AbstractRaw white clay was acidified with H2SO4 (2 M) and then activated in a furnace for 2 h at 150°C. The resulting acid-activated clay product was used as an adsorbent for the removal of radioactive substances such as Cs+, Sr2+ and Co2+ ions in aqueous solution. The specific surface area and the pore volume of acid-activated clay were three times higher compared to raw white clay. At 100 mg L− of initial concentration and 1.0 g of dosage, removal efficiencies of Cs+, Sr2+ and Co2+ ions showed 60.0%, 27.3%, and 88.1%, respectively. It was found that the Langmuir isotherm was described well to the adsorption behavior of Cs+, Sr2+ and Co2+ ions on acid-activated clay rather than Freundlich isotherm. The Langmuir isotherm constant (Q) for Cs+, Sr2+ and Co2+ ions was found to be 8.81, 3.78 and 10.20 mg g−1, respectively. Compared to the pseudo-first-order kinetic model, the pseudo-second-order kinetic model was more suitable for adsorption of Cs+, Sr2+ and Co2+ ions in water/acid-activated clay medium owing to the higher correlation coefficient (R2) and the more proximity value of the experimental value qe,exp and the calculated value qe,cal.
Graphical Abstract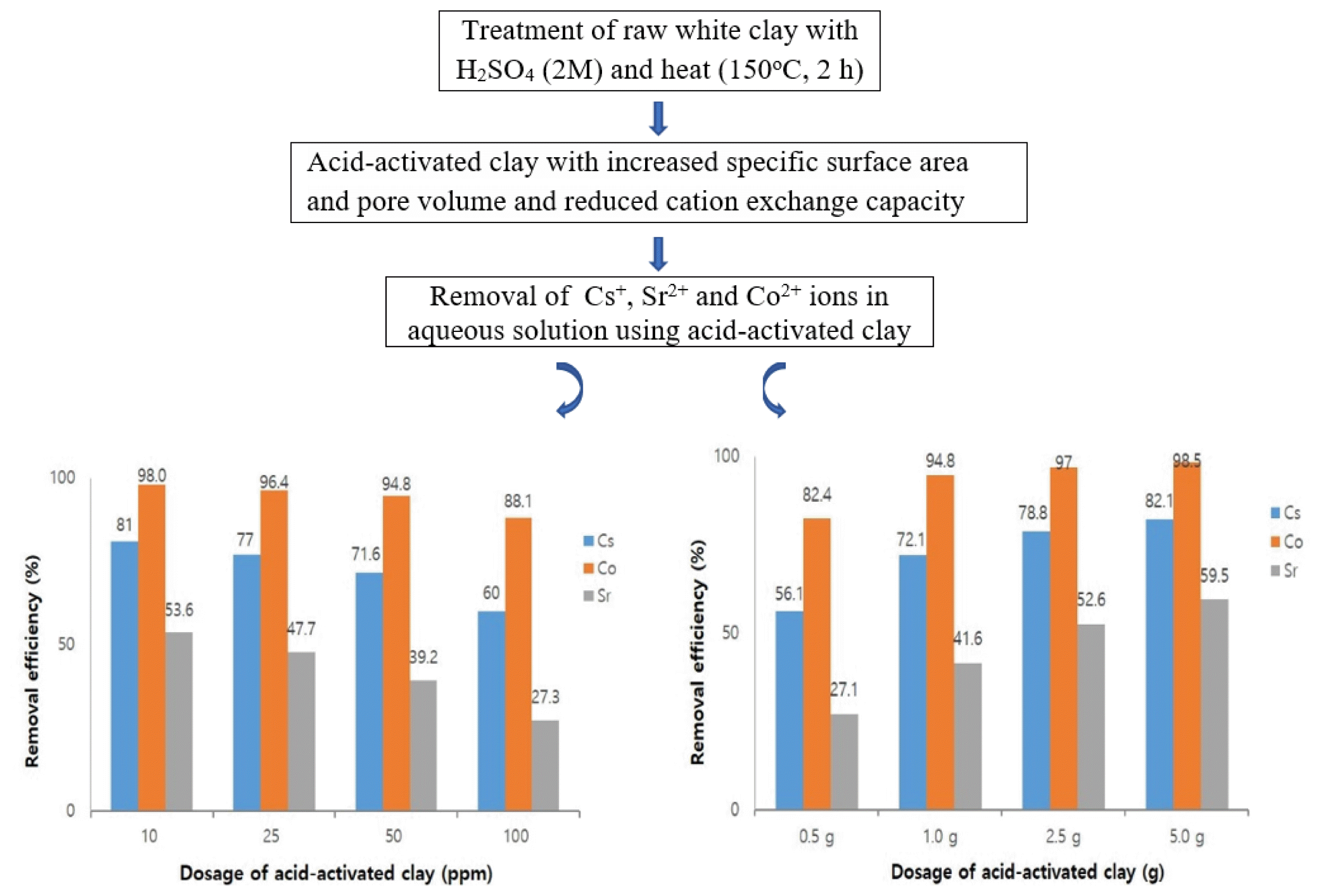
1. IntroductionNuclear power plants provide economically and stably producing optimal energy among the current electricity supply technologies. However, when they are operated, repaired and disposed, or damaged by an external shock, harmful radioactive pollutants can be inevitably generated. As an example, the Fukushima Daiichi Nuclear Power Plant disaster in Japan in 2011 released a large amount of radioactive materials such as Cs, Sr and Co, which rarely exist in the nature, into the environment [1]. Due to this incident, the surrounding atmosphere, soil, and seawater were significantly contaminated with these radioactive substances. The half-lives of 137Cs, 90Sr and 60Co are known to be 30.2 years, 28.7 years and 5.27 years, respectively, and these long half-lives leads to severe diseases when exposed to humans for a long time [2]. In particular, Cs+ and Sr2+ ions have similar chemical properties as K+ and Ca2+ ions, which are essential elements of life [3]. For this reason, they are easily absorbed and concentrated into the biological organization. It is generally well known that Cs+ ions bring about various cancers, general paralysis, and infertility in the human body [4]. When Sr2+ ions are ingested into the body, it accumulates in the bones and is difficult to be excreted from the body, so it arouses bone marrow cancer and leukemia, and adversely affects animals and plants such as genetic mutations [5]. Long-term exposure to Co2+ ions has also been known to cause heartburn, dizziness, and local skin necrosis [6].
