (−OH), the intermolecularly-bonded hydrogen molecules that were on the surface of the SiNC adsorbent. The sharp, asymmetric stretching of peaks that appeared at the wavelength region from 1100 cm−1 to 1000 cm−1 were attributed to the silanol functional group (Si-O-H/Si-O-R). The Si-O-H/Si-O-R functional group, which made up the main structure or the framework of the SiNC adsorbent, was present in high concentration. The high concentration of Si-O-H/Si-O-R was characterised by the strong sharp peaks in these wavelength regions. The weak but broad peaks in the wavelength region from 1650 cm−1 to 1630 cm−1 could be observed in all SiNC spectra. This peak represented the aliphatic alkane functional group (C–C) that emanated from CTAB and TEOS which were some of the chemicals that were utilised during the synthesis of SiNC adsorbent.
The spectra reveal a consistent trend of non-calcined samples with higher transmittance intensity as compared to their calcined counterparts. Higher transmittance of all non-calcined samples was ascribed to the higher hydration of non-calcined SiNC samples as compared to the calcined samples. As the calcined samples were subjected to elevated temperature treatment, the SiNC adsorbent lost its hydration, resulting in lower transmittance reading in the IR spectra. The trend shown in Fig. 1 is in line with the result that was reported by Chebli et al.[55] in their study. In respect to the use of different solvents in the synthesis of the SiNC adsorbent, few notes were observed. Ethanol as solvent resulted in higher intensity of the O-H peaks in the wavelength region at ~3400 cm−1. As ethanol was used as the solvent, the −OH from ethanol contributed to the stronger band appearance of the −OH peaks in the SEU and SEC spectra. The appearance of weak peaks in SEEU and SEEC spectra at the wavelength region of 2900 cm−1 to 2800 cm−1 characterised the presence of methyl (−CH3) functional group. The appearance of −CH3 functional group was due to the utilisation of diethyl ether during the SiNC synthesis. The peak at the wavelength region of 1700 cm−1 to 1600 cm−1 can also be assigned to the conjugated alkene group (C=C). The C=C peaks appeared as the toluene appear from the benzene ring and a conjugated methyl structure which made up the toluene, the solvent used during the synthesis.
Imaging analysis from transmission electron microscopy (TEM) enables the physical and structural characteristics of the SiNC particles to be examined and ascertained. Fig. 2 (a–f) represented the particle morphologies of the prepared SiNC adsorbent. The main objectives of utilising different solvents for SiNC synthesis are to ascertain which solvent will produce SiNC adsorbent with nanocapsules structure. The morphologies of the STU, STC, SEEU and SEEC shows toluene and diethyl ether as solvent resulted in SiNC adsorbent to have hollowed-core or capsules-like structure. Fig. 2(a) and 2(b) represented the non-calcined and calcined SiNC adsorbent synthesised from using toluene as solvent, STU and STC, respectively, which showed a non-uniform ellipsoidal structure. The particles were in agglomerated states, whereby the particles nearly merged with each other. Fig. 2(c) and 2(d) exhibit the morphologies of SEEU and SEEC, the non-calcined and calcined SiNC samples by using diethyl ether as solvent. Although the size of the particles is non-uniform, the SEEU and SEEC particles appeared to have corrugated surfaces, more spherical in shape and less agglomerated as compared to STU and STC samples. The high agglomeration of STU and STC could be accredited to the high viscosity of toluene. As viscosity is higher, hydrolysis rate of the silica precursor during the synthesis were slower, resulting in higher agglomeration. In comparisons, SEEU and SEEC are of lower particle agglomeration due to diethyl ether have lower viscosity. Fig. 2(e) and 2(f) which represent the SEU and SEC particles, respectively, showed that the particles were of solid nanoparticle structure. Contradictory to the use of toluene and diethyl ether as solvent, use of ethanol as solvent does not produce capsules-like structure. SEU and SEC particles are spherical in shape, nearly-uniform in size, and no appearance of particle agglomeration. The morphologies displayed by Fig. 2(e) and 2(f) conform with the morphologies reported by Yoo and Stein [45] in the synthesis of the mesoporous silica materials.
The N2 adsorption-desorption isotherm of the SiNC adsorbents at 77K and the corresponding pore size distribution are given by Fig. 3. The adsorption-desorption isotherm shows a typical Type IV isotherm as per IUPAC classification, which characterised the SiNC adsorbent as a mesoporous material. The narrow presence of hysteresis looping could be an indication that the SiNC adsorbent materials have uniform mesoporous structures. The Type IV adsorption-desorption isotherm also indicate that the SiNC adsorbents possess an average pore size of 2 – 50 nm. This finding is consistent with the shown pore size distribution shown in Fig. 3, where the distribution of the pore size concentrated around the region of 2 – 50 nm.
