AbstractIn the present work, ZnO/g-C3N4/biochar was prepared, and it was used for visible-light driven photocatalytic degradation of some colorants (methylene blue, rhodamine B, methyl orange) and some antibiotics (doxycycline, ciprofloxacin, amoxicilline). Biochar was prepared by pyrolysing Phragmites australis biomass. The ZnO/g-C3N4/biochar composite was synthesized with the alkaline hydrolysis method. The obtained materials were characterized by X-ray diffraction, scanning electron microscopy, transition electron microscopy, energy dispersive X-ray/elemental mapping, ultraviolet-visible-diffuse reflectance spectroscopy, photoluminescence spectroscopy, nitrogen adsorption/desorption isotherms and X-ray photoelectron spectroscopy. The results show that ZnO nanoparticles with a large surface area are highly dispersed on the g-C3N4 particle surface and biochar. The composite exhibits superior photocatalytic degradation ability toward doxycycline, a broad-spectrum antibiotic of the tetracycline compared with individual components (ZnO or g-C3N4) and satisfies stability after six treatment cycles. The kinetics and degradation mechanisms of doxycyline were also addressed. In addition, the present catalyst also exhibits the photocatalytic degradation of methylene blue, rhodamine B, methyl orange, ciprofloxacin and amoxicillin in visible-light regions.
Graphical Abstract
1. IntroductionAntibiotics are widely utilized for human and veterinary disease treatments, and they are present in various domestic wastewater sources, from hospitals to livestock farms [1]. During use, only a part of the antibiotics is absorbed and metabolized in the human/animal body, and most of them are excreted unchanged. Numerous antibiotics are stable and do not decompose naturally, and their residues left in wastewater and sludge can be dispersed to receiving sources and accumulate in the ecosystems, increasing the risk of antibiotic-resistance genes in livestock and humans [2]. Doxycycline (DC, C22H24N2O8) is a broad-spectrum antibiotic of the tetracycline class, used in the treatment of infections caused by bacteria and certain parasites in humans and animals [3, 4]. Because of its antibacterial properties, even at low concentrations, doxycycline can induce antibiotic resistance to microorganisms, altering the microbial structure and function and posing a serious risk to humans and animals [4, 5]. Since contaminated wastewater is a major source of antibiotics, the development of effective wastewater treatment methods is necessary to remove these harmful compounds [5, 6]. Up to the present, numerous methods, such as biodegradation, electrochemistry, flocculation, and membrane filter, are used to treat antibiotics [2, 7]. However, the complex and persistent structure of antibiotics, the formation of toxic by-products, and high operating or maintenance costs make these methods less effective. Photocatalytic oxidation is currently considered as one of the effective technique in wastewater treatment, especially for wastewater containing toxic and persistent organic pollutants that are not easily degraded and removed with biological or other traditional methods [8, 9]. Photocatalytic processes can completely decompose organic pollutants via the formation of hydroxyl and superoxide radicals that produce less toxic compounds without generating sludge [10, 11].
Although TiO2 photocatalysts have been widely used for environmental applications, zinc oxide (ZnO), a semiconductor, is found to be an ideal material to replace TiO2 because of its unique properties, such as low cost, sustainability, versatile applications, and high photocatalytic activity [11, 12]. However, pristine ZnO has a large energy bandgap (about 3.37 eV), which is only excited in the ultraviolet-light region, thus limiting its ability to work under sunlight conditions [12]. To overcome these limitations, various techniques related to the modification of ZnO have been developed, such as morphology and composition control [13, 14], metal ion doping [15, 16], and non-metal ion doping [17, 18] coupled with other photocatalysts [19].
Graphitic carbon nitride (g-C3N4), a non-metallic polymer semiconductor, is suitable for combining with ZnO because of its narrow band gap (2.7 eV) [20]. Numerous studies show that ZnO/g-C3N4 heterojunction coupling can promote the separation of electron-hole pairs, thus improving the photocatalytic degradation efficiency [21, 22]. Kuang et al. reported that the heterojunction not only increases the separation efficiency of the photogenerated electron-hole pairs but also provides a long shift of the optical absorption to the visible light region [23]. They coated the surface of ZnO nanotubes with a g-C3N4 layer of 20–30 nm thickness. As a result, the g-C3N4/ZnO materials have significantly higher photo-degradability for methylene blue under visible light than individual components (g-C3N4 or ZnO) [23]. To further enhance the photocatalytic activity of ZnO/g-C3N4 materials and expand the application range of this material, researchers have studied immobilizing them on different substrates such as outer non-woven layer [24], NiFe2O4 [25], perlite [26], stainless steel mesh [27], and graphene oxide [28].
Biochar is super charcoal fabricated by calcining any biomass, such as rice husk, corncob, husk or stalk, potato or soy or wheat straw, without oxygen [29, 30]. Phragmites australis, a common reed, is a vigorous and aggressive species. It grows at rivers, the upper edges of estuaries, streams and other wetlands. Phragmites australis stems are composed of tissues with porous structure, which is an ideal biomass source to form porous biochar [31]. Biochar is a promising material in environmental remediation thanks to its excellent physicochemical properties, such as large surface area, large pore diameter, and numerous active functional groups on the surface. Besides its superior adsorption capacity, biochar can act as an electron acceptor and participate in electron transport. In addition, its significant stability allows biochar to be an excellent support for photocatalysts [32]. In recent years, biochar-based materials, such as ZnO/biochar [33], TiO2/biochar [34], Co3O4/biochar [35], CuO/biochar [36], and BiOBr/biochar [37], have been used as photocatalysts for the decomposition of various pollutants. However, to our best knowledge, there is a lack of research on loading ZnO/g-C3N4 on biochar matrix.
In this work, biochar was fabricated from Phragmites australis stems. The ZnO/g-C3N4 loaded biochar photocatalysts (ZnO/g-C3N4/biochar) were synthesized via a simple alkaline hydrolysis method. The photocatalytic activity of the photocatalysts was evaluated based on the photodegradation of doxycycline under visible-light (λ > 420 nm) irradiation.
2. Materials and Methods2.1 Materials
Phragmites australis samples were collected from a wetland in Dong Thap province, Vietnam, and cleaned with tap water to remove dirt and other impurities adhered to their surfaces. The stems were dried under sunlight for four days before being finely ground to approximately 1–2 mm sizes. The obtained biomass was rinsed with water and dried in a vacuum oven at 70°C to get a constant weight. The product was stored in a desiccator and used as raw P. australis biomass (cellulose 43.31%, hemicellulose 30.82%, and lignin 20.37%) [38].
Sodium hydroxide (NaOH, ≥99%), hydrochloric acid (HCl, 37%), potassium iodide (KI, ≥99.5%), potassium bromate (KBrO3, 99.8%), urea (CO(NH2)2, >99%), and sodium chloride (NaCl, ≥99.5%) were purchased from Merck. Zinc nitrate hexahydrate (Zn(NO3)2·6H2O, ≥99.0%), tert-butanol ((CH3)3COH, tBA, 99.5%), doxycycline hyclate (C22H24N2O8·HCl·0.5H2O·0.5C2H6O, DC, 93.5% (HPLC)) and L-ascorbic acid (C6H8O6, AA, 99%) were obtained from Sigma – Aldrich. All chemicals used were of analytical grade and were used as received without any further purification.
2.2. Methods2.2.1. Synthesis of ZnO/g-C3N4/biocharAdd 3.0 g of raw P. australis biomass to a porcelain boat and calcinate it under N2 atmosphere at 400°C for 2 h (heating rate 3°C·min−1). The product was first washed with 1 M HCl solution and then with distilled water several times until the filtrate was neutral. The washed product was dried at 105°C to constant weight and stored in a desiccator to obtain biochar for further use.
5.0 g of urea was placed in a porcelain boat, wrapped in aluminum foil and calcined in an N2 atmosphere at 520°C for 2 h (heating rate 3°C·min−1). The obtained yellow powder was placed in a beaker containing distilled water and sonicated for 30 min. The solid was then filtered and dried at 105°C for 12 h, yielding pristine g-C3N4.
In the present work, the ZnO/g-C3N4 with the ratio of ZnO to g-C3N4 (2.0/1.0 in weight) were mixed with biochar according to different ratios. Briefly, dissolve 7.437 g of zinc nitrate hexahydrate in a 250 mL beaker containing 80 mL of distilled water. Add a certain amount of biochar (0.25; 0.50; 0.75 and 1.00 g) and 1.0 g of g-C3N4 to the zinc nitrate solution and stir the obtained mixture for 12 h. Next, slowly add 50 mL of a 1 M NaOH solution dropwise, hydrolyse the mixture at ambient temperature for 4 h, and let the mixture stand for 1 h. Then, the mixture was filtered and washed with distilled water until the filtrate became neutral. After washing, the product was dried at 105°C for 12 h (heating rate 3°C·min−1), then finely ground and calcined in the N2 atmosphere at 450°C for 2 h to obtain the ZnO/g-C3N4/biochar composites. The final products were calculated so that the theoretical mass ratios of ZnO/g-C3N4/biochar correspond to 2/1.0/0.25 (ZCNB-0.25); 2/1.0/0.50 (ZCNB-0.50); 2/1.0/0.75 (ZCNB-0.75) and 2/1.0/1.00 (ZCNB-1.00). The photocatalytic activity of ZCNB with different mass ratios was tested toward DC degradation (Table S1). Since the sample of ZCNB-0.50 exhibited the highest DC degradation the samples with similar compositions of ZCNB-0.50 synthesized were calcined at different temperatures of 400; 450; 500; 550 and 600°C for 2 hours. Its photocatalytic activity toward DC degradation was presented in Table S2. It was found that the sample calcined at 450°C for 2 hours was able to achieve the highest catalytic activity. Therefore, this sample was selected for further experiments. For the sake of comparison, ZnO/g-C3N4 (ZCN) was synthesised under the same conditions without biochar.