To date, methods for treating liquid radioactive waste have been introduced in many literatures. The available methods include ionic exchange, coagulative precipitation, reverse osmosis filtration, membrane distillation, adsorption, electrochemical separation, etc., and these methods were treated alone or in combination of several methods [7, 8]. Among the methods for removing radioactive substances from liquid radioactive waste, adsorption has turned out to be diverse, economical, and convenient to operate. Therefore, a plenty of reviews have been reported concerning the materials of adsorbent that can be removed radioactive substances from liquid radioactive waste [9–11]. The adsorption materials that have been developed and applied for practical use so far are Prussian blue, which is used as a paint or dye in a core material (acrylic acid polymerization) [12, 13], crown ether and calixarene derivatives [14], metal (Fe3+/Cu2+) ferrocyanide [15], carbonated cement slurry powder [16], crystalline silicotitanate [17], iron hexacyanoferrate/graphene/carbon fiber composite [18], metal hexacyanoferrates (MHCF) [19, 20], hybrid porous magnetic bentonite-chitosan beads [21] and etc. However, although these adsorbents are very effective in removing radioactive substances, they have limitations in terms of cost and flexibility of process for producing the adsorbent.
As an alternative, it is necessary to introduce an adsorbent that can be supplied cheaply and stably, and is environmentally friendly. Raw clay has the potential capability as core or supporting material to eliminate metal ions from wastewater by providing a large amount of active sites in its layer structure [22]. However, in general, raw clay itself is known to be significantly less capable of adsorbing various adsorbates [23. 24]. Therefore, in order to enhance this, there needs to increase the adsorption capacity of raw clay through activation. One of the most appropriate activation techniques is an inorganic acid treatment process. It showed that acid-activated clay improves the adsorption capability by increasing the specific surface area and porosity as well as active sites as compared to raw clay [25, 26]. In the process of treating raw clay with acid, the most commonly used acids are hydrochloric acid and sulfuric acid. In industry, H2SO4 is relatively chosen more than HCl because it is less expensive and is not as harsh as HCl [27]. This makes the acid-activated clay more economical and effective when remove radioactive substances from aqueous solution.
