AbstractPeroxymonosulfate (PMS, HSO5-) is an increasingly popular oxidant for groundwater remediation via in situ chemical oxidation (ISCO). However, it is desired to evaluate the effect of phosphate on the PMS oxidation of organics in detail due to the saline characteristics of groundwater. In this study, ofloxacin (OFX) as a typical antibiotic was chosen as a model pollutant. The results indicated that 64% of OFX was degraded by PMS in the presence of HPO42− (PMS/HPO42− system), and the efficiency increased from 64% to 80% when the HPO42− concentration was increased from 0.1 mol/L to 0.3 mol/L. The corresponding pseudo-first-order reaction constants were 0.0086 and 0.02 min−1, respectively. In comparison, PO43− and H2PO4− showed almost no reactivity. Electron spin response (ESR) analysis and quenching tests proved that SO4− and 1O2 were the main oxidizing species for OFX degradation in PMS/HPO42− system. Hence, HPO42− was the most effective form in PMS activation and its performance was highly pH and ionic strength sensitive. Given the ambient pH and phosphate concentration in saline groundwater, a considerable amount of HPO42− can be produced, which then serves as a homogeneous PMS activator in ISCO process. This finding provides new insights into ISCO process using PMS.
Graphical Abstract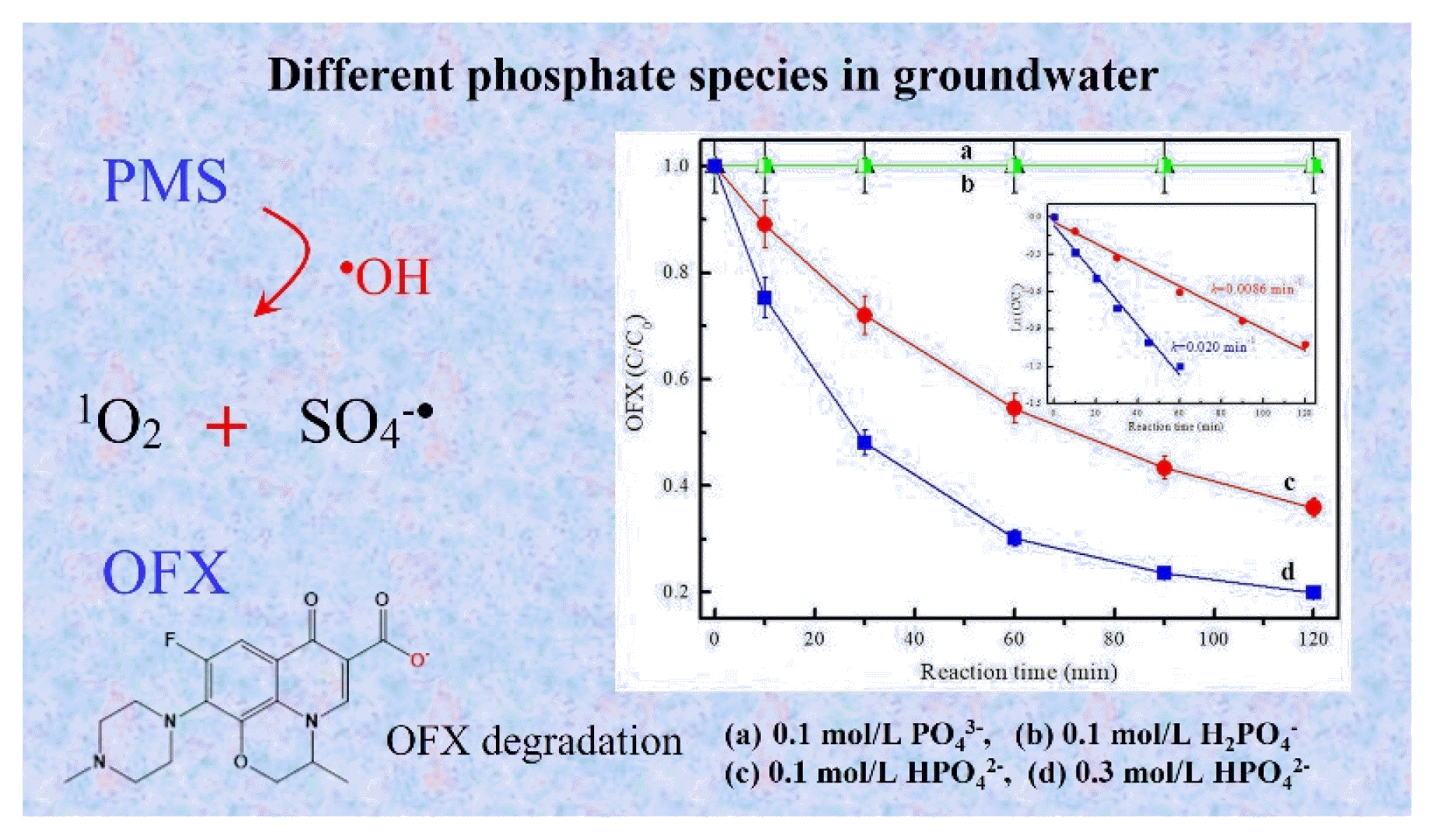
1. IntroductionAt present, a lot of organic and inorganic pollutants in groundwater have become a considerable and growing concern due to their ubiquitous presence, recalcitrance in the environment, and toxicity to humans and wildlife [1–3]. Based on the recent research on both the laboratory and pilot scales, in situ chemical oxidation (ISCO) has proven to be a widely used technology for the remediation of contaminated groundwater [4, 5].
Compared with the most common ISCO reagents such as H2O2, and permanganate, peroxymonosulfate (PMS) is more popular because it is significantly more stable and cheaper, which provides the potential for transport from the point of injection to contaminants in lower permeability regions of the subsurface [6–9]. Actually, PMS has a low reactivity towards the degradation of organic pollutants. When initiated by an activator, sulfate radicals (SO4˙−) and hydroxyl radicals (˙OH) can be generated from PMS