2.2.2. Material CharacterizationX-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance X-ray diffractometer (Bruker, Germany) with an operating voltage of 40 kV, a current of 40 mA, and a CuKα radiation source, and λ = 0.15401 nm. The transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images were obtained with a JEOL JEM-1010 and a FEI Nova NanoSEM 450, respectively. The elemental analysis was conducted by using EDX mapping spectroscopy on a TEAM Apollo XL EDS machine. Fourier transform-infrared (FT-IR) spectra of the samples were recorded on an IR Affinity-1S spectrophotometer (Shimadzu). Ultraviolet-visible-diffuse reflectance (UV-Vis-DRS) spectra were obtained with the UV-2600 (Shimadzu) instrument. The nitrogen adsorption/desorption isotherms were determined by using a Quantachrome TriStar 3000 V6.07A adsorption instrument. The photoluminescence (PL) spectra were recorded by using a Horiba Fluorolog 3 FL3–22 with the excitation light at 370 nm. X-ray photoelectron spectra (XPS) were recorded by ESCA-3400 (Shimadzu) with Mg Kα (1253.6 eV, 10 kV, 20 mA). Binding energy was corrected using peak Au 4f7/2 (powder) at 84.0 eV. Ultraviolet-visible (UV-Vis) spectra were obtained by a UV-Vis spectrophotometer (Spectro UV-2650, Labomed-USA).
2.2.3. Photocatalytic Degradation of DoxycyclineFirst, 0.1 g of catalyst was added to a 500-mL beaker containing 200 mL of an aqueous suspension of 25 mg·L−1 DC. Then, a light source (50 W, 220 V Compact lamps (Dien Quang)) equipped with a wavelength cut-off filter (λ ≤ 420 nm, d = 77 mm) was applied to the mixture. Before illumination, the suspension was stirred magnetically in the dark for 60 min to ensure adsorption/desorption equilibrium. A 5 mL of the suspension was withdrawn at a specified time and centrifuged to remove the solid catalyst. Finally, the concentration of DC in the supernatant was determined with a UV-Vis spectrophotometer (Spectro UV-2650, Labomed, USA) at the maximum wavelength of 346 nm [39]. The adsorption efficiency (AE) of DC was calculated according to Eq. (1):
where C0 (mg·L−1) is the initial concentration of DC, and C0e (mg·L−1) is the concentration of DC at sorption equilibrium time t (min).
The photodegradation efficiency (PE) of the photocatalyst was calculated according to Eq. (2):
where C0e and Ct (mg·L−1) are the DC concentration at sorption equilibrium and at irradiated time of t (min). According to the Langmuir-Hinshelwood kinetics model, the photocatalytic degradation of DC can be described according to the apparent pseudo-first-order kinetic equation [40, 41]:
where k is the apparent pseudo-first-order rate constant; C0e and Ct (mg·L−1) are the DC concentrations at equilibrium and at irradiated time of t (min).
3. Results and Discussion3.1. Characterization of PhotocatalystsXRD patterns of ZnO, g-C3N4, biochar, ZCN, and ZCNB are shown in Fig. 1a. We can see that the XRD pattern of biochar has almost no diffraction peaks because of its amorphous structure [42]. The XRD pattern of ZnO presents characteristic diffraction peaks of the ZnO hexagonal wurtzite-phase structure at 2θ angles of 31.7, 34.4, 36.2, 47.5, 56.6, 62.8, 66.4, 67.9, 69.1, 72.6, and 76.9°, indexed as (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202) planes, respectively (JCPDS-01-075-9743). As for g-C3N4, the diffraction characteristic peaks at 13.1 and 27.4° are attributed to the (100) and (002) planes, as reported previously [43–45]. The ZCN sample also shows characteristic diffraction peaks for the ZnO wurtzite structure, but their intensity is lower than that of pristine ZnO. This decrease indicates the formation of g-C3N4/ZnO composite, and it is possible that g-C3N4 inhibits ZnO crystal growth, as reported in previous works [22, 46]. Similar to g-C3N4, ZCNB exhibits a new phase of zinc cyanamide (ZnNCN) with low diffraction intensity at 2θ angles of approximately 19.1, 27.9, and 28.6° for faces (101), (211) and (220), respectively, besides the characteristic diffraction peaks of ZnO [47]. This structure might result from condensation between the amino triazine group and the surface hydroxyl groups of ZnO to form Zn-N bonds [48].
The average crystal size of the ZnO, ZCNB, and ZCNB samples is about 30.3, 26.6, and 25.7 nm, according to the Scherrer equation at the diffraction peak (101), respectively (Table S3). The variation in grain size indicates that g-C3N4 and biochar can significantly inhibit the crystal growth of ZnO in the composites.
The functional groups of ZnO, g-C3N4, biochar, ZCN, and ZCNB are assessed via the FTIR spectroscopic spectra (Fig. 1b). We can see that all samples have absorption bands at around 3400 cm−1 and 2350 cm−1, related to the stretching vibrations of O–H in water and C–C in biochar, oxygen in the carbon dioxide molecule is adsorbed on the sample surface [21]. The absorption bands of ZnO observed at around 3400 cm−1, 1640 cm−1, and 498 cm−1 are related to the –OH, H–O–H and Zn–O stretching vibrations, respectively [48, 49]. For biochar, in addition to the absorption bands related to the –OH and C–O vibrations as mentioned above, there are also absorption bands at 2924 cm−1 and 1619 cm−1, assigned to asymmetry vibrations of C–H and sp2 character of C=C [37, 49]. The band at 1462 cm−1 can be attributed to the stretching vibration of –COO– [50]. The region between 900–1300 cm−1 and 1385 cm−1 is thought to be involved with C–O vibration and the binding vibrations of O–H, C–C, and C–H [51, 52]. For g-C3N4, the wide absorption region between 3200 cm−1 and 3600 cm−1 is thought to be responsible for the stretching vibrations of N–H and O–H [49]. Furthermore, sample g-C3N4 has several typical bands for the stretching vibrations of the C–N heterojunctions (1636 cm−1, 1459 cm−1, and 1410 cm−1) and high-intensity strain vibrations of tri-unit s-triazine at 813 cm−1 [44, 48]. The bands at 1317 cm−1 and 1240 cm−1 are associated with aromatic C–N stretching [44, 48]. More importantly, the absence of absorption bands at 3000 cm−1 and 2200 cm−1 rules out the –C≡C– bond formation. The ZCN and ZCNB samples also have characteristic bands but with a lower intensity than that of the component materials, demonstrating the successful formation of heterojunction in the composite [22, 53]. In addition, from Fig. 1b, we also observe that both ZCN and ZCNB composite samples have a new absorption band at 2050 cm−1, which is typical for the asymmetric stretching vibration of –N=C=N– [54], and this is also consistent with the XRD analysis. Xie et al. suggest that the formation of the g-C3N4/ZnNCN heterostructure can enhance the separation efficiency between photogenerated electrons and holes [54], which is one of the crucial factors for improving the photocatalytic activity of photocatalysts. It is possible that g-C3N4 uniformly coats ZnO nanoparticles, leading to blocking the order stacking pattern of g-C3N4 in the long-range order [20, 21].
The optical properties of ZnO, g-C3N4, biochar, ZCN, and ZCNB are estimated by using UV-Vis absorption spectroscopy. The absorption spectra exhibit the adsorption band covering the ultraviolet visible light region (Fig. 1c) [55]. It can be seen that the pristine ZnO has an upward-moving optical absorption edge at a wavelength of about 390 nm. The g-C3N4 sample has an upward-moving optical absorption edge in the wavelength region of about 445 nm. After the surface of g-C3N4 or both g-C3N4 and biochar was coated with ZnO, the optical absorption edge of the two composites ZCN and ZCNB has a significant blue shift towards the wavelength regions of 466 nm and 490 nm. This shift indicates a small crystal size of the ZCN and ZCNB nanocomposites. To calculate the band gap values, we use the Tauc plot relation [56].
where α is the absorption coefficient; h is the Planck constant; ν is the wavenumber; A is a constant, and Ebg is the energy band gap. The bandgap energy value from the absorption data was calculated by plotting (α × h × ν)2 against the photon energy (Ebg = h × ν) (Fig. 1d) [22, 56]. Accordingly, the Ebg values of ZnO, g-C3N4, ZCN, and ZCNB are 3.18, 2.79, 2.66, and 2.53 eV, respectively (Table S3). Thus, a transition to lower energy occurs for the ZCNB nanocomposite, and its ability to absorb visible light increases [53].
Fig. 1e presents PL spectra of the obtained materials. The photoluminescence of ZnO, g-C3N4 and ZCN peak at 509; 438 and 473 nm, respectively. Since the PL intensity of g-C3N4 is significantly larger than that of ZnO, hence the photoluminescence of ZCN is determined by g-C3N4 and peaks at around 473 nm. The PL intensity of the ZCN composite is lower than that of the ZnO and g-C3N4 components, suggesting that g-C3N4 on the ZnO surface can inhibit electron-hole recombination [22]. The ZCNB composite exhibits a much lower absorption intensity than the others, indicating that the combination of ZnO with both g-C3N4 and biochar can enhance the separation efficiency of the photogenerated electron-hole pairs [53, 57].