In this study, authors used H2SO4 as an acid modifier to activate raw white clay, and compared the change of physicochemical properties for raw white clay and acidic activated product. After that, adsorption experiments were conducted to evaluate the possibility of removing radioactive substances such as Cs+, Sr2+ and Co2+ ions from aqueous solution using acid-activated clay manufactured in this study. Various experimental conditions such as contact time, dosage of acid-activated clay, and initial concentrations of radioactive substances were investigated in detail to determine optimal conditions. Aside from these, the adsorption isotherm and kinetic models were used to establish the adsorption rate and the maximum adsorption capacity as well as the mechanism for adsorption of radioactive substances.
2. Materials and Methods2.1. Materials2.1.1. Preparation of acid (H2SO4)-activated clayRaw white clay was obtained from the ocean shore in Pohang, Gyeongsangbuk-do. The obtained white clay contains a montmorillonite ((MgAl)4Si8O20(OH)4·nH2O) as the main component, which are member of 2 : 1 layer silicate family which share the common feature that two tetrahedral sheets consisting of Al sandwich a sheet of octahedrally coordinated Si. The raw white clay was firstly calcined in a muffle furnace at 600°C for 2 h. The calcined raw white clay was then heated at 80°C for 6 h under mechanical stirring after adding H2SO4 (2 M) solution. In succession, the resulting acidified white clay was washed several times with distilled water for the purpose of removing sulfate ions and impurities. Then, acidified white clay was dried in an oven at 105°C overnight. Following drying in an oven, the acidified clay was activated in a furnace for 2 h at 150°C using a heating rate of 10°C per min and cooled in a desiccator at room temperature prior to experiment. Acid-activated white clay in the particle size range of 100 μm to 200 μm was employed as adsorbent for the adsorption of Cs+, Sr2+ and Co2+ ions.
2.1.2. ReagentsCesium chloride (CsCl), Strontium chloride hexahydrate (SrCl2· 6H2O), and Cobalt chloride hexahydrate (CoCl2·6H2O) were supplied by Daejeong Chemicals & Metals, Co, Ltd. Siheung-si, Korea. All reagents were used as received without further purification. A stock solution containing 1000 mg L−1 of Cs+, Sr2+ and Co2+ ions was provided by dissolving above-mentioned reagents with distilled water. The desired concentrations in this experiment were prepared from a stock solution through continuous dilutions. The cation exchange capacity (CEC) of raw white clay and acid-activated clay was measured according to the Agricultural Environmental Resource Analysis Method.
2.2. Methods2.2.1. Instrumental analysisThe specific surface area, BJH (Barret-Joyner-halenda) pore size, and BJH pore volume were investigated with a gravimetric nitrogen BET (Brunauer-Emmett-Teller) surface area analyzer (BET, 3 Flex, Micrometrics, Atlanta, USA). The mineral oxides were measured by X-ray fluorescence spectroscopy (XRF, ZSX Primus II, Rigaku, Japan). The elemental composition was determined using energy dispersive X-ray spectroscopy (EDX, X-MaxN, Oxford, UK). The pH was observed using a pH meter (Radiometer, PHM 250 ion analyzer, Woonsocket, USA). Cs+, Sr2+ and Co2+ ions were analyzed using an inductively coupled plasma-atomic emission spectrometer (ICP/AES, Flame Modula S, Spectro, Kleve, Germany). CECs were quantified by ion chromatography with a conductivity detector (940 professional IC Vario, Metrohm, Swiss).
2.2.2. Adsorption studyThe adsorption experiments were conducted in a batch type. Acid-activated white clay (0.5 – 5.0 g) was added into 500 mL of solution (25 – 100 mg L−) containing Cs+, Sr2+ and Co2+ ions and shaken on a laboratory shaker at the ambient temperature without adjusting pH. A certain amount of solution was collected at a designated time of 5, 10, 30, 60, 120 mins and filtered with a GF/C (0.45 μm) filter paper. The supernatant was stored in the refrigerator prior to analysis.
where, V (L) is the volume of the solution and W (g) is the weight of adsorbent. C0 (mg/L) and Ce (mg/L) are initial and equilibrium concentration of the adsorbate, respectively. qe (mg/g) is the amount of adsorbate adsorbed per g of adsorbent at equilibrium.