Tabulated in Table 1 are the result of surface area and porosity measurement of the SiNC adsorbents. The measurements were conducted at 77K, under nitrogen flow atmosphere at 1 bar pressure for 1h holding time. Results of surface area measurement showed SEEC adsorbent had the highest recorded surface area of 644 m2/g, followed by STC and SEC, whereas the measured surface area were 575 m2/g and 533 m2/g, respectively. The high surface area of the SEEC could be attributed to the physical structure of the SEEC adsorbent, whereby the surface was corrugated, and the core was hollowed. The corrugation and the hollowed core of the adsorbent provided more spaces for N2 adsorption/desorption during the BET measurement, resulting in bigger surface area reading. In comparisons to SEEC adsorbent, the STC and SEC adsorbents have moderately big surface area measurement in the range of 530 cm2/g to 580 cm2/g. STC had high surface area as compared to the SEC adsorbent on account of the capsules structure of the STC adsorbent, whereas the SEC was only of solid structure. As the SEC was the only solid structure with no hollowed core, the reading of BET measurement was lower as compared to the STC adsorbent. The measurement results also showed the effect of calcination process on the surface area and porosity of the SiNC adsorbent. It was found out that the calcination of the SiNC adsorbent resulted in increase of surface area and pore volume. The surface area and pore volume were adsorbent characteristics that were generally correlated as the increase in surface area measurement was typically associated with increased pore volume [56]. The increase in surface area of the SiNC adsorbent was attributed to the formation and expansion of pores (i.e., increase in pore volume) during the calcination process. As the non-calcined SiNC adsorbent might have contain water during its synthesis in the form of adsorbed water, capillary water, free water and water structure, these water forms were vapourised and released during the calcination at elevated temperature [57]. As a result of the water released from the SiNC adsorbent, pores became developed on the surface and subsequently expanded, resulting in bigger surface area reading of the calcined samples as compared to the non-calcined SiNC adsorbent samples. In addition, the smaller surface area measurement of the non-calcined SiNC adsorbent could be caused by the occupation of surfactant molecules in the non-calcined SiNC pores [58]. As the calcination process also vaporise the surfactant molecules, pores were vacant, resulting in bigger surface area reading.
The structural ordering of the SiNC adsorbent were analysed by the powder X-ray diffraction measurement. Fig. 4 displays the XRD pattern for the SiNC adsorbents. The XRD pattern show that the SiNC adsorbent shows similar pattern characteristics of regardless of its solvent type and calcination process indicating it have no apparent effect on the structural ordering of the SiNC adsorbent. It can be seen from Fig. 4 that the XRD patterns of all the SiNC adsorbent presents with one broad peak appearance around 2Θ = ~22° indicating that the SiNC adsorbents were of amorphous structure [59, 60].
The thermogravimetric of the SiNC adsorbent in a temperature range of 30°C to 600°C under atmospheric conditions is depicted by Fig. 5. In general, the thermogravimetric curves showed that the SiNC adsorbent experienced a two-stage weight loss process. For STU, STC, and SEU adsorbent, the samples experienced an initial weight loss process in a temperature range of 30°C to 170°C. This initial weight loss of the adsorbent could be attributed to the release of physically adsorbed CO2 gas from the surface of the adsorbent samples as well as other physically adsorbed volatile gas [61]. Besides, this weight loss could be explained by the desorption and desorption of adsorbed water molecules which are commonly released in this temperature range [62].
Fig. 5 also shows that the SEU adsorbent experienced a higher degree of weight loss characterised by the steeper gradient. SEU adsorbent might experience higher degree of weight loss as the adsorbent contained more adsorbed H2O molecules as compared to the STU and STC adsorbent samples. In contrast to the first stage, the second stage of the weight loss which occurred at temperature range of 200 – 300°C, was experienced by all of the SiNC adsorbent samples, shown by the DTG curve. In a heating temperature higher than 300°C, the weight loss experienced by the SiNC adsorbents were attributed by the particle template removal process. As reported by Park et al. [63] and Goworek et al. [64], the particle templating source, which was CTAB, utilised during the synthesis of the SiNC adsorbents, were volatilised at a temperature higher than 300°C. During the synthesis, CTAB were utilised in small amount, which conformed the minimal weight loss experienced by all SiNC adsorbents. It was interesting to note that SEEU and SEEC adsorbents experienced a different trend of weight change during the heating process. During the initial heating process, Fig. 5 reveals that the SEEU and SEEC adsorbents underwent a weight gain process, which was in contrast with other SiNC adsorbents. The weight gain trend shown by SEEU and SEEC adsorbents might be caused by the oxidation of diethyl ether during the heating process. As the diethyl ether was highly reactive with temperature change, it reacted with atmospheric oxygen, resulting in creation of by-products, which was characterised by the weight gained. Nevertheless, as the temperature was further increased, SEEU and SEEC adsorbents underwent weight-loss process, similar to the other SiNC adsorbents. This could also indicate that any by-products that were formed during the oxidation process might have been volatilised during the heating process. In addition, Fig. 5 also shows during the initial stage of the TGA measurement, the SEEC adsorbent experienced a faster weight gain compared to the SEEU adsorbent. As a result of the calcination process, the SEEC adsorbent possesses a larger surface area (644 m2/g), compared to the SEEU adsorbent, which are only 129 m2/g. Owing to the large surface area of the SEEC adsorbent, this may have caused more chemical reactions to take place, thus resulting in an increased amount of product formed on the surface of the adsorbent. Compared to the low surface area of the SEEU adsorbent, this may hinder any additional chemical reaction, thus resulting in slower weight gain trend which is observed in Fig. 5. In addition, the faster weight gain of the SEEC adsorbent can also be attributed to the large pore volume and smaller pore size of the adsorbent. On the account that the SEEC adsorbent have large pore volume and small pore size, the adsorbent can retain the product that may have formed better, resulting in more spaces for product storage, and consequently, slower weight loss, due to its smaller pore size.