The textural properties of the ZnO, g-C3N4, biochar, ZCN, and ZCNB samples were studied from the nitrogen adsorption/desorption isotherms at 77 K (Fig. 1f and Table S4). Their N2 isotherms belong to type IV with an H3 hysteresis loop, according to the International Union of Pure and Applied Chemistry (IUPAC), and they indicate that the obtained samples possess a mesoporous structure [58, 59]. The H3 hysteresis loop is assigned to the non-rigid materials with plate-like particles, forming slit-shaped pores [60]. The individual components of the composite have a specific surface area (SBET) as follows: 5.5 m2·g−1 for ZnO, 22.8 m2·g−1 for biochar, and 36.8 m2·g−1 for g-C3N4 (Table S2). Meanwhile, their two- and three-component composites exhibit a higher specific surface area: 28.4 m2·g−1 for ZCN and 25.1 m2·g−1 for ZCNB. These values demonstrate that g-C3N4 and biochar significantly improve the surface characteristics of ZnO, and the resulting composites have a capillary structure. Therefore, it can be concluded that the dispersion of ZnO onto g-C3N4 and biochar increases the surface area, which is beneficial for the surface reactions of the adsorbents. In general, an increased specific surface area can provide more potential active sites. Therefore, the large surface area, volume, and pore diameter of ZCNB could be one of the reasons for higher catalytic activity since the large surface area helps to increase the mass transfer rate, leading to an improved photocatalytic activity [61]. Furthermore, g-C3N4 in the composite not only enhances the surface area and reduces the particle size of ZnO but also forms heterojunctions. This structure is beneficial for improving the efficiency of migration of the photogenic charged particles, providing more photocatalytic sites and further improves the photodegradation efficiency [58].
The morphology of the ZnO, g-C3N4, biochar, ZCN, and ZCNB samples can be seen on SEM and TEM images. Fig. 2a and 2b show that ZnO exists as agglomerated spherical particles with an average diameter of about 30–35 nm. Fig. 2c displays the structure of g-C3N4 as thin flake-like particles with diameters ranging from a few hundred nanometres to several micrometres, overlapping the slits. Fig. 2d shows the structure of biochar in the plate form with a smooth surface. Fig. 2e and 2f display the SEM and TEM images of the ZCN composite and indicate that this material exists in the form of spherical particles with a ZnO particle size of about 30–35 nm. Fig. 2g and 2h show the SEM and TEM images of the ZCNB composite and reveal that ZCNB consists of both nodular and plate-shaped particles with a ZnO grain size of about 20–30 nm. The grains are more even than those of ZnO itself, and they seem to be successfully attached to g-C3N4 and biochar surfaces. Finally, Fig. 2i shows a high-resolution TEM (HRTEM) with lattice fringes with distances d(002) (2.6 Å) and d(101) (2.4 Å) for the ZnO wurtzite in ZCNB composite [62, 63].
The EDX elemental mapping of a selected area on the ZCNB composite sample is shown in Fig. 3. Carbon and nitrogen are densely distributed in the ZCNB lattice (Fig. 3d and 3e). Meanwhile, O and Zn are uniformly distributed on the surface of g-C3N4 and biochar in the ZCNB composite (Fig. 3f and 3g). The existence of C, N, O, and Zn on ZCNB and the merged image of C, N, O, and Zn (Fig. 3c) confirm the spatial distribution of the elements in the ZCNB composite structure. The EDX analysis further indicates the presence of C, N, O, and Zn in ZCNB (Fig. 3b). The atomic composition of the surface is as follows: C 29.17, N 36.69, O 16.9, and Zn 17.24% (the inset of Fig. 3b). These results demonstrate that ZnO nanoparticles are highly dispersed on g-C3N4 and biochar.
The core-level XPS of Zn 2p, O 1s, C 1s and N 1s of ZCNB are presented in Fig. 4. The Zn 2p spectrum at 1024.7 eV and 1047.8 eV are acribed Zn 2p3/2 and Zn 2p1/2 of hexagonal structure (Fig. 4a) [48]. O 1s spectrum of Fig. 4b presents the peaks at 524.5 eV and 526.3 eV contributed to O2− in ZnO, while peaks at 533.5 eV, 534.5 eV and 535.4 eV belong to O2− in oxigen deffect in ZnO or the OH and O2 adsorbed on the catalyst surface, respectively [48, 64]. The presence of O2 in defected region make it form O2− · and improve catalytic ativity (Fig. 4b) [64]. C 1s spectrum peaks at 281.6 eV, 287.5 eV that assigned to C–N [65] and 290.6 eV corresponding to bonding energy of C in C–(N)3 [66, 67] or C=O [68] (Fig. 4c). The core-level XPS of N 1s is deconvulated into three peaks at 397.2 eV, 399.1 eV and 407.6 eV, which are assigned to the sp2 hybridized nitrogen C=N–C, tertiary N in N–C3 and N atoms in amino moieties, respectively (Fig. 4d) [64, 65, 69].
3.2. Photocatalytic Degradation of Doxycycline over ZCNB CatalystFor primary tests, the effect of the different catalyst loadings (0.05, 0.10, 0.15, and 0.20 grams) in 200 mL of 25 mg.L−1 DC at pH 5 on DC degradation is depicted in Fig. S1a. DC decomposition efficiency increased from 90.9 to 99.8% corresponding to an increase in the amount of ZCNB from 0.05 to 0.20 grams. This may be due to the presence of more active sites with increasing ZCNB dosage and thus facilitating the generation of oxidation agents [70]. The sample at the amount of ZCNB 0.10 gram showed a fairly high DC decomposition efficiency (98.9%), and therefore we chose this amount of catalyst as the appropriate condition for the further experiments.
The photocatalytic activity of pristine ZnO, pristine g-C3N4, ZCN, and ZCNB was studied via doxycycline photodegradation under visible light irradiation (λ > 420 nm) (Fig. 5a). When no catalysts are applied, no photolysis of DC is observed after 2 h of visible light irradiation, demonstrating that DC is stable under visible light [40, 71]. Similarly, ZnO does not favour DC decomposition under visible light radiation. Like previously reported results [72, 73], pristine g-C3N4 exhibits good performance in photodegradation under visible light in the present work. The ZCN composite has a much higher photocatalytic activity than pristine g-C3N4 for DC degradation, with an efficiency of 69.74% after 120 min. Meanwhile, the ZCNB photocatalyst is the most active in DC degradation, with an efficiency of 98.9%.
To better understand the reaction kinetics of DC degradation catalyzed by different photocatalysts, we fitted the experimental data with the Langmuir-Hinshelwood first-order model (Eq. 3). As can be seen from Fig. 5b, the photocatalytic degradation curves in all cases are consistent with the apparent first-order kinetic model with high determination coefficients (R2>0.95). Furthermore, a significant difference in the photocatalytic activity of ZCNB from the others implies that the incorporation of ZnO with g-C3N4 and biochar substantially improves the photocatalytic activity of ZnO, and this composite has the highest degradation rate constant for DC photodegradation. The rate constant for ZnO, g-C3N4, ZCN, and ZCNB is about 2.39×10−4, 0.007, 0.016, and 0.069 min−1, respectively. The ZCNB composite is about 9.31 and 4.18 times more effective than pristine g-C3N4 and ZCN. A comparison of the first-order apparent degradation rate constant of the present catalyst with the literature is presented in Table S5. The data show that the catalytic activity of the ZCNB composite is relatively high compared with what was reported in previous papers [6, 39, 72–74].
The COD values were determined to assess the mineralisation of DC over ZCNB (Fig. 5c). It is clear that the COD gradually decreases with time, indicating a gradual decomposition of DC. The reduction of COD up to 93.52% is achieved after 120 min of illumination with the ZCNB composite. Therefore, it can be concluded that the DC is decomposed into carbon dioxide over the ZCNB almost completely.
Since photocatalytic degradation involves photo-induced electron/hole pairs (e−/h+), superoxide radicals (•O2−) and hydroxyl radicals (•OH), several scavengers were used to study the effect of free radicals on DC degradation (Fig. 5d). For these experiments, 10 mM of potassium iodide (KI), potassium bromate (KBrO3), tert-butanol (t-BA), and ascorbic acid (AA) was added to the DC solution to capture h+, e−, •OH, and •O2−, respectively. Fig. 5d shows that the degradation efficiency drops to 33.61, 58.58, 80.14, and 71.88% upon the addition of KI, KBrO3, tBA, and AA, respectively. First, tBA is considered a scavenger for •OH [72], it is considered that t-BA presents a strong interaction with the •OH through an electron transfer process [75].
If the •OH radicals play a crucial role in the DC degradation, the reaction rate is expected to decrease significantly. As shown in Fig. 5d, adding an excess amount of t-BA to the reaction mixture significantly suppresses the DC degradation (by ~ 80.1%) compared with the mixtures without the scavenger. Secondly, the addition of AA drops the decomposition efficiency by around 71.9% compared with the absence of scavenger. Like benzoquinone, AA exhibits ability to capture •O2− by an electron transfer mechanism [76],
Thirdly, the BrO3− ion captures photogenerated electrons and then reduces to bromide (Br−) [77],
and the addition of the KBrO3 makes the degradation efficiency dropping around 58.58% compared with the absence of scavenger.
Finally, the iodide ion is a common scavenger that reacts with vacancy-band holes [78].
The addition of the KI reduces the degradation efficiency only to 33.62%. Therefore, it can be concluded that the formation of •OH and •O2− play an essential role in the photocatalytic degradation of DC.
The electrons and holes generated in the photocatalyst react with the water and oxygen molecules adsorbed on the catalyst surface and generate •OH and •O2− that react to decompose the DC molecules in solutions [44,45].