3. Results and Discussion3.1. Characterization of Acid-Activated Clay3.1.1. XRF analysis
Table 1 shows the constituents of oxide present in raw white clay and acid-activated clay obtained by XRF. As shown in Table 1, major oxides consisting of raw white clay and acid-activated clay were Al2O3, SiO2, SO3, CaO and Fe2O3, along with other minor constituents. It was found that the content of Al2O3, SO3, CaO and Fe2O3 in acid-activated clay was reduced compared to those in raw white clay. On the other hand, the content of SiO2 was significantly increased. During acid treatment of raw white clay, most of oxides including calcium, magnesium, aluminum, and iron are partially dissolved from the lattice except for silicone that stay in solid state. For instance, the weight percentage of MgO, Al2O3 and Fe2O3 dropped by 51.9%, 30.2% and 75.8%, respectively. This confirms that the leaching of interlayer cations gives rise to the structural changes in the raw white clay, which leads to opening of the lattice and an increase in internal surface area. Consequentially, acid-activated clay is almost saturated with hydrogen ions through the replacement of cations such as Mg2+, Al3+, Ca2+ and Fe3+ located at the surface of the lattice with H+ from acid and exhibits acidic property (Fig. 1). It suggests that acid-activated clay can have better adsorption capacity than raw white clay.
3.1.2. EDX analysisEDX was used to indentify the elemental constituents of raw white clay and acid-activated clay, and its analysis results were listed in Table 2. The main elements constituting raw white clay and acid-activated clay were O, Al, Si, S and Fe. The wt.% of Al, S, Ca, Mg and Fe of acid-activated clay showed lower than raw white clay, whereas Si showed the opposite trend and TiO2 remained unchanged. These results are almost completely consistent with data by XRF. When raw white clay is treated with sulfuric acid, above-mentioned main elements are more easily eluted, causing deformation of the the lattice of raw white clay. This phenopenon results in the further formation of pore, which in turn leads to an increase in surface acidity.
3.1.3. Textural analysis
Table 3 represents the analysis results for the textural properties of raw white clay and acid-activated clay. The values of the BET surface area and BJH pore volume of acid-activated clay were about 3 times higher in comparison to those of the raw white clay, while BJH pore size of acid-activated clay was slightly reduced. All of these changes are attributed to a consequence of the leaching of the several cations such as Fe3+, Al3+ and Mg2+ during acid activation, which could develop not only more porous structure and internal surface area but also smaller particles. The pH value of acid-activated clay was 2.9, indicating it can be regarded as an acidic adsorbent.
3.1.4. Analysis of cation exchange capacity (CEC)The CEC by raw white clay and acid-activated clay is shown in Fig. S1. As seen, when raw white clay was treated with sulfuric acid, there was a significant change in the CEC. The CEC of the acid-activated clay was largely decreased compared to raw white. The reduction of CEC may be due to the reason that main cations existing interlayers of raw white clay are eluted by acid and substituted with hydrogen ions as stated earlier.
3.2. Effects of Initial Concentration
Fig. S2 (a, b, c) show a comparison of the amount of Cs+, Sr2+ and Co2+ ions adsorbed by acid-activated clay as a function of contact time (5–120 min). Where, the dosage of acid-activated clay was 1.0 g and the initial concentrations of Cs+, Sr2+ and Co2+ ions were 10, 25, 50 and 100 mg L−, respectively. In the case of Cs+ and Sr2+ ions, a rapid adsorption appeared with in 5 min of contact time. Thereafter, the adsorbed amount remained constant after 10 min of contact time, implying that equilibrium was reached. On the contrary of this, the equilibrium of Co2+ ions appeared later than that of Cs+ and Sr2+ ions. The removal efficiencies for the initial concentrations of Cs+, Sr2+ and Co2+ ions at equilibrium were depicted in Fig. 2. When the initial concentration of Cs+ ions increased from 10 mg L− to 100 mg L−, removal efficiency of Cs+ ions steadily decreased from 81% to 60%. This trend was similar for Sr2+ and Co2+ ions. This is because the gradual increase in initial concentration gradually saturates the active adsorption sites at fixed adsorbent amount. For this reason, it can be seen that even if the initial concentration of Cs+, Sr2+ and Co2+ ions were increased further, it does not have any effect on the improvement of adsorption efficiency of the acid-activated clay. It was found that the higher removal efficiency by acid-activated clay regardless of the initial concentrations was in order of Co2+ > Cs+ > Sr2+.