decomposition that can ideally destroy a wide range of toxic organic compounds [10–21]. The above properties make PMS a promising alternative for ISCO technology due to the fact that PMS could minimize the loss during the oxidant delivery process to the targeted area and could become highly reactive in the contaminated zone by the effect of additional catalysts or pollutants themselves.
In the ISCO studies of natural waters, it needs to be carefully considered is the complexity of the water matrices that contain different kinds of water parameters. As reported, reactive radicals can react with both organic and inorganic compounds. Common water quality parameters such as inorganic anions may compete with target compound for radical species leading to positive or negative impacts on the degradation kinetics [22, 23]. Therefore, it is important to consider and evaluate the impact of the co-existed anions such as phosphate on the remediation efficiency of PMS when PMS is employed for water treatment and environmental remediation.
The interactions of phosphate and PMS as well as the subsequent oxidation processes have been investigated. Generally, the PMS decomposition and the generation of reactive species are mainly affected by the presence of phosphate species [24]. It is reported that the sodium pyrophosphate (PA, Na4P2O7) is active to degrade acid orange 7, rhodamine B and 2,4,6-trichlorophenol in PMS/base system [25–28]. Moreover, monohydrogen phosphate (HPO42−) promoted PMS activation, while dihydrogen phosphate (H2PO4−) played a negative role in sulfamethoxazole degradation [29]. In comparison, phosphate (PO43− and HPO42−) adversely affected the oxidation efficiency of reactive species towards target pollutants because they can scavenge SO4˙− and ˙OH radicals to form less reactive species [30, 31]. Besides that, Yang et al. found in their study that the addition of phosphate had no effect on the removal of furfuryl alcohol (FFA) and the PMS decomposition [6]. However, no direct evidence can be obtained to clarify the above issue and the important role played by phosphate species in PMS ISCO process is unknown. Hence, it is necessary to investigate the OFX degradation efficiency by PMS in the presence of different phosphate species and the dominant reactive species (ROS).