3.2. CO2 Adsorption Performance of SiNC AdsorbentsThe adsorption capacity of the SiNC adsorbents towards CO2 gas under experimental conditions of 1 bar pressure and 30°C is shown in Fig. 6. Under the stated condition, the results show the calcined SiNC adsorbents performed a higher adsorption capacity towards CO2 gas compared to its non-calcined adsorbent counterparts. Among the calcined SiNC adsorbent, the STC adsorbent, which was synthesised using toluene as the solvent, demonstrated the highest adsorption capacity towards CO2 gas (Q = 2.59 mmol/g), followed by SEEC adsorbent (Q = 1.45 mmol/g), and SEC (Q = 1.28 mmol/g). Meanwhile, among the non-calcined SiNC adsorbents, a similar trend in adsorption capacity towards CO2 gas is observed, represented as QSTU> QSEEU> QSEU, based on the type of solvent used during the synthesis.
Adsorption capacity of an adsorbent can be closely correlated to its textural characteristics. As shown in Table 1, the STC adsorbent had a surface area of 575 m2/g, with highest pore volume of 1.64 cm2/g. The sufficiently big surface area and large pore volume provided sufficient active spaces for adsorption of CO2 molecules during the adsorption process. In addition, the big pore size of the STC adsorbent, 11.9 nm enabled higher diffusion of CO2 molecules into its pores framework, which in turn resulted in higher adsorption capacity of the STC towards CO2. Although Table 1 shows SEEC had a higher surface area as compared to the STC and SEC adsorbent, its adsorption capacity was lower than STC but higher than SEC. The sufficiently high adsorption capacity of the SEEC adsorbent were attributed to the high surface area of the SEEC adsorbent but might be limited by the low pore volume (1.26 cm3/g) and low pore diffusion caused by small pore size (8.09 nm). Amongst all the SiNC synthesised by different solvent, SEU and SEC adsorbent had the lowest adsorption capacity, 1.24 mmol/g, and 1.28 mmol/g, respectively.
In comparison to the existing studies, the STC adsorbent performed relatively higher CO2 removal capabilities as compared to the SBA-15 adsorbent by Zhao et al. [65] and hierarchical porous silica (HPS) adsorbent by Zhang et al. [66] and Ji et al. [67] whereas the reported CO2 adsorption capacity was 0.61 mmol/g, 0.56 mmol/g, and 0.3 mmol/g, respectively. The high CO2 adsorption capacity of the STC adsorbent might be attributed to the high surface area (575 m2/g) as compared to the SBA-15 (189 m2/g) [65] and HPS (544 m2/g) [67]. This was not the case with the HPS adsorbent by Zhang et al. [66], with a surface area of 978 m2/g, which showed that a high surface might contribute to the performance of CO2 removal of the silica materials. However, its functionalisation with amine increased its CO2 removal capacity, which showed that surface area and surface chemistry played a role in the CO2 removal.
To further test the adsorptive performance of the SiNC adsorbent, the highest performance SiNC adsorbent were subjected to CO2 adsorption/desorption experiment for over several cycles. The sample that was used for the CO2 adsorption/desorption experiment was STC adsorbent. In the cyclic adsorption study of the STC adsorbent, the CO2 adsorption was performed at 30°C under flow of pure CO2 gas, subsequently followed with thermal CO2 desorption at 110°C. Fig. 7 represents the result obtained from the cyclic adsorption study of the STC adsorbent. The result shows the STC adsorbent performed relatively stable CO2 adsorption/desorption performance for over five (5) cycles. This result shows STC not only performed the highest CO2 adsorption among the SiNC adsorbent, but also performed stable CO2 adsorption/desorption performance over several adsorption cycles suggesting promising capabilities as CO2 adsorbent.
Distinctly from activated carbon adsorbent materials, SiNC is a type of silica-based adsorbent materials with excellent adsorption performance that are not limited by their low selectivity towards CO2 during adsorption process [68]. It has been agreed that utilisation of adsorbent with excellent selectivity will result in a more efficient separation process, minimisation of process operational cost, improvement on process flexibility, produced product of higher purity, and reduced waste generation. In this study, the SiNC adsorbent are successfully synthesised and utilised in powdered form as it is more scale-appropriate for a lab-scaled CO2 adsorption process. Thus, further comprehensive investigation and studies will be required for the SiNC adsorbent to be commercialised and utilised for industrial application.