3.3. Effects of DosageThe effect of adsorbent dosage on the adsorption of Cs+, Sr2+ and Co2+ ions in aqueous solution were studied by varying the amount of the acid-activated clay according to contact time. In this study, the dosage of acid-activated clay was 0.5 g to 5.0 g and the initial concentration of Cs+, Sr2+ and Co2+ ions was 50 mg L−. The degree of adsorption obtained were plotted versus the dosage of acid-activated clay for Cs+, Sr2+ and Co2+ ions (Fig. S3). As seen in Fig. 5(a, b, c), adsorption took place quickly in the few minutes and then the adsorption rate became slow. Fig. 3 shows that the adsorption capacity of Cs+, Sr2+ and Co2+ ions gradually increased as the dosage of acid-activated clay was increased from 0.5 g to 5.0 g. The removal efficiency of Cs+ ions showed 56.1%, 72.1%, 78.8% and 82.1% at 0.5 g, 1.0 g, 2.5 g and 5.0 g of acid-activated clay, respectively. Unexpectedly, removal efficiency of Cs+ ions did not improve proportionally even if the dosage was increased. Effects of further increase in adsorbent dosage were only slight. At higher adsorbent dosage, anyway, the increase in removal percentage of Cs+ ions was suppressed. This trend was similar in the cases of Sr2+ and Co2+ ions. It is assumed that the reduction of available adsorption sites of acid-activated clay is caused by agglomeration of acid-activated clay at higher dosages. Therefore, it is thought that it is preferable to use an appropriate amount of acid-activated clay rather than excessive when trying to adsorb radioactive substances in aqueous solution.
3.4. Adsorption IsothermsIn this study, the Freundlich and Langmuir adsorption isotherm models were employed to investigate the isothermal adsorption characteristics of Cs+, Sr2+ and Co2+ ions in aqueous solution using acid-activated clay. The adsorption isothermal model shows detailed information for the maximum amount of adsorbate removed by the adsorbent and the adsorption affinity when the adsorption reachs the equilibrium at a certain temperature. The Freundlich adsorption isotherm is an empirical model representing the adsorbate that forms a multilayer on the heterogeneous surface of the adsorbent. This isotherm equation is defined by the following qe = KF ·Ce1/n and can be transformed into a linear equation by taking the logarithm on both sides, as shown in Eq. (3).
The Langmuir adsorption isotherm is a model based on the assumption that adsorption occurs at specific homogeneous adsorption sites of the adsorbent without mutual attraction between neighboring active sites. The Langmuir adsorption isotherm is expressed as the Eq. (4) as follows:
where, KF and Q (mg/g) are the maximum adsorption amount of the adsorbent per unit mass of adsorbate at equilibrium, and n and KL represent the adsorption affinity of the adsorbate for the adsorbent.
The obtained correlation coefficient R2 of Freundlich and Langmuir adsorption isotherms for Cs+, Sr2+ and Co2+ ions were depicted in Figs. S4 and S5. The R2 is a measure of the fit of the regression linear equation, and a value closer to 1 indicates that the adsorption isotherm model is more suitable. The R2 of Freundlich and Langmuir adsorption isotherms for Cs+, Sr2+ and Co2+ ions were 0.9702, 0.9766 and 0.9714 and 0.9907, 0.9986, and 0.9903, respectively. It was found that, regarding adsorption of Cs+, Sr2+ and Co2+ ions on acid-activated clay, the fit of experimental data with Langmuir adsorption isotherm was rather better than that with Freundlich adsorption isotherm.
The values of Freundlich and Langmuir constants were given in Table 4. The maximum adsorption capacity of acid-activated clay for Cs+, Sr2+ and Co2+ ions was 8.91, 3.78, and 10.20 mg/g, respectively. Freundlich and Langmuir constants 1/n and KL are related with the affinity between adsorbent and adsorbate in adsorption process. In general, when the constants mentioned above are in the range of 0.1 to 0.5 or less than, adsorption is known to be effective. Whereas, when they are 2 or more, it shows poor adsorption. Considering the obtained constant values, it was found that acid-activated clay is a relatively good adsorbent for adsorption of Cs+, Sr2+ and Co2+ ions in aqueous solution.
Langmuir constant KL is related to Gibbs free energy as shown in Eq. (5). Where, R is the ideal gas constant (8.314 J/mol·K) and T is the Kelvin temperature. If the calculated value of Gibbs free energy is negative, adsorption process proceeds as a spontaneous reaction. Commonly, the change in Gibbs free energy for physisorption is between −20 and 0 kJ/mol, but chemisorption is in the range of −80 and −400 kJ/mol.