The valence-band energy potential (EVB) and conduction-band energy potential (ECB) of a semiconductor can be calculated as follows [22]:
where χ is the absolute electronegativity of the semiconductor, and the values of χ were 4.74 and 5.6 eV for g-C3N4 and ZnO [22]. Ee is the energy of free electrons versus hydrogen (4.5 eV), and Ebg is the bandgap value of the semiconductor. The calculated values of EVB and ECB are 2.69 and –0.49 eV for ZnO and 1.635 and −1.155 eV for g-C3N4. Because of the different positions of valence-band and conduction-band potentials between ZnO and g-C3N4, type II heterojunction can occur with coupling effects [79]. The ZnO semiconductor cannot be excited under visible-light illumination since it has a large band gap (3.18 eV). Pristine g-C3N4, with its narrow bandgap energy (2.79 eV), is excited by visible light to create photo-generated electron-hole pairs (Eq. 13). Since the CB edge potential (−1.155 eV) of g-C3N4 is more negative than that of ZnO (−0.49 eV), the photoinduced electrons in the conduction band of g-C3N4 transfer directly to the conduction band of ZnO and subsequently to the surface of ZCNB [80]. On the other hand, biochar on the ZCNB composite surface can also accept electrons on the conduction band of ZnO or g-C3N4 [77, 78]. The photosensitive electrons on the catalyst surface react with O2 dissolved in the solution and create •O2− (Eq. 14). These radicals continue to react with adsorbed H2O on the surface of the catalyst or H+ ions present in the solution to form •OH (Eq. 15–18), and the resulting •OH decomposes DC [22] (Eq. 19). On the opposite side, since the VB edge potential of ZnO (2.69 eV) is more positive than the potential of the •OH/OH− pair (1.99 eV) [81], the holes in the valence band cannot react with the OH− ion to form active •OH, but they can instead directly oxidize DC molecules (Eq. 20 and 21) [23, 82]. Based on the literature [22, 82–84] and the aforementioned results, we propose a possible visible light-driven photocatalytic mechanism at the interface of the ZCNB heterojunction. Briefly, visible light excites g-C3N4 to form photoinduced electron/hole pairs. Then these photogenerated electrons transferred directly to the conduction band of ZnO and the surface of ZCNB lead the promotion of separation of photogenerated electron-hole pairs. These free electrons will react with oxygen to form •O2−, followed by the reactions of these radicals with H2O or H+ to form •OH. This process is illustrated as shown in Fig. 6.
In addion, we applied ZCNB composites to decompose other types of pollutants under visible light illumination. As presented in Fig. S1b, ZCNB exhibited an excellent degradation efficiency within 120 min for ciprofloxacin (98.2%), amoxicillin (90.3%), methylene blue (95.2%), rhodamine B (99.9%), and methyl orange (82.4%). These results show that composites have great potential for applications in treating organic pollutants in wastewater.
Inorganic anions can trap oxidants that play a prominent role in photocatalysis, reducing the decomposition ability of the photocatalytic treatment system. For this reason, this study incorporated 1.0 mM of ions commonly present in natural waters namely Cl−, PO43−, CO32−, NO3− and SO42− into the reactor (anion/DC molar ratio of 20.5) as coexisting anions to show their impact on the DC degradation rate. The reactions were conducted under selected conditions with a pH of 5.0, a catalyst amount of 0.1 g, and 200 mL of DC solution at a concentration of 25 mg L−1. The results are illustrated in Fig. S1c. It is observed that all these anions have the effect of reducing DC decomposition efficiency. Adsorption of these anions onto the active sites of the catalyst will reduce the available surface sites for DC adsorption, as will the reaction of the anions with oxidizing agents to produce oxygen species less strong oxidation, are the main reasons for the smaller DC removal efficiency in the presence of these anions [85]. Among the combined anions, phosphate and nitrate showed a slight decreasing effect on the degradation rate, reducing the removal efficiency from 98.9 to 90.1 and 93.5%, respectively. In contrast, the addition of carbonate, chloride and sulfate resulted in a significant decrease in DC degradation efficiency to 55.6, 62.5 and 73.9%, respectively. It seems that the stronger competition of carbonate, chloride and sulfate anions with DC for adsorption on the ZCNB surface and the scavenging of oxidative radicals by these ions is responsible for their destructive effects on the ZCNB surface. with effective DC removal [86]. The quenching effect of carbonate, chloride and sulfate anions can be described by equations (22)–(24).
3.3. Recyclability and Real Sample TestFor the purposes of practical applications, it is necessary to evaluate the long-term stability of the photocatalyst during the reaction. The ZCNB catalyst was reused four times. After each test, the catalyst was separated by using centrifugation; then, it was eluted several times with methanol [87] to ensure complete removal of residual DC, and finally was dried at 105°C for 24 h. The photocatalytic degradation efficiency of ZCNB decreases from 98.9 to 90.1% after six cycles (Fig. 7a). The XRD patterns of the ZCNB samples before and after the fourth test remain almost unchanged; therefore, it can be concluded that ZCNB is stable in the photocatalytic degradation reactions (Fig. 7b).
The shrimp aquaculture wastewater was collected from a shrimp aquaculture pool at Mekong delta. The characteristics of shrimp farming wastewater samples are shown in Table S6. It was used to test DC degradation over the present catalyst (Fig. 7c). Around 90% of DC is degraded after 120 minutes. In addition, ZCNB also showed a certain degradation effect on other pollution (Table S6). The TOC and COD removal efficiency reached values of 73.1% and 87.1%, respectively, showing that ZCNB is a promising material in practical wastewater treatment.
4. ConclusionsA tertiary nanocomposite ZnO/g-C3N4/biochar was synthesised with the hydrolysis method. The composite consists of ZnO nanoparticles with a size of about 25–30 nm, highly dispersed on the g-C3N4 and biochar matrix. The ZnO/g-C3N4/biochar composite exhibits a significantly higher photodegradation efficiency for doxycycline than g-C3N4 and ZnO under visible light. The catalyst is stable during operation. The present study proposes a premise to obtain an inexpensive photocatalyst from biomass waste. This ZnO/g-C3N4/biochar catalyst can be used for the antibiotics photodegradation in aqueous solutions. However, some limitation of this process need acknowledgement including g-C3N4 production in anaerobic conditions in large-scale, large volumes of wastewater treatment, the complex wastewater compositions and the difficult recovery of catalysts due to small particle sizes.
AcknowledgementsThis research was supported by The Ministry of Education and Training, Vietnam, under the project coded B2022.SPD.562.07.
NotesAuthor Contributions The authors confirm contribution to the paper as follows: N.V.H. (Associate Professor) and D.Q.K. (Professor) study conception, design, draft, and final manuscript preparation; B.T.M.N. (Ph.D.), N.H.N. (Master), N.M.L. (Master) and N.N.B (Ph.D.) data collection; L.V.T.S. (Master); N.T.K. (Ph.D.), Đ.N.N. (Associate Professor) and N.T.T. (PhD student) analysis and interpretation of results. All authors reviewed the results and approved the final version of the manuscript. References1. Ahmed MJ. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017;50:1–10.
https://doi.org/10.1016/j.etap.2017.01.004
2. Wang J, Zhuan R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020;701:135023.
https://doi.org/10.1016/j.scitotenv.2019.135023
3. Liu W, Zhou J, Hu Z. Nano-sized g-C3N4 thin layer @CeO2 sphere core-shell photocatalyst combined with H2O2 to degrade doxycycline in water under visible light irradiation. Sep. Purif. Technol. 2019;227:115665.
https://doi.org/10.1016/j.seppur.2019.06.003
4. Bolobajev J, Trapido M, Goi A. Effect of iron ion on doxycycline photocatalytic and Fenton-based autocatatalytic decomposition. Chemosphere. 2016;153:220–226.
https://doi.org/10.1016/j.chemosphere.2016.03.042
5. Mohammadi A, Pourmoslemi S. Enhanced photocatalytic degradation of doxycycline using a magnetic polymer-ZnO composite. Water Sci. Technol. 2018;2017(3)791–801.
https://doi.org/10.2166/wst.2018.237
6. Yan X, Qian J, Pei X, et al. Enhanced photodegradation of doxycycline (DOX) in the sustainable NiFe2O4/MWCNTs/BiOI system under UV light irradiation. Environ. Res. 2021;199:111264.
https://doi.org/10.1016/j.envres.2021.111264
7. Ouyang J, Zhou L, Liu Z, Heng JYY, Chen W. Biomass-derived activated carbons for the removal of pharmaceutical mirco-pollutants from wastewater: A review. Sep. Purif. Technol. 2020;253:117536.
https://doi.org/10.1016/j.seppur.2020.117536
8. Cheng S, Chen Q, Xia H, et al. Microwave one-pot production of ZnO/Fe3O4/activated carbon composite for organic dye removal and the pyrolysis exhaust recycle. J. Clean. Prod. 2018;188:900–910.
https://doi.org/10.1016/j.jclepro.2018.03.308
9. Vinayagam M, Ramachandran S, Ramya V, Sivasamy A. Photocatalytic degradation of orange G dye using ZnO/biomass activated carbon nanocomposite. J. Enviro. Chem. Eng. 2018;6(3)3726–3734.
https://doi.org/10.1016/j.jece.2017.06.005
10. Khan ZUH, Gul NS, Sabahat S, et al. Removal of organic pollutants through hydroxyl radical-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2023;267:115564.
https://doi.org/10.1016/j.ecoenv.2023.115564
11. Okab AA, Jabbar ZH, Graimed BH, Alwared AI, Ammar SH, Hussein MAA. A comprehensive review highlights the photocatalytic heterojunctions and their superiority in the photo-destruction of organic pollutants in industrial wastewater. Inorg. Chem. Commun. 2023;158:111503.
https://doi.org/10.1016/j.inoche.2023.111503
12. Pallavolu MR, Maddaka R, Viswanath SK, Banerjee AN, Kim MD, Joo SW. High-responsivity self-powered UV photodetector performance of pristine and V-doped ZnO nano-flowers. Opt. Laser Techno. 2023;157:108776.