4. ConclusionsIn this work, synthesis of SiNC adsorbent as affected by varying the type of solvent used and calcination process were characterised and assessed. The infrared spectra of the SiNC adsorbent showed a typical spectra of a silica materials with presence of hydroxyl (−OH) and silanol (Si-O-R) functional group detected. Ethanol-based SiNC had higher transmittance of −OH peaks, diethyl ether had an extra peak that was associated with methyl functional group (−CH3). The morphological image showed that only toluene and diethyl ether produced SiNC with capsules structure. The BET measurement showed calcined SiNC had bigger surface area as compared to their non-calcined counterparts, whereas SEEC adsorbent had the biggest surface area (644 m2/g), followed by STC (575 m2/g) and SEC (533 m2/g). The preliminary CO2 adsorption study exhibited a similar trend whereby calcined SiNC adsorbent performed higher CO2 adsorption capacity as compared to the non-calcined SiNC adsorbent. STC adsorbent performed the highest CO2 adsorption capacity of 2.59 mmol/g, higher than SEEC (Q = 1.45 mmol/g) and SEC (1.28 mmol/g), with relatively stable cyclic adsorption performance for over five (5) cycle. Establishing from these findings, SiNC adsorbent presented as a promising adsorbent application as CO2 adsorbent.
AcknowledgementsThe authors would like to acknowledge the financial supports received from the Ministry of Higher Education (MOE) of Malaysia under the Fundamental Research Grant Scheme (FRGS/1/2020/TK0/UTP/02/24) and from Universiti Teknologi PETRONAS under YUTP-FRG Grant (Project Cost Centre: 015LC0-408).
NotesAuthor Contribution A.R.A.R. (PhD student) Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Visualization), K.J. (Supervisor) Conceptualization, Methodology, Supervision, Writing - Review & Editing, Funding acquisition), M.H. (Supervisor) Supervision, Conceptualization) References1. Policicchio A, Conte G, Agostino RG, et al. Hexagonal Mesoporous Silica for carbon capture: Unrevealing CO2 microscopic dynamics by Nuclear Magnetic Resonance. J. CO
2
. Util. 2022;55:101809.
https://doi.org/10.1016/j.jcou.2021.101809
2. Mukherjee S, Akshay , Samanta AN. Amine-impregnated MCM-41 in post-combustion CO2 capture: Synthesis, characterization, isotherm modelling. Adv. Powder Technol. 2019;30:3231–3240.
https://doi.org/10.1016/j.apt.2019.09.032
3. Shim JG, Lee DW, Lee JH, Kwak NS. Experimental study on capture of carbon dioxide and production of sodium bicarbonate from sodium hydroxide. Environ. Eng. Res. 2016;21:297–303.
https://doi.org/10.4491/eer.2016.042
4. Tiwari SK, Kim K-H, Singh RS, et al. A critical review on CO2 sequestration using construction and demolition waste: Future scope and perspective. Environ. Eng. Res. 2023;29:230256.
https://doi.org/10.4491/eer.2023.256
5. Rezazadeh H, Salahshoor Z, Ahmadi F, Farshad N. Reduction of carbon dioxide by bio-façades for sustainable development of the environment. Environ. Eng. Res. 2022;27:200580–200583.
https://doi.org/10.4491/eer.2020.583
6. Lei L, Cheng Y, Chen C, Kosari M, Jiang Z, He C. Taming structure and modulating carbon dioxide (CO2) adsorption isosteric heat of nickel-based metal organic framework (MOF-74(Ni)) for remarkable CO2 capture. J. Colloid Interface Sci. 2022;612:132–145.
https://doi.org/10.1016/j.jcis.2021.12.163
7. Olabi AG, Rezk H, Sayed ET, Ghoniem RM, Abdelkareem MA. Boosting carbon dioxide adsorption capacity applying Jellyfish optimization and ANFIS-based modelling. Ain Shams Eng. J. 2023;14:101931.
https://doi.org/10.1016/j.asej.2022.101931
8. Zhang H, Xue K, Cheng C, Gao D, Chen H. Study on the performance of CO2 capture from flue gas with ceramic membrane contactor. Sep. Purif. Technol. 2021;265:118521.
https://doi.org/10.1016/j.seppur.2021.118521
9. Niu M, Yang H, Zhang X, Wang Y, Tang A. Amine-Impregnated Mesoporous Silica Nanotube as an Emerging Nanocomposite for CO2 Capture. ACS Appl. Mater. Interfaces. 2016;8:17312–17320.
https://doi.org/10.1021/acsami.6b05044
10. Wang S, Lee YR, Won Y, et al. Development of high-performance adsorbent using KOH-impregnated rice husk-based activated carbon for indoor CO2 adsorption. Chem. Eng. J. 2022;437:135378.