In this work, ofloxacin (OFX) was chosen as a model contaminant because of its widespread and frequently detected in groundwater. H2PO4−, HPO42− and PO43− were used to evaluate the effect of phosphate species on remediation efficiency of PMS. The reactive oxygen species and their contribution to OFX degradation were studied using electron spin response (ESR) and radical quenching tests. The performance of H2PO4− on PMS activation at different solution pH and ionic strength was conducted and the relative kinetics analysis was utilized to quantify the impact of H2PO4− on ISCO using PMS.
2. Materials and Methods2.1. MaterialsOxone® (2KHSO5˙KHSO4˙K2SO4, PMS) and ofloxacin were obtained from Aladdin. Ethanol, tert-butyl alcohol (TBA, 99.9%), sodium azide (NaN3, 99.5%), acetonitrile, and methanol (MeOH) of HPLC grade were purchased from Sinopharm Chemical Reagent Co., Ltd. 5,5-Dimethyl-1-pyrroline (DMPO, >99.0%) and 2,2,6,6- Tetramethyl-4-piperidinol (TEMP, 99%) for EPR-spectroscopy were purchased from Sigma-Aldrich. All chemicals were used as received without further purification.
2.2. Experimental ProcedureAll experiments were carried out in a batch reactor with a magnetic stirrer in a water bath maintained at 25°C. In a typical experiment, the desired amount of PMS and phosphate were added into 100 mL 10 mg/L OFX solution. The reaction solution was not buffered and the pH changes during the reaction process were monitored by a pH meter. The solution pH was maintained at 5.0 and 9.0 and adjusted using HNO3 or NaOH dilute solution, which remained the same within 0.3 units at the end. At given time intervals, 2.5 mL of sample was withdrawn and filtered by a 0.45 μm membrane, and the filtrate was quickly mixed with 150 μL of a Na2S2O3 solution to quench ROS and terminate the oxidation. All the experiments were repeated three times and the data represented the average of the triplicates with a standard deviation of less than 5%.
2.3. AnalysisThe concentration of OFX was determined by high-performance liquid chromatography (HPLC) with a UV-DAD detector. The chromatographic separation was performed by a reverse-phase C18 column (250 mm×4.6 mm, 5 μm). The mobile phase was composed of 15% acetonitrile and 85% ultrapure water acidified with 1% formic acid at 0.5mL/min. The injection volume was 20 μL. Temperature of the column chamber was maintained at 25°C and the detection wavelength was 288 nm. Electron paramagnetic resonance (EPR) experiments were performed on a JES-FA200 EPR spectrometer with DMPO and TEMP as the spin-trapping agents. The ROS trapping experiments were performed using tert-butanol (TBA), Ethanol (MeOH), and sodium azide (NaN3) as the quenching agents.
3. Results and Discussion3.1. Performance of Phosphate Species on PMS Activation for OFX DegradationFirst, H2PO4−, HPO42− and PO43− were adopted to evaluate their performance on PMS activation towards OFX degradation. As shown in Fig. 1, at a PMS concentration of 0.8 g/L, phosphate concentration of 0.1 mol/L at pH 9, almost no OFX degradation was observed by PMS in the presence of H2PO4− or PO43−. However, 64% of OFX was removed at 120 min by the addition of HPO42− under the same conditions. Importantly, the removal efficiency was further increased from 64.1% to 80.1% when HPO42− concentration was increased from 0.1 mol/L to 0.3 mol/L. The corresponding pseudo-first-order reaction rate constants of HPO42− were 0.0086 and 0.020 min−1 respectively. Moreover, Fig. 2 compared the OFX degradation efficiency by PMS at pH 9 and PMS/HPO42− system at pH 9, respectively. During the reaction process, the pH value remained the same within 0.3 units adjusted by a diluted aqueous solution of NaOH or HCl. It was found that the OFX removal efficiency was only 27.4% in PMS/pH 9 system with a reaction rate constant of 0.0027 min−1. Obviously, the addition of HPO42− in the above system can greatly increase the OFX degradation efficiency to 80% with a reaction rate constant of 0.020 min−1. Hence, HPO42− is proven to be effective in PMS activation for OFX degradation.