https://doi.org/10.1016/j.optlastec.2022.108776
13. Han C, Li X, Shao C, et al. Composition-controllable p-CuO/n-ZnO hollow nanofibers for high-performance H2S detection. Sens. Actuators B: Chem. 2019;285:495–503.
https://doi.org/10.1016/j.snb.2019.01.077
14. Grau PB, Domene RMF, Tovar RS, Antón JG. Control on the morphology and photoelectrocatalytic properties of ZnO nanostructures by simple anodization varying electrolyte composition. J. Electro. Chem. 2021;880:114933.
https://doi.org/10.1016/j.jelechem.2020.114933
15. Devi LR, Sundar SM. Investigation on structural, photoluminescence and magnetic behavior of Mn-ZnO nanoparticles via solvothermal route. J. Indian Chem. Soc. 2023;100(7)101036.
https://doi.org/10.1016/j.jics.2023.101036
16. Dymshits O, Gorokhova E, Alekseeva I, et al. Transparent materials based on semiconducting ZnO: glass-ceramics and optical ceramics doped with rare-earth and transition-metal ions. J. Non Cryst. Solids. 2022;588:121625.
https://doi.org/10.1016/j.jnoncrysol.2022.121625
17. Li Y, Xu R, Qiao L, et al. Controlled synthesis of ZnO modified N-doped porous carbon nanofiber membrane for highly efficient removal of heavy metal ions by capacitive deionization. Micro. Meso. Mater. 2022;338:111889.
https://doi.org/10.1016/j.micromeso.2022.111889
18. Yılmaz G, Dindar B. Non-metal doped ZnO photocatalyst prepared by sonication-assisted sol-gel method and use for dye degradation. Inorg. Chem. Commun. 2023;157:111320.
https://doi.org/10.1016/j.inoche.2023.111320
19. Sawunyama L, Oyewo O, Onwudiwe DC, Makgato SS. Photocatalytic degradation of tetracycline using surface defective black TiO2-ZnO heterojunction photocatalyst under visible light. Heliyon. 2023;9(11)e21423.
https://doi.org/10.1016/j.heliyon.2023.e21423
20. Wang L, Ma C, Guo Z, et al. In-situ growth of g-C3N4 layer on ZnO nanoparticles with enhanced photocatalytic performances under visible light irradiation. Mater. Lett. 2017;188:347–350.
https://doi.org/10.1016/j.matlet.2016.11.113
21. Chen Q, Hou H, Zhang D, et al. Enhanced visible-light driven photocatalytic activity of hybrid ZnO/g-C3N4 by high performance ball milling. J. Photochem. Photobiol. A Chem. 2018;350:1–9.
https://doi.org/10.1016/j.jphotochem.2017.09.015
22. Prabhu S, Pudukudy M, Harish S, et al. Facile construction of djembe-like ZnO and its composite with g-C3N4 as a visible-light-driven heterojunction photocatalyst for the degradation of organic dyes. Mater. Sci. Semicond. Process. 2020;106:104754.
https://doi.org/10.1016/j.mssp.2019.104754
23. Kuang PY, Su YZ, Chen GF, et al. g-C3N4 decorated ZnO nanorod arrays for enhanced photoelectrocatalytic performance. Appl. Surf. Sci. 2015;358:296–303.
https://doi.org/10.1016/j.apsusc.2015.08.066
24. Nag I. Development of an engineered face mask with optimized nanoparticle layering for filtration of air pollutants and viral pathogens. Environ. Eng. Res. 2023;28(6)230003.
https://doi.org/10.4491/eer.2023.003
25. Stiadi Y, Wendari TP, Zilfa , Zulhadjri , Rahmayeni . Tuning the structural, magnetic, and optical properties of ZnO/NiFe2O4 heterojunction photocatalyst for simultaneous photodegradation of Rhodamine B and Methylene Blue under natural sunlight. Environ. Eng. Res. 2022;28(3)220074.
https://doi.org/10.4491/eer.2022.074
26. Shokrollahzadeh S, Abassi M, Ranjbar M. A new nano-ZnO/perlite as an efficient catalyst for catalytic ozonation of azo dye. Environ. Eng. Res. 2019;24(3)513–520.
https://doi.org/10.4491/eer.2018.322
27. Ravichandran K, Kalpana K, Uma R, Sindhuja E, Seelan KS. Cost-effective fabrication of ZnO/g-C3N4 composite film coated stainless steel meshes for visible light responsive photocatalysis. Mater. Res. Bulletin. 2018;99:268–280.
https://doi.org/10.1016/j.materresbull.2017.11.010
28. Škuta R, Kostura B, Ritz M, Foniok K, Pavlovský J, Matýsek D. Comparing the photocatalytic performance of GO/ZnO and g-C3N4/ZnO composites prepared using metallurgical waste as a source of zinc. Inorg. Chem. Commun. 2023;152:110728.
https://doi.org/10.1016/j.inoche.2023.110728
29. Xiao Y, Lyu H, Yang C, Zhao B, Wang L, Tang J. Graphitic carbon nitride/biochar composite synthesized by a facile ball-milling method for the adsorption and photocatalytic degradation of enrofloxacin. J. Environ. Sci. 2021;103:93–107.
https://doi.org/10.1016/j.jes.2020.10.006
30. Zheng Y, Yang Y, Zhang Y, et al. Facile one-step synthesis of graphitic carbon nitride-modified biochar for the removal of reactive red 120 through adsorption and photocatalytic degradation. Biochar. 2019;1(1)89–96.
https://doi.org/10.1007/s42773-019-00007-4
31. Guo J, Jiang S, Lin Z, et al. Pyrolysis of torrefied Phragmites australis from atmospheric and gas-pressurized torrefaction: Pyrolysis kinetic and product analysis. J. Anal. Appl. Pyrolysis. 2022;167:105670.
https://doi.org/10.1016/j.jaap.2022.105670
32. Sousa JGMD, Silva TVCD, Moraes NPD, et al. Visible light-driven ZnO/g-C3N4/carbon xerogel ternary photocatalyst with enhanced activity for 4-chlorophenol degradation. Mater. Chem. Phys. 2020;256:123651.
https://doi.org/10.1016/j.matchemphys.2020.123651
33. Guan K, Zhou P, Zhang J, Zhu L. Synthesis and characterization of ZnO@RSDBC composites and their Photo-Oxidative degradation of Acid Orange 7 in water. J. Mol. Structure. 2020;203:127425.
https://doi.org/10.1016/j.molstruc.2019.127425
34. Zhai S, Li M, Wang D, Zhang L, Yang Y, Fu S. In situ loading metal oxide particles on bio-chars: Reusable materials for efficient removal of methylene blue from wastewater. J. Clean. Prod. 2019;220:460–474.
https://doi.org/10.1016/j.jclepro.2019.02.152
35. Chen XL, Li F, Zhang M, Liu B, Chen H, Wang H. Highly dispersed and stabilized Co3O4/C anchored on porous biochar for bisphenol A degradation by sulfate radical advanced oxidation process. Sci. Total Environ. 2021;777:145794.
https://doi.org/10.1016/j.scitotenv.2021.145794
36. Wei X, Wang X, Gao B, Zou W, Dong L. Facile ball-milling synthesis of CuO/Biochar nanocomposites for efficient removal of Reactive Red 120. ACS Omega. 2020;5(11)5748–5755.
https://doi.org/10.1021/acsomega.9b03787
37. Geng A, Xu L, Gan L, et al. Using wood flour waste to produce biochar as the support to enhance the visible-light photocatalytic performance of BiOBr for organic and inorganic contaminants removal. Chemosphere. 2020;250:126291.
https://doi.org/10.1016/j.chemosphere.2020.126291
38. Nguyet BTM, Nghi NH, Tien NA, Khieu DQ, Duc HD, Hung NV. Enhanced adsorption of methylene blue by chemically modified materials derived from Phragmites australis stems. Acta. Chim. Slov. 2022;69(4)7567.
https://doi.org/10.17344/acsi.2022.7567
39. Liu W, Zhang J, Kang Q, Chen H, Feng R. Enhanced photocatalytic degradation performance of In2O3/g-C3N4 composites by coupling with H2O2
. Ecotoxicol. Environ. Saf. 2023;252:114611.
https://doi.org/10.1016/j.ecoenv.2023.114611
40. Liu J, Zhou S, Gu P, et al. Conjugate Polymer-clothed TiO2@V2O5 nanobelts and their enhanced visible light photocatalytic performance in water remediation. J. Colloid Interface Sci. 2020;578:402–411.
https://doi.org/10.1016/j.jcis.2020.06.014
41. Raizada P, Singh P, Kumar A, et al. Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: Role of adsorption in dye degradation. Appl. Catal. A-Gen. 2014;486:159–169.
https://doi.org/10.1016/j.apcata.2014.08.043
42. He Y, Wang Y, Hu J, et al. Photocatalytic property correlated with microstructural evolution of the biochar/ZnO composites. J. Mater. Res. Technol. 2021;11:1308–1321.
https://doi.org/10.1016/j.jmrt.2021.01.077
43. Djoko SYT, Bashiri H, Njoyim ET, et al. Urea and green tea like precursors for the preparation of g-C3N4 based carbon nanomaterials (CNMs) composites as photocatalysts for photodegradation of pollutants under UV light irradiation. J. Photochem. Photobiol. A: Chem. 2020;398:112596.
https://doi.org/10.1016/j.jphotochem.2020.112596
44. Huang L, Liu H, Wang Y, Zhang TC, Yuan S. Construction of ternary Bi2O3/biochar/g-C3N4 heterojunction to accelerate photoinduced carrier separation for enhanced tetracycline photodegradation. Appl. Surf. Sci. 2023;616:156509.