https://doi.org/10.1016/j.cej.2022.135378
11. Yan H, Zhang G, Xu Y, et al. High CO2 adsorption on amine-functionalized improved macro-/mesoporous multimodal pore silica. Fuel. 2022;315:123195.
https://doi.org/10.1016/j.fuel.2022.123195
12. Chen Y, Wu J, Wang X, Liu M, Liu Y. Synthesis, Characterization and Application of Amine-Functionalized Hierarchically Micro-Mesoporous Silicon Composites for CO2 Capture in Flue Gas. Molecules. 2022;27:3429.
https://doi.org/10.3390/molecules27113429
13. Gopalan J, Buthiyappan A, Abdul Raman AA. Insight into metal-impregnated biomass based activated carbon for enhanced carbon dioxide adsorption: A review. J. Ind. Eng. Chem. 2022;113:72–95.
https://doi.org/10.1016/j.jiec.2022.06.026
14. Liu RS, Shi XD, Wang CT, et al. Advances in Post-Combustion CO2 Capture by Physical Adsorption: From Materials Innovation to Separation Practice. ChemSusChem. 2021;14:1428–1471.
https://doi.org/10.1002/cssc.202002677
15. Serafin J, Cruz OF. Promising activated carbons derived from common oak leaves and their application in CO2 storage. J. Environ. Chem. Eng. 2022;10:107642.
https://doi.org/10.1016/j.jece.2022.107642
16. Yang R, Yang Q. A review of emerged constructed wetlands based on biochar filler: Wastewater purification and carbon sequestration/greenhouse gas reduction. Environ. Eng. Res. 2023;29:230105–0.
https://doi.org/10.4491/eer.2023.105
17. Fu D, Park Y, Davis ME. Confinement effects facilitate low-concentration carbon dioxide capture with zeolites. Proc. Natl. Acad. Sci. U. S. A. 2022;119:1–7.
https://doi.org/10.1073/pnas.2211544119
18. Jun HJ, Yoo DK, Jhung SH. Metal-organic framework (MOF-808) functionalized with ethyleneamines: Selective adsorbent to capture CO2 under low pressure. J. CO
2
. Util. 2022;58:101932.
https://doi.org/10.1016/j.jcou.2022.101932
19. Francia V. Towards responsive gas-solid operations: Oscillating and vortex flows. Chem. Eng. Process. - Process Intensif. 2023;186:109324.
https://doi.org/10.1016/j.cep.2023.109324
20. Sigonya S, Mokhothu TH, Mokhena TC, Makhanya TR. Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review. Appl. Sci. 2023;13:772.
https://doi.org/10.3390/app13020772
21. Maia F, Tedim J, Lisenkov AD, Salak AN, Zheludkevich ML, Ferreiraa MGS. Silica nanocontainers for active corrosion protection. Nanoscale. 2012;1287–1298.
https://doi.org/10.1039/c2nr11536k
22. Qiao Y, Li W, Wang G, Zhang X, Cao N. Application of ordered mesoporous silica nanocontainers in an anticorrosive epoxy coating on a magnesium alloy surface. RSC Adv. 2015;5:47778–47787.
https://doi.org/10.1039/c5ra05266a
23. Cao Z, Xu C, Ding X, Zhu S, Chen H, Qi D. Synthesis of fragrance/silica nanocapsules through a sol–gel process in mini-emulsions and their application as aromatic finishing agents. Colloid Polym. Sci. 2015;293:1129–1139.
https://doi.org/10.1007/s00396-015-3502-2
24. Liu N, Dunphy DR, Atanassov P, et al. Photoregulation of mass transport through a photoresponsive azobenzene-modified nanoporous membrane. Nano Lett. 2004;4:551–554.
https://doi.org/10.1021/nl0350783
25. Zhang Y, Hsu BYW, Ren C, Li X, Wang J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2015;44:315–335.
https://doi.org/10.1039/c4cs00199k
26. Huang J-L, Nayak P. Processing and Characterization of Alumina/Chromium Carbide Ceramic Nanocomposite. Hashim A, editorAdvances in Nanocomposite Technology. Rijeka: IntechOpen; 17899.
https://doi.org/10.5772/17899
27. Jiang X, Brinker CJ. Aerosol-assisted self-assembly of single-crystal core/nanoporous shell particles as model controlled release capsules. J. Am. Chem. Soc. 2006;128:4512–4513.
https://doi.org/10.1021/ja058260+
28. Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Deliv. Rev. 2002;54:S77–S98.
https://doi.org/10.1016/S0169-409X(02)00116-3
29. Santiago AM, Ribeiro T, Rodrigues AS, et al. Multifunctional Hybrid Silica Nanoparticles with a Fluorescent Core and Active Targeting Shell for Fluorescence Imaging Biodiagnostic Applications. Eur. J. Inorg. Chem. 2015;2015:4579–4587.
https://doi.org/10.1002/ejic.201500580
30. Chen B, Zhou D, Zhu L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008;42:5137–5143.
https://doi.org/10.1021/es8002684
31. Maryam Hafezian S, Biparva P, Bekhradnia A, Naser Azizi S. Amine and thiol functionalization of SBA-15 nanoparticles for highly efficient adsorption of sulforaphane. Adv. Powder Technol. 2021;32:779–790.