The values of ΔG obtained for Cs+, Sr2+ and Co2+ ions were − 9.697, −9.144, and −9.890 kJ/mol, respectively. Since ΔG is negative value of about −10 kJ/mol, it can be seen that the adsorption process of Cs+, Sr2+ and Co2+ ions in the water/acid-activated clay medium is spontaneous and are favored by physisorption.
3.5. Adsorption KineticsThe adsorption kinetics of Cs+, Sr2+ and Co2+ ions on acid-activated clay were investigated by applying pseudo-first-order and pseudo-second-order kinetic models. The obtained kinetic parameters provide useful information for determining adsorption rate and the amounts of adsorbate adsorbed per unit mass. The pseudo-first-order kinetic equation is given as Eq. (6).
where, k1 (min−1) is the rate constant of the pseudo-first-order kinetics. qe (mg g−1) and qt (mg g−1) are the amounts of adsorbate adsorbed per unit mass of adsorbent at the equilibrium and at any time t (min), respectively.
The pseudo-second-order kinetic equation is expressed in the following Eq. (8).
where k2 (g mg−1 min−1) is the rate constant of pseudo-second-order kinetics.
Fig. S6 shows the pseudo-first-order and the psedo second-order kinetic plots for adsorption of Cs+, Sr2+ and Co2+ ions on acid-activated clay. The obtained R2 values of the pseuso-second-order kinetic are found to be greater than those of the pseudo-first-order kinetic and nearly equal to 1, which indicates that the kinetic experimental data were better fitted by the pseuso-second-order kinetic model. The derived kinetic parameters of the pseudo-first-order and the pseudo-second-order kinetic models are shown in Table 5. The calculated values qe,cal derived by fitting the second-order kinetic equation are quite similar to qe,exp obtained the experimentally, indicating that adsorption of Cs+, Sr2+ and Co2+ ions follows the the pseudo-second-order kinetic. In addition, it was investigated that when the dosage of acid-activated clay was increased, the reaction rate value (k2) was also increased.
4. ConclusionsIn this study, we explored the possibility of removing Cs+, Sr2+ and Co2+ ions in aqueous solution using acid-activated clay. While activating raw white clay with acid, most of oxides such as calcium, magnesium, aluminum, and iron were partially dissolved from the lattice except for silica that remains in solid state, resulting in the opening of the lattice and an increase in internal surface area. The values of the BET surface area and BJH pore volume of acid-activated clay were approximately 3 times higher compared with those of the raw white clay. Applicability of acid-activated clay for Cs+, Sr2+ and Co2+ ions removal was carried out in a batch mode. It was observed that the rapid adsorption of Cs+ and Sr2+ relative to Co2+ occured within the first few minutes and equilibrium was reached in about 10 minutes. The effects of initial concentrations and dosage on adsorption capacity were tested according to designated contact time. In the case of Cs+ ions, the removal efficiency of Cs+ ions steadily decreased from 81% to 60% when the initial concentration of Cs increased from 10 mg L− to 100 mg L− at fixed 1 g of acid-activated clay. It is thought that the gradual increase in initial concentration is due to saturating the available adsorption active sites of acid-activated clay. On the contrary of this, when the dosage of acid-activated clay increased from 0.5 g to 5.0 g at a 50 mg L− of concentration, the removal efficiency of Cs+ ionas efficiency gradually increased from 56.1% to 82.1%. However, the effect of further increase in dosage was inadequate than expected. It is presumed that this is because the agglomeration of acid-activated clay at higher dosages reduces available adsorption sites of acid-activated clay. The experimental adsorption data were applied by adsorption isotherm models in order to know the adsorprion behavior of Cs+, Sr2+ and Co2+ ions on acid-activated clay. When considering correlation coefficient (R2), it turned out that the Langmuir adsorption isotherm is more appropriate than the Freundlich adsorption isotherm. The Langmuir maximum adsorption capacity (Q) of acid-activated clay for Cs+, Sr2+ and Co2+ ions was found to be 8.91, 3.78 and 10.20 mg/g, respectively. Furthermore, since the Langmuir constant (KL) is in the range of 0.1 to 1.0, acid-activated clay can be considered as a good adsorbent for the adsorption of Cs+, Sr2+ and Co2+ ions. Aside from these, the experimental results obtained were fitted by the pseudo-first-order kinetic and pseudo-second-order kinetic models to elucidate the rate and mechanism of adsorption. The pseudo-second-order kinetic was revealed to the better fitting model for Cs+, Sr2+ and Co2+ ions removal, where R2 value was almost 1.0 and the calculated value qe,cal showed similar value to the experimental value qe,exp.