https://doi.org/10.1016/j.apsusc.2023.156509
45. Li Y, Liu Z, Li Z, Wang Q. Renewable biomass-derived carbon-supported g-C3N4 doped with Ag for enhanced photocatalytic reduction of CO2
. J. Colloid Interface Sci. 2022;606(Pt2)1311–1321.
https://doi.org/10.1016/j.jcis.2021.08.176
46. Martins NDJ, Gomes ICH, Silva GTSTD, et al. Facile preparation of ZnO:g-C3N4 heterostructures and their application in amiloride photodegradation and CO2 photoreduction. J. Alloys Compd. 2021;856:156798.
https://doi.org/10.1016/j.jallcom.2020.156798
47. Zhang W, Yin J, Chen C, Qiu X. Carbon nitride derived nitrogen-doped carbon nanosheets for high-rate lithium-ion storage. Chem. Eng. Sci. 2021;241:116709.
https://doi.org/10.1016/j.ces.2021.116709
48. Zhong Q, Lan H, Zhang M, Zhu H, Bu M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram Int. 2020;46(8)12192–12199.
https://doi.org/10.1016/j.ceramint.2020.01.265
49. Liang S, Sui G, Guo D, et al. g-C3N4-wrapped nickel doped zinc oxide/carbon core-double shell microspheres for high-performance photocatalytic hydrogen production. J. Colloid Interface Sci. 2023;635:83–93.
https://doi.org/10.1016/j.jcis.2022.12.120
50. Hu H, Sun L, Gao Y, et al. Synthesis of ZnO nanoparticle-anchored biochar composites for the selective removal of perrhenate, a surrogate for pertechnetate, from radioactive effluents. J. Hazard. Mater. 2020;387:121670.
https://doi.org/10.1016/j.jhazmat.2019.121670
51. Li Y, Liu X. Activated carbon/ZnO composites prepared using hydrochars as intermediate and their electrochemical performance in supercapacitor. Mater. Chem. Phys. 2014;148(1–2)380–386.
https://doi.org/10.1016/j.matchemphys.2014.07.058
52. Jafari M, Rahimi MR, Ghaedi M, Javadian H, Asfaram A. Fixed-bed column performances of azure-II and auramine-O adsorption by Pinus eldarica stalks activated carbon and its composite with zno nanoparticles: Optimization by response surface methodology based on central composite design. J. Colloid Interface Sci. 2017;507:172–189.
https://doi.org/10.1016/j.jcis.2017.07.056
53. Gholami P, Dinpazhoh L, Khataee A, Orooji Y. Sonocatalytic activity of biochar-supported ZnO nanorods in degradation of gemifloxacin: Synergy study, effect of parameters and phytotoxicity evaluation. Ultra. Sonochem. 2019;55:44–56.
https://doi.org/10.1016/j.ultsonch.2019.03.001
54. Xie Y, Zhuo Y, Liu S, et al. Ternary g-C3N4/ZnNCN@ZIF-8 hybrid photocatalysts with robust interfacial interactions and enhanced CO2 reduction performance. Sol. RRL. 2020;4(8)1–12.
https://doi.org/10.1002/solr.201900440
55. Wang S, Zhou Y, Han S, et al. Carboxymethyl cellulose stabilized ZnO/biochar nanocomposites: Enhanced adsorption and inhibited photocatalytic degradation of methylene blue. Chemosphere. 2018;197:20–25.
https://doi.org/10.1016/j.chemosphere.2018.01.022
56. Chand P, Gaur A, Kumar A. Structural and optical properties of ZnO nanoparticles synthesized at different pH values. J. Alloys Compd. 2012;539:174–178.
https://doi.org/10.1016/j.jallcom.2012.05.104
57. Guan R, Li J, Zhang J, et al. Photocatalytic Performance and Mechanistic Research of ZnO/g-C3N4 on degradation of methyl orange. ACS Omega. 2019;4(24)20742–20747.
https://doi.org/10.1021/acsomega.9b03129
58. Peng X, Wang M, Dai H, Qiu F, Hu F. In situ growth of carbon nitride on titanium dioxide/hemp stem biochar toward 2D heterostructured photocatalysts for highly photocatalytic activity. Environ. Sci. Pollut. Res. 2020;27(31)39198–39210.
https://doi.org/10.1007/s11356-020-09381-0
59. Tautua BMA, Fakayode OJ, Songca SP, Oluwafemi OS. Effect of synthetic conditions on the crystallinity, porosity and magnetic properties of gluconic acid capped iron oxide nanoparticles. Nano Struct. Nano Obj. 2020;23:100480.
https://doi.org/10.1016/j.nanoso.2020.100480
60. Hong S, Bruyn KD, Bescher E, Ramseyer C, Kang THK. Porosimetric features of calcium sulfoaluminate and Portland cement pastes: testing protocols and data analysis. J. Struct. Int. Maint. 2018;3(1)52–66.
https://doi.org/10.1080/24705314.2018.1426168
61. Zhang H, Su T, Yu S, et al. Fabrication of Anderson-Polyoxometalates/TiO2/C3N4 heterojunction composite for efficient visible-light-driven photooxidative desulfurization. Mol. Catal. 2023;536:112916.
https://doi.org/10.1016/j.mcat.2023.112916
62. Fernandez LM, Villalba LSG, Milošević O, Rabanal ME. Influence of nanoscale defects on the improvement of photocatalytic activity of Ag/ZnO. Mater. Charact. 2022;185:111718.
https://doi.org/10.1016/j.matchar.2021.111718
63. Alsulmi A, Mohammed NN, Soltan A, Messih MFA, Ahmed MA. Engineering S-scheme CuO/ZnO heterojunctions sonochemically for eradicating RhB dye from wastewater under solar radiation. RSC Adv. 2023;13(19)13269–13281.
https://doi.org/10.1039/D3RA00924F
64. Gupta B, Gupta AK, Tiwary CS, Ghosal PS. A multivariate modeling and experimental realization of photocatalytic system of engineered S-C3N4/ZnO hybrid for ciprofloxacin removal: Influencing factors and degradation pathways. Environ. Res. 2020:110390.
https://doi.org/10.1016/j.envres.2020.110390
65. Guo F, Shi W, Guan W, Huang H, Liu Y. Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep. Purif. Technol. 2017;173:295–303.
https://doi.org/10.1016/j.seppur.2016.09.040
66. Li X, Li M, Yang J, et al. Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J. Phys. Chem. Solids. 2014;75(3)441–446.
https://doi.org/10.1016/j.jpcs.2013.12.001
67. Das D, Nandi P. ZnO/g-C3N4 heterostructures: Synthesis, characterization and application as photoanode in dye sensitized solar cells. Sol. Energy Mater. Sol. Cells. 2022;248:112002.
https://doi.org/10.1016/j.solmat.2022.112002
68. Jung H, Pham TT, Shin EW. Interactions between ZnO nanoparticles and amorphous g-C3N4 nanosheets in thermal formation of g-C3N4/ZnO composite materials: The annealing temperature effect. Appl. Surf. Sci. 2018;458:369–381.
https://doi.org/10.1016/j.apsusc.2018.07.048
69. Nie N, Zhang L, Fu J, Cheng B, Yu J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018;441:12–22.
https://doi.org/10.1016/j.apsusc.2018.01.193
70. Thi NT, Nam SN. Sunlight-driven photocatalysis of dissolved organic matter: Tracking by excitation emission matrix-parallel factor analysis and optimization using response surface methodology. Environ. Eng. Res. 2021;26(3)200201.
https://doi.org/10.4491/eer.2020.201
71. Berdini F, Otalvaro JO, Avena M, Brigante M. Photodegradation of doxycycline in water induced by TiO2-MCM-41. Kinetics, TOC evolution and reusability. Results Eng. 2022;16:100765.
https://doi.org/10.1016/j.rineng.2022.100765
72. Swetha S, Maksoud MAA, Okla MK, et al. Triple-mechanism driven Fe-doped n-n hetero-architecture of Pr6O11-MoO3 decorated g-C3N4 for doxycycline degradation and bacterial photoinactivation. Chem. Eng. J. 2023;461:141806.
https://doi.org/10.1016/j.cej.2023.141806
73. Dhiman P, Rana G, Alshgari RA, et al. Magnetic Ni-Zn ferrite anchored on g-C3N4 as nano-photocatalyst for efficient photo-degradation of doxycycline from water. Environ. Res. 2023;216(Pt3)114665.
https://doi.org/10.1016/j.envres.2022.114665
74. Wen Q, Li D, Li H, et al. Synergetic effect of photocatalysis and peroxymonosulfate activated by Co/Mn-MOF-74@g-C3N4 Z-scheme photocatalyst for removal of tetracycline hydrochloride. Sep. Purif. Technol. 2023;313:123518.
https://doi.org/10.1016/j.seppur.2023.123518
75. Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17(2)513–886.
https://doi.org/10.1063/1.555805
76. Palominos R, Freer J, Mondaca MA, Mansilla HD. Evidence for hole participation during the photocatalytic oxidation of the antibiotic flumequine. J. Photochem. Photobiol. A Chem. 2008;193(2–3)139–145.
https://doi.org/10.1016/j.jphotochem.2007.06.017
77. Oosawa Y, Grätzel M. Effect of surface hydroxyl density on photocatalytic oxygen generation in aqueous TiO2 suspensions. J. Chem. Soc., Faraday Trans. 1. 1988;84(1)197–205.
https://doi.org/10.1039/F19888400197
78. Li Y, Wang J, Yao H, Dang L, Li Z. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A: Chem. 2011;334(1–2)116–122.
https://doi.org/10.1016/j.molcata.2010.11.005
79. Shi YC, Chen SS, Feng JJ, Lin XX, Wang W, Wang AJ. Dicationic ionic liquid mediated fabrication of Au@Pt nanoparticles supported on reduced graphene oxide with highly catalytic activity for oxygen reduction and hydrogen evolution. Appl. Surf. Sci. 2018;441:438–447.