https://doi.org/10.1016/j.apt.2021.01.025
32. Benamor T, Miesh J, Montégut G, Nouali H, Marichal C, Lebeau B. Post-synthesis grafting of fluoropropyl groups on SBA-15 type ordered mesoporous silica. Eur J Inorg Chem. 2012;5371–5379.
https://doi.org/10.1002/ejic.201201001
33. Rajabzadeh M, Khalifeh R, Eshghi H, Hafizi A. Design and synthesis of CuO@SiO2 multi-yolk@shell and its application as a new catalyst for CO2 fixation reaction under solventless condition. J. Ind. Eng. Chem. 2020;89:458–469.
https://doi.org/10.1016/j.jiec.2020.06.020
34. Shi J, Cui H, Xu J, Yan N, Liu Y. Design and fabrication of hierarchically porous carbon frameworks with Fe2O3 cubes as hard template for CO2 adsorption. Chem. Eng. J. 2020;389:124459.
https://doi.org/10.1016/j.cej.2020.124459
35. Singh B, Polshettiwar V. Solution-phase synthesis of two-dimensional silica nanosheets using soft templates and their applications in CO2 capture. Nanoscale. 2019;11:5365–5376.
https://doi.org/10.1039/c8nr10119a
36. Chen D, Fu Y, Yu W, Yu G, Pan C. Versatile Adamantane-based porous polymers with enhanced microporosity for efficient CO2 capture and iodine removal. Chem. Eng. J. 2018;334:900–906.
https://doi.org/10.1016/j.cej.2017.10.133
37. Montaño-Priede JL, Coelho JP, Guerrero-Martínez A, Peña- Rodríguez O, Pal U. Fabrication of Monodispersed Au@SiO2 Nanoparticles with Highly Stable Silica Layers by Ultrasound-Assisted Stöber Method. J. Phys. Chem. C. 2017;121:9543–9551.
https://doi.org/10.1021/acs.jpcc.7b00933
38. Wibowo D, Hui Y, Middelberg APJ, Zhao CX. Interfacial engineering for silica nanocapsules. Adv. Colloid Interface Sci. 2016;236:83–100.
https://doi.org/10.1016/j.cis.2016.08.001
39. Savic S, Vojisavljevic K, Počuča-Nešić M, Zivojevic K, Mladenovic M, Knezevic N. Hard Template Synthesis of Nanomaterials Based on Mesoporous Silica. Metall. Mater. Eng. 2018;24:1.
https://doi.org/10.30544/400
40. Xie Y, Kocaefe D, Chen C, Kocaefe Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016.
https://doi.org/10.1155/2016/2302595
41. Zakaria J, Abd Shukor SR, Abdul Razak K. Effect of Surfactant, Solvent and Stirring Rate on the Synthesis of Silica Nanoparticles Entrapped Rifampicin. J. Chem. Eng. Ind. Biotechnol. 2020;5:36–47.
https://doi.org/10.15282/jceib.v5i2.3677
42. Flood-Garibay JA, Méndez-Rojas MA. Effects of co-solvent nature and acid concentration in the size and morphology of wrinkled mesoporous silica nanoparticles for drug delivery applications. Molecules. 2021;26:4186.
https://doi.org/10.3390/molecules26144186
43. Salabat A, Eastoe J, Mutch KJ, Tabor RF. Tuning aggregation of microemulsion droplets and silica nanoparticles using solvent mixtures. J. Colloid Interface Sci. 2008;318:244–251.
https://doi.org/10.1016/j.jcis.2007.10.050
44. Themis M, Erdogan G. Monomer-addition growth with a slow initiation step: A growth model for silica particles from alkoxides. J. Colloid Interface Sci. 1989;132:13–21.
45. Yoo WC, Stein A. Solvent effects on morphologies of mesoporous silica spheres prepared by pseudomorphic transformations. Chem. Mater. 2011;23:1761–1767.
https://doi.org/10.1021/cm102829m
46. Thangarajoo N, Abdul Rahim AR, Johari K, Saman N. (3-Aminopropyl) triethoxysilane-functionalized silica nanocapsule adsorbent: Synthesis and analysis of physicochemical characteristics. J. Solid State Chem. 2022;310:123019.
https://doi.org/10.1016/j.jssc.2022.123019
47. Abdul Rahim AR, Hao TT, Wan Azhari AA, Saman N, Mat H, Johari K. Synthesis and characterization of secondary amine-functionalized silica for CO2 capture. IOP Conf. Ser. Earth Environ. Sci. 2021;765:012091.
https://doi.org/10.1088/1755-1315/765/1/012091
48. Yang J, Fan W, Bell CM. Effect of calcination atmosphere on microstructure and H2/CO2 separation of palladium-doped silica membranes. Sep. Purif. Technol. 2019;210:659–669.