AcknowledgementsThis work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1F1A1060823).
NotesAuthor Contributions K.S.R (professor) established the concept of the experiment, wrote a draft thesis, and revised the draft thesis. D.K (professor) gave a lot of advice in conducting the experiment. S.J.H (professor) collected data from the experiment and prepared pictures and tables in the paper. S.H.B (professor) mainly conducted experiments and achieved the tangible results. References1. Sakamoto S, Kawase Y. Adsorption capacities of poly-γ-glutamic acid and its sodium salt for cesium removal from radioactive wastewaters. J. Environ. Radio. 2016;165:151–158.
https://doi.org/10.1016/j.jenvrad.2016.10.004
2. Nazmul HMD, Shenashen MA, Munjur HMD, Znad H, Rabiul AMD. Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent.
Chemosphere
. 2021;270:128668.
https://doi.org/10.1016/j.chemosphere.2020.128668
3. Long H, Wu P, Yang L, Huang Z, Zhu N, Hu Z. Efficient removal of cesium from aqueous solution with vermiculite of enhanced adsorption property through surface modification by ethylamine. J. Colloid. Inter. Sci. 2014;428:295.
https://doi.org/10.1016/j.jcis.2014.05.001
4. Yang X, Jiang L, Peng S, Zhang L, Huang G. Removal of cesium from liquid radioactive waste by in situ electrochemical synthesis of cesium zinc ferrocyanide. Coll. Surf. 2021;626:127050.
https://doi.org/10.1016/j.colsurfa.2021.127050
5. Zhang P, Cao J, Yang Z, Wu Z, Wu L. Adsorption of Sr (II) in aqueous solution by multilayer titanium carbon nitrogen (Ti3CNTx) MXene: Box-Behnken modeling design and experimental study. J. Environ. Chem. Eng. 2022;10:109019.
https://doi.org/10.1016/j.jece.2022.109019
6. Lujaniene G, Novikau G, Karalevciute K, et al. Chitosan-minerals-based composite for adsorption of cesium, cobalt and europium. J. Hazard. Mat. 2024;462:132747.
https://doi.org/10.1016/j.jhazmat.2023.132747
7. Seema H, Khan N, Ali SAH, Muhammad A. Fabrication of self-assembled Prussian blue graphene hydrogel for highly selective removal of radioactive cesium in water: Adsorption study. Mat. Chem. Phys. 2023;306:128003.
https://doi.org/10.1016/j.matchemphys.2023.128003
8. Trung ND, Ping N, Dan HK. Application of mesopores copper hexacyanoferrate (II) nanomaterials for cesium adsorption. Environ. Eng. Res. 2023;28(6)220389.
https://doi.org/10.4491/eer.2022.389
9. Emara AM, Elsharma EM, Abdelmonem IM. Adsorption of radioactive cesium using synthesized chitosan-g-poly(acrylic acid/N-vinylcaprolactam) by γ-irradition. Rad. Phys. Chem. 2023;208:110892.
https://doi.org/10.1016/j.radphyschem.2023.110892
10. Bok-Badura J, Kazek-Kesik JA, Karon K, Jakobik-Kolon A. Highly efficient copper hexacyanoferrate embedded pectine sorbent for radioactive cesium ions removal. Wat. Res. Ind. 2022;28:100190.
https://doi.org/10.1016/j.wri.2022.100190
11. Chen S, Hu J, Han S, Guo Y, Belzile N, Deng T. A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Sep. Purf. Technol. 2020;251:117340.
https://doi.org/10.1016/j.seppur.2020.117340
12. Chen GR, Chang YR, Liu X, et al. Cesium removal from drinking water using Prussian blue adsorption followed by anion exchange process. Sep. Purif. Technol. 2017;172:147–151.
https://doi.org/10.1016/j.seppur.2016.07.005
13. Liu X, Chen G, Lee GD, et al. Adsorption removal of cesium from drinking waters: A mini review on use of biosorbents and other adsorbents. Bio. Technol. 2014;160:142–149.