https://doi.org/10.1016/j.apsusc.2018.01.240
80. Qamar MA, Shahid S, Javed M. Synthesis of dynamic g-C3N4/Fe@ZnO nanocomposites for environmental remediation applications. Ceram. Int. 2020;46(14)22171–22180.
https://doi.org/10.1016/j.ceramint.2020.05.294
81. Mengting Z, Duan L, Zhao Y, et al. Fabrication, characterization, and application of BiOI@ZIF-8 nanocomposite for enhanced photocatalytic degradation of acetaminophen from aqueous solutions under UV-vis irradiation. J. Environ. Manage. 2023;345:118772.
https://doi.org/10.1016/j.jenvman.2023.118772
82. Lv H, Ji G, Yang Z, et al. Enhancement photocatalytic activity of the graphite-like C3N4 coated hollow pencil-like ZnO. J. Colloid Interface Sci. 2015;450:381–387.
https://doi.org/10.1016/j.jcis.2015.03.038
83. Liu W, Wang M, Xu C, Chen S. Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem. Eng. J. 2012;209:386–393.
https://doi.org/10.1016/j.cej.2012.08.033
84. Mousavi M, Habibi-Yangjeh A. Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites: Novel visible-light-driven photocatalysts based on graphitic carbon nitride. J. Colloid Interface Sci. 2016;465:83–92.
https://doi.org/10.1016/j.jcis.2015.11.057
85. Payan A, Akbar IA, Gholizade N. Catalytic decomposition of sulfamethazine antibiotic and pharmaceutical wastewater using Cu-TiO2@functionalized SWCNT ternary porous nanocomposite: Influential factors, mechanism, and pathway studies. Chem. Eng. J. 2019;361:1121–1141.
https://doi.org/10.1016/j.cej.2018.12.118
86. Shao B, Liu X, Liu Z, et al. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019;368:730–745.
https://doi.org/10.1016/j.cej.2019.03.013
87. He C, Ma J, Xu H, Ge C, Lian Z. Selective capture and determination of doxycycline in marine sediments by using magnetic imprinting dispersive solid-phase extraction coupled with high performance liquid chromatography. Mar. Pollut. Bull. 2022;184:14215.