https://doi.org/10.1016/j.seppur.2018.08.041
49. Sari Yilmaz M. Graphene oxide/hollow mesoporous silica composite for selective adsorption of methylene blue. Microporous Mesoporous Mater. 2022;330:111570.
https://doi.org/10.1016/j.micromeso.2021.111570
50. Li T, Geng T, Md A, Banerjee P, Wang B. Novel scheme for rapid synthesis of hollow mesoporous silica nanoparticles (HMSNs) and their application as an efficient delivery carrier for oral bioavailability improvement of poorly water-soluble BCS type II drugs. Colloids Surfaces B Biointerfaces. 2019;176:185–193.
https://doi.org/10.1016/j.colsurfb.2019.01.004
51. Ruggiero L, Di Bartolomeo E, Gasperi T, et al. Silica nanosystems for active antifouling protection: nanocapsules and mesoporous nanoparticles in controlled release applications. J. Alloys Compd. 2019;798:144–148.
https://doi.org/10.1016/j.jallcom.2019.05.215
52. Lu H, Wei D, Zheng R, Xu S. Post-imprinting modification based on multilevel mesoporous silica for highly sensitive molecularly imprinted fluorescent sensors. Analyst. 2019;144:6283–6290.
https://doi.org/10.1039/c9an01503e
53. Raganati F, Alfe M, Gargiulo V, Chirone R, Ammendola P. Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite- based sorbent. Chem. Eng. J. 2019;372:526–535.
https://doi.org/10.1016/j.cej.2019.04.165
54. Yang FM, Zhou XY, DaLi X, Yi ZC, Feng R, He GW. Hollow urchin-shaped NCM811 ternary-structure for high rate charge/discharge capability and efficient CO2 adsorption. J Environ Chem Eng. 2023;11:109445.
https://doi.org/10.1016/j.jece.2023.109445
55. Chebli D, Bouguettoucha A, Reffas A, et al. Removal of the anionic dye Biebrich scarlet from water by adsorption to calcined and non-calcined Mg–Al layered double hydroxides. Desalin. Water Treat. 2016;57:22061–22073.
https://doi.org/10.1080/19443994.2015.1128365
56. Blankenship LS, Mokaya R. Modulating the porosity of carbons for improved adsorption of hydrogen, carbon dioxide, and methane: A review. Mater. Adv. 2022;3:1905–1930.
https://doi.org/10.1039/d1ma00911g
57. Sun ZX, Zheng TT, Bo QB, Du M, Forsling W. Effects of calcination temperature on the pore size and wall crystalline structure of mesoporous alumina. J. Colloid Interface Sci. 2008;319:247–251.
https://doi.org/10.1016/j.jcis.2007.11.023
58. Şahin RZY. Understanding the Effect of Calcination Process on the Mesoporous MCM-41 Material Morphology. J. Turkish Chem. Soc. 2021;4:27–34.
59. Ali R, Shaghaghi Z, Aghahosseini H, Asiabi PA, Woo Joo S. Silica nanoparticles as a highly efficient catalyst for the onepot synthesis of sterically congested 2-(dibenzylamino)-2-aryl acetamide derivatives from by phthaldehyde isomers, isocyanides and dibenzylamine. Bull. Chem. Soc. Ethiop. 2014;30:22–31.
60. Purnawira B, Purwaningsih H, Ervianto Y, et al. Synthesis and characterization of mesoporous silica nanoparticles (MSNp) MCM 41 from natural waste rice husk. IOP Conf. Ser. Mater. Sci. Eng. 2019;541:012018.
https://doi.org/10.1088/1757-899X/541/1/012018
61. Kishor R, Ghoshal AK. High molecular weight polyethyleneimine functionalized three dimensional mesoporous silica for regenerable CO2 separation. Chem. Eng. J. 2016;300:236–244.
https://doi.org/10.1016/j.cej.2016.04.055
62. Bae JY, Jang SG. Preparation and Characterization of Amine-Functionalized Mesoporous Hollow Silica for CO2 Capture. J. Nanosci. Nanotechnol. 2020;20:7070–7074.
https://doi.org/10.1166/jnn.2020.18843
63. Park HS, Kim TH, Lee MH, Song HK. Catalytic carbonization of an uncarbonizable precursor by transition metals in olivine cathode materials of lithium ion batteries. J. Mater. Chem. 2012;22:20305–20310.
https://doi.org/10.1039/c2jm33841f
64. Goworek J, Kierys A, Gac W, Borówka A, Kusak R. Thermal degradation of CTAB in as-synthesized MCM-41. J. Therm. Anal. Calorim. 2009;96:375–382.
https://doi.org/10.1007/s10973-008-9055-6
65. Zhao P, Zhang G, Hao L. A novel blended amine functionalized porous silica adsorbent for carbon dioxide capture. Adsorption. 2020;26:749–764.
https://doi.org/10.1007/s10450-020-00238-z
66. Zhang G, Zhao P, Xu Y. Development of amine-functionalized hierarchically porous silica for CO2 capture. J. Ind. Eng. Chem. 2017;54:59–68.