https://dx.doi.org/10.1016/j.biortech.2014.01.012
14. Wang J, Zhuang S. Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes. Nuclear Eng. Technol. 2020;52:328.
https://doi.org/10.1016/j.net.2019.08.001
15. Abd-EIhamid A, Abu EEM, Aly H. Sugarcane bagasse decorated by metal (Fe3+/Cu2+) ferrocyanide for effective removal of cesium from aqueous solutions. J. Wat. Proc. Eng. 2024;57:104641.
https://doi.org/10.1016/j.jjwpe.2023.104641
16. Hong T, Pan Y, Liu Y, Yang G, Leng Y. The mechanism and behavior of cesium adsorption from aqueous solutions onto carbonated cement slurry powder. J. Environ. Radio. 2024;272:107350.
https://doi.org/10.1016/j.jenvrad.2023.107350
17. Lee EH, Lee KY, Kim KW, et al. Removal of Cs adsorption with IE911 (crystalline silicotitanate) from high-radioactive seawater waste. J. Kor. Radio. Waste Soc. 2021;13(3)171–180.
https://dx.doi.org/10.7733/jnfcwt.2015.13.3.171
18. Chen FP, Jin GP, Peng SY, Liu XD, Tian JJ. Recovery of cesium from residual saltlake brine in Qarham playa of Qaidam Basin with Prussian blue functionalized graphene/carbon fibers composite. Coll. Surf. 2016;509:359–366.
https://dx.doi.org/10.1016/j.colsurfa.2016.09.030
19. Wang J, Zhuang S, Liu Y. Metal hexacyanoferrates-based adsorbents for cesium removal. Coord. Chem. Rev. 2018;374:430–438.
https://doi.org/10.1016/j.CCR.2018.07.014
20. Kim Y, Eom Hh, Kim D, Harbottle D, Lee JW. Adsorptive removal of cesium by electrospun nanofibers embedded with potassium copper hexacyanoferrate. J. Sep. Purif. Technol. 2021;255:117745.
https://doi.org/10.1016/j.seppur.2020.117745
21. Wang K, Ma H, Pu S, et al. Hybrid porous magnetic bentonite-chitosan beads for selective removal of radioactive cesium in water. J. Hazard. Meter. 2019;362:160–169.
https://doi.org/10.1016/j.JHAZMAT.2018.08.067
22. Zhao Y, Qj W, Chen G, Ji M, Zhang Z. Behavior of Cr (II) removal from wastewater by adsorption onto HCl activated Akadama clay. J. Taiwan Ins. Chem. Eng. 2015;60:190–197.
https://dx.doi.org/10.1016/j.jtice.2014.12.016
23. Chaari I, Medhioub M, Jamoussi F, Hamzaoui AH. Acid-treated clay materials (Southwestern Tunisia) for removing sodium Leuco-vat dye: Characterization, adsorption study and activation mechanism. J. Mol. Struct. 2021;1223:128944.
https://doi.org/10.1016/j.molstruc.2020.128944
24. Joseph CG, Taufiq-Yap YH, Krishnan V, Puma GL. Application of modified red mud in environmentally-benign applications: A review. Environ. Eng. Res. 2020;25:2020.
https://doi.org/10.4491/eer.2016.2019.374
25. Komadel P. Acidactivated clays: Materials in continuous demand. Appl. Clay Sci. 2016;131:84–99.
https://dx.doi.org/10.1016/j.clay.2016.05.001
26. Aris Espana VA, Sarkar B, Biswas B, Rusmin R, Naidu R. Environmental applications of thermally modified and acid activated clay materials: Current status of the art. Environ. Technol. Innova. 2019;13:383–397.
https://dx.doi.org/10.1016/j.eti.2016.11.005
27. Dodoo D, Fynn GE, Yawson ESC, Appiah G, Suleiman N, Yaya A. Eco-efficient treatment of hazardous bauxite liquid-residue using acid-activated clays. Cleaner Chem. Eng. 2022;3:100040.
https://doi.org/10.1016/j.clce.2022.100040
Table 1Oxide Constituents of raw white clay and acid-activated clay obtained by XRF Table 2Elemental constituents of raw white clay and acid-activated clay obtained by EDX
Table 3Textural properties of raw white clay and acid-activated clay
Table 4Freiundlich and Langmuir constants for adsorption of Cs+, Sr2+ and Co2+ ions on acid-activated clay
Table 5Adsorption kinetic parameters for adsorption of Cs+, Co2+ and Sr2+ ions on acid activated clay |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||