https://doi.org/10.1016/j.marpolbul.2022.114215
Fig. 1The XRD patterns (a), FTIR spectra (b), UV-Vis DR spectra (c), Tauc’s plots (d), PL spectra (e), N2 sorption/desorption isotherms of ZnO, g-C3N4, biochar, ZCN, and ZCNB (f). 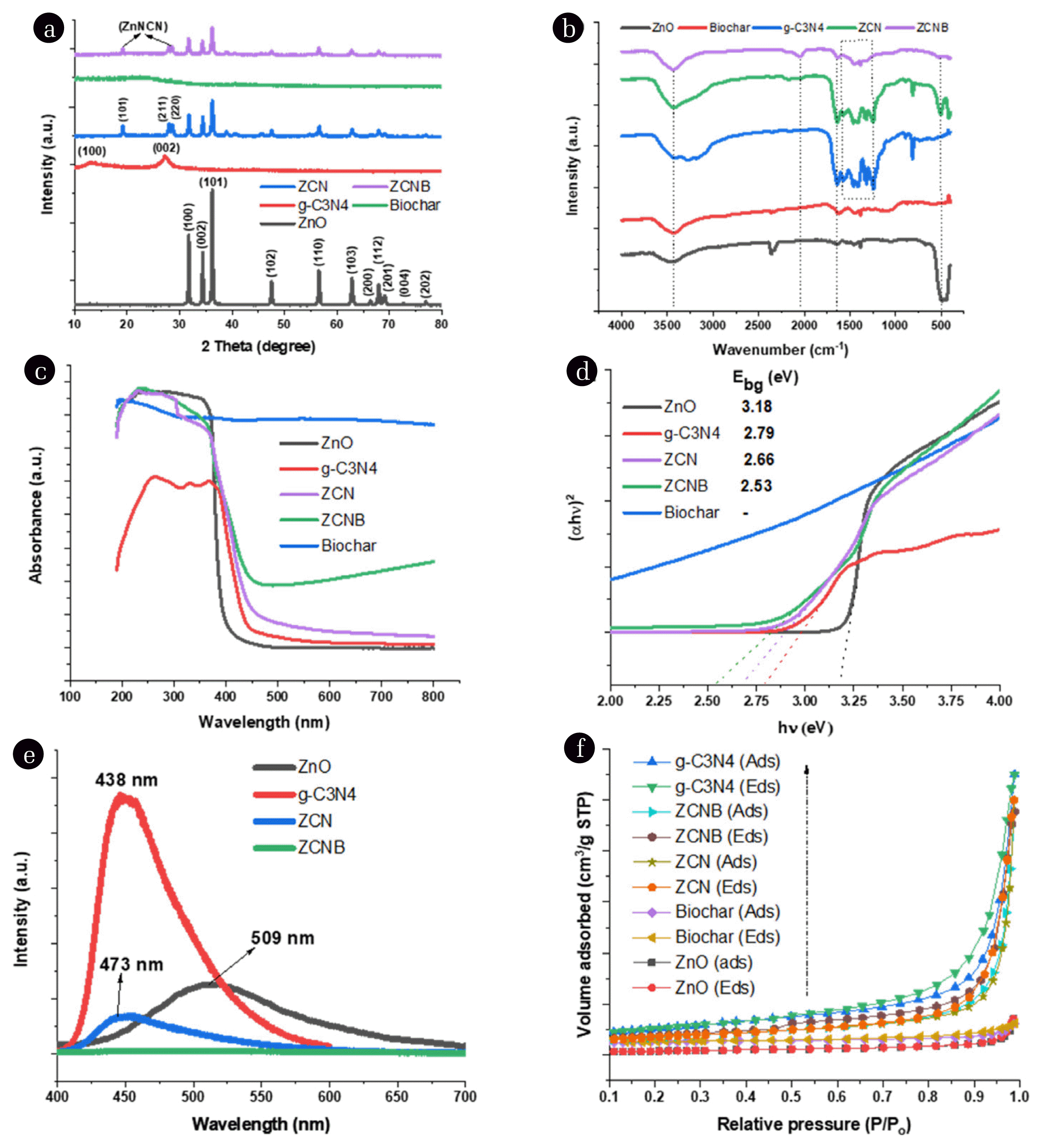
Fig. 2SEM observations of ZnO (a); TEM observation of ZnO (b); SEM observations of g-C3N4 (c); biochar (d); ZCN (e); ZCN (f), ZCNB (g); and ZCNB (h); and HRTEM observations of ZCNB (i). 
Fig. 3a) electron image and b) EDX spectrum of ZCNB sample; c) elemental mapping image of C, N, O, and Zn on ZCNB; EDX elemental mapping of d) C, e) N, f) O, and g) Zn. 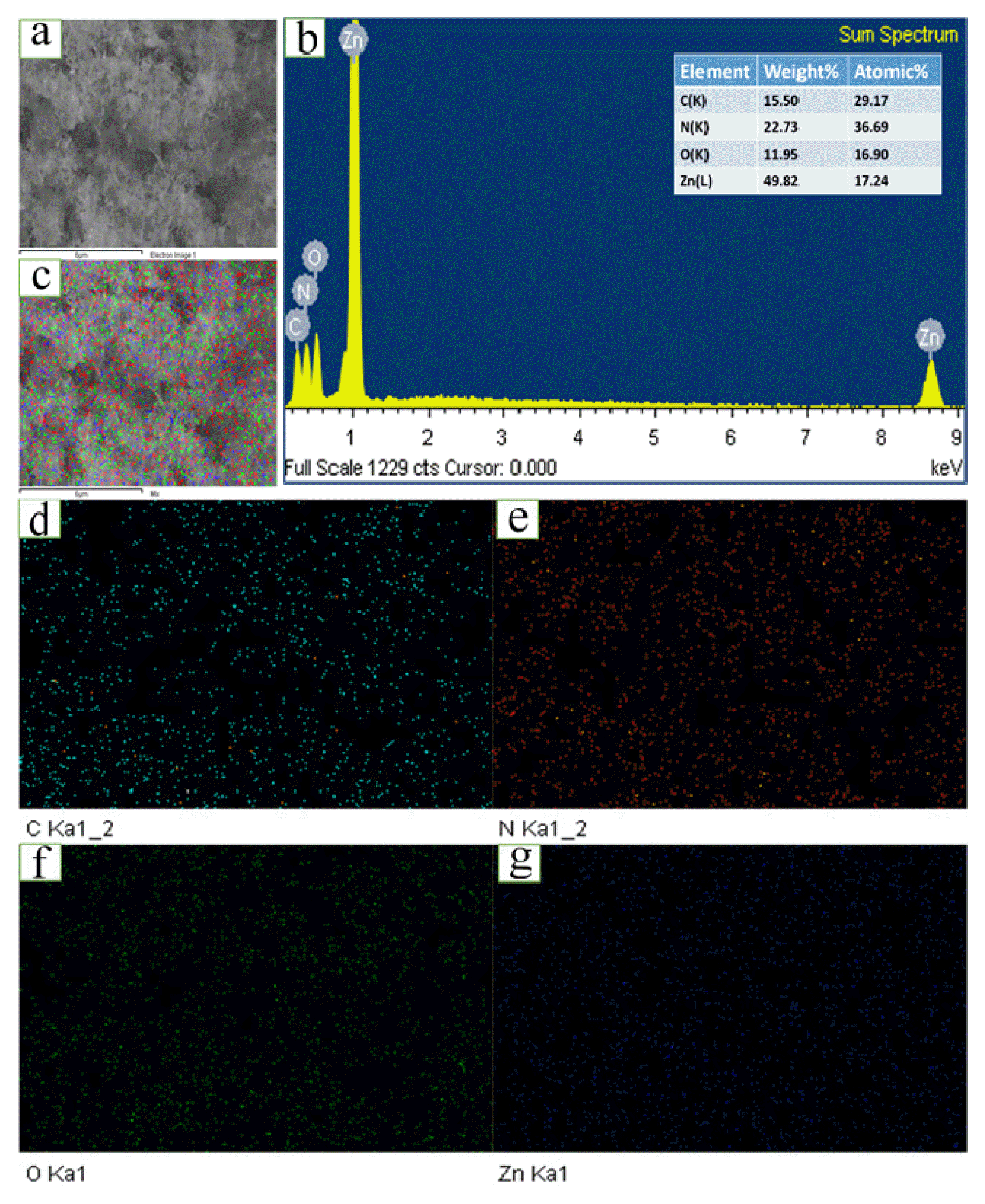
Fig. 5a) Photodegradation of doxycycline over no catalyst, ZnO, g-C3N4, ZCN, and ZCNB. b) First-order kinetics plot for the photodegradation of doxycycline by ZnO, g-C3N4, ZCN, and ZCNB samples; c) COD value versus irradiation time; d) Effect of different scavengers on doxycycline photodegradation over ZCNB samples. (Mass of catalyst: 0.1 gram; DC concentration of 25 mg·L−1; the volume of 200 mL (pH~5); time for dark adsorption is 60 min and for illumination is 120 minutes). 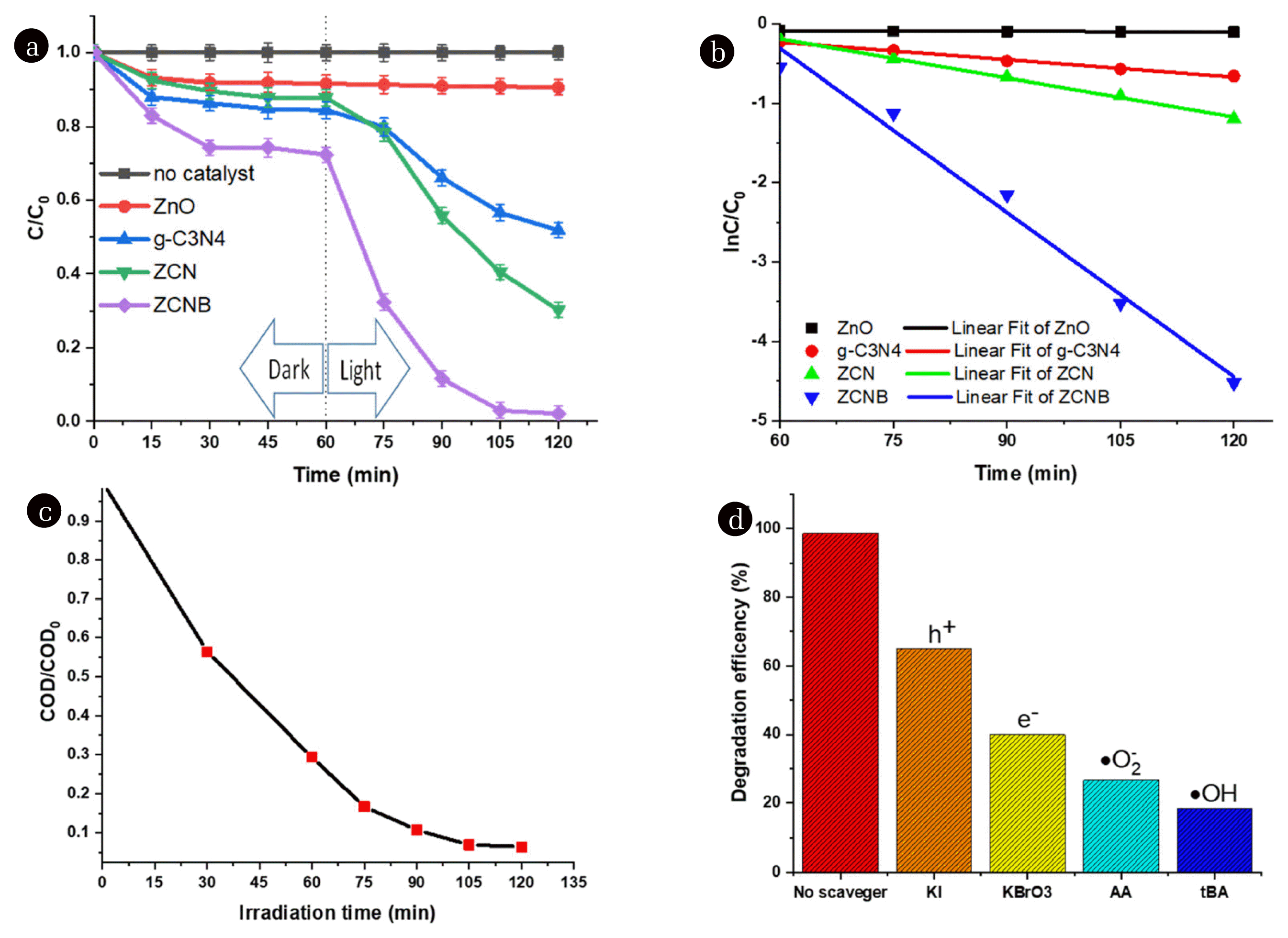
Fig. 7a) Reusability of the ZCNB nanocomposite in six successive runs; b) XRD patterns of used ZCNB after third and sixth cycle (Mass of catalyst: 0.1 gram; DC concentration of 25 mg·L−1; the volume of 200 mL (pH~5); time for dark adsorption is 60 min and for illumination is 120 minutes) and c) DC degradation in the real shrimp aquaculture wasterwater over ZCNB catalyst. 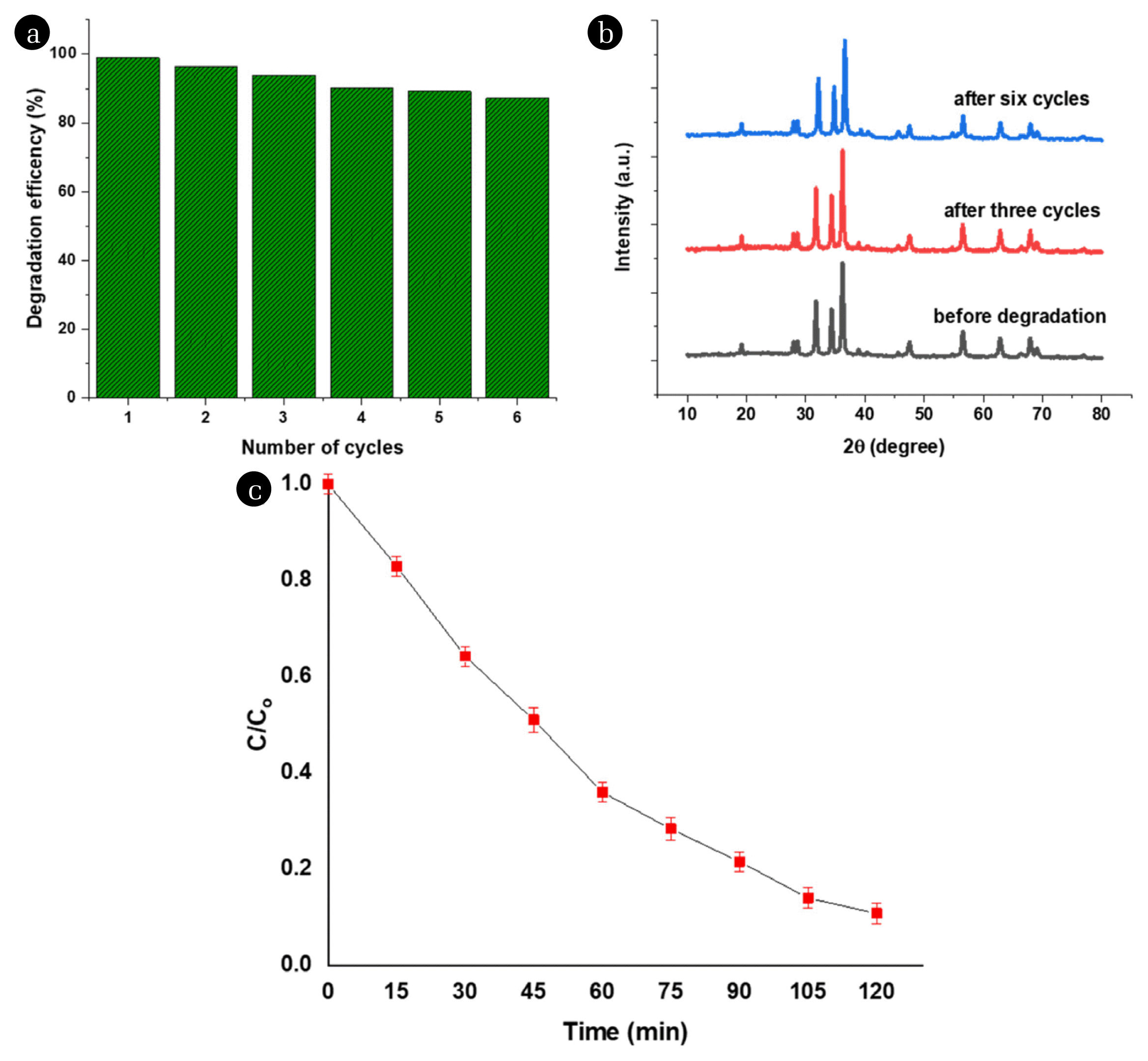
|
|
|||||||||||||||||||||||||||||||||||