https://doi.org/10.1016/j.jiec.2017.05.018
67. Ji C, Huang X, Li L, Xiao F, Zhao N, Wei W. Pentaethylenehexamine-loaded hierarchically porous silica for CO2 adsorption. Materials (Basel). 2016;9:835.
https://doi.org/10.3390/ma9100835
68. Adegoke KA, Oyedotun KO, Ighalo JO, et al. Cellulose derivatives and cellulose-metal-organic frameworks for CO2 adsorption and separation. J. CO
2
. Util. 2022;64:102163.
https://doi.org/10.1016/j.jcou.2022.102163
Fig. 2Morphological imaging of the SiNC adsorbent a) STU, b) STC, c) SEEU, d) SEEC, e) SEU and f) SEC as visualised by TEM imaging. 
Fig. 3The N2 adsorption-desorption isotherm at 77K and the corresponding pore size distribution curve of the SiNC adsorbents. 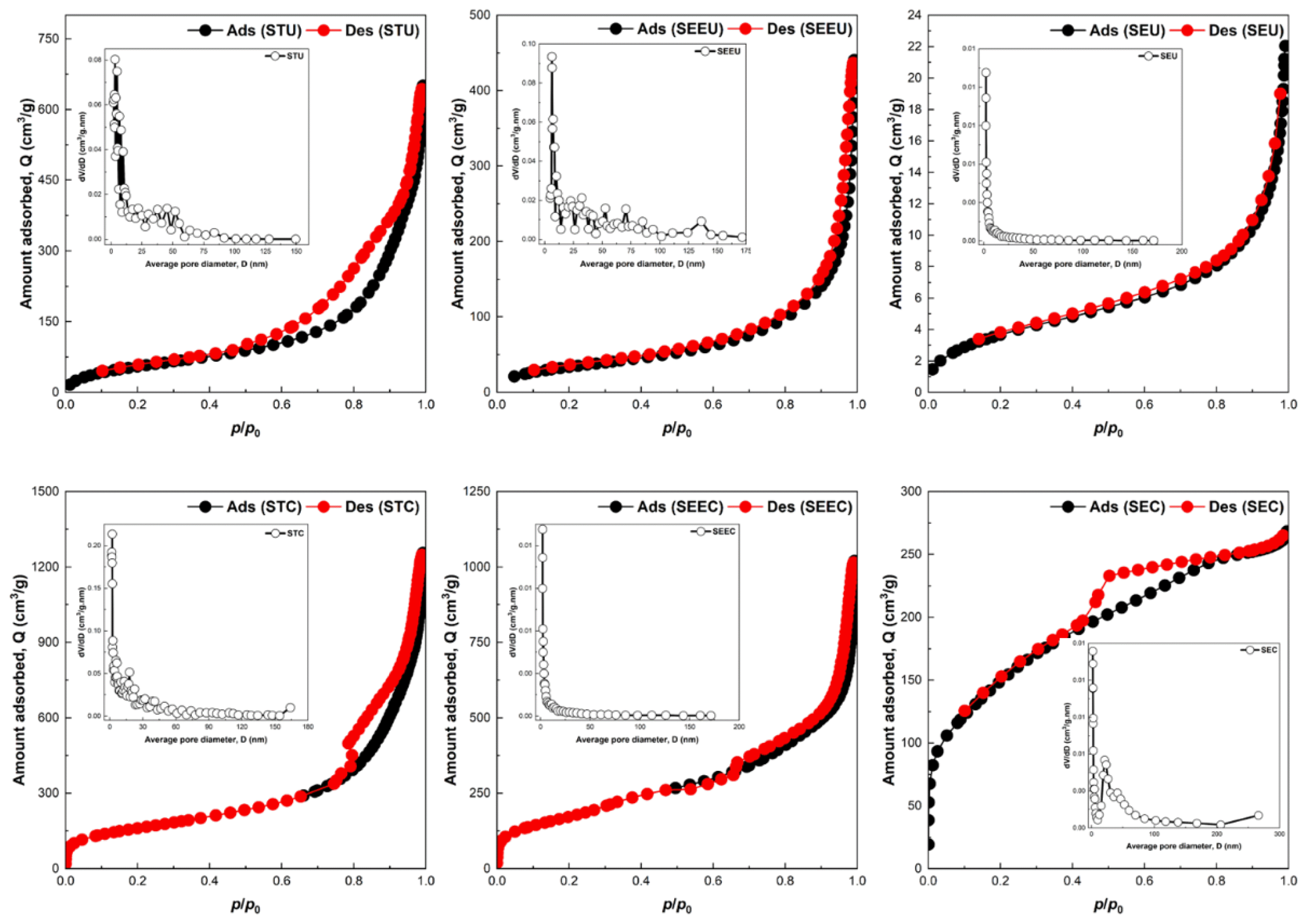
Table 1Textural characteristics of the SiNC adsorbent synthesis under different solvent and calcination process. |
|
||||||||||||||||||||||||||||||||||||