The effect of initial solution pH on the catalytic performance of PMS/HPO42− system was further investigated and the results were shown in Fig. 3a. At pH 9, 80% of OFX can be degraded at 120 min, in comparison, the removal rate apparently decreased at pH 5 and only 58.3% of OFX was removed under the same conditions. Apart from acidic and alkaline conditions, the efficiency of OFX removal in the PMS/HPO42− system is estimated in the neutral solution. As depicted in Fig. 3a, about 74.7% of OFX was removed at 120 min in the PMS/HPO42− system at pH 7, which was contributed to the mixed phosphate species (HPO42− and H2PO4−) presented in the reaction solution. It has also been reported that phosphate can accelerate the degradation of organic compounds in the presence of PMS and H2O2 [28, 32]. While Yang et al. found that phosphate had no effect on the removal of furfuryl alcohol (FFA) and the PMS decomposition [6]. Based on the distribution of H2PO4−, HPO42− and PO43− in aqueous solution as a function of pH in Fig. 4, at pH 3–7, H3PO4, H2PO4− and HPO42− are the species present in solution and H2PO4− is the dominant species. While at pH 7–12, H2PO4−, HPO42− and PO43− present, and HPO42− is the dominant species. Therefore, at pH 5, the dominant species of phosphate was H2PO4−, while H2PO4− was ineffective to activate PMS as depicted in Fig. 1. This result indicated that the decrease of OFX degradation efficiency at pH 5.0 can be contributed to the partial conversion of HPO42− to H2PO4−. PMS activation efficiency by phosphate was dependent on the steady concentration of HPO42− in the solution.
Moreover, it was reported from previous studies that high-phosphate groundwater ranging from 1.53 to 40.204±6.024 mg/L was observed in the Pearl River Delta (China), Oguta Lake located in Southeast Nigeria, and lower Vamsadhara River basin (India) [33–35]. Hence, the effect of phosphate with the lower concentration (0.1 and 10 mmol/L) was conducted and the results were shown in Fig 3b. Obviously, approximately 43.8% and 47.7% of OFX removal efficiency can be found at 120 min in the presence of 0.1 and 10 mmol/L HPO42−, respectively. Most importantly, 82.4% of OFX can be removed within 4 h at 0.1 mmol/L of HPO42− (inset of Fig. 3b). It demonstrated that PMS activation for OFX oxidation can be achieved at extremely low concentration of phosphate which can meet the wastewater discharge standard.
3.2. PMS Activation Mechanism by HPO42−ESR studies were carried out to identify the kind of reactive oxygen species involved in PMS/HPO42− system. As depicted in Fig. 5, the strong 1:1:1 triplet signal characteristic of TEMPO was quickly observed when TEMP was added, indicating the existence of singlet oxygen (1O2) in the reaction system. Simultaneously, the sextet peaks with special hyperfine coupling constants (a(N) 1.38 mT, a(H) 1.02 mT, a(H) 0.14 mT, a(H) 0.08 mT, all ± 0.05 mT) of DMPO-SO4˙− were also detected. The seven-line spectrum labeled as ♣ can be assigned to the DMPO oxidation product by SO4˙−. Radical quenching tests were further conducted to clarify the contribution of ROS and PMS direct oxidation to OFX degradation. In this study, Ethanol can scavenge both SO4˙− (1.6–7.8×108 M−1×s−1) and ˙OH (1.2–2.8×109 M−1×s−1), whereas tert-butanol can only scavenge ˙OH with a reaction rate constant (3.8–7.6×108 M−1×s−1) 1000 times higher than with SO4˙− (4.0–9.1×105 M−1×s−1). Besides, sodium azide (NaN3) is a promising scavenger of both 1O2 and ˙OH with reaction rate constants of 2×109 M−1s−1 and 1×1010 M−1s−1. As shown in Fig. 6, the addition of tert-butanol had almost no inhibitory effect on the OFX degradation. While the OFX degradation decreased by 48.4% and only 32% of OFX was removed after the addition of ethanol. It indicated that SO4˙− rather than ˙OH was generated in the PMS/HPO42− system. Most important, the OFX degradation was nearly completely depressed in the presence of NaN3. Hence, both 1O2 and SO4˙− were generated and involved in the PMS-HPO42− system.
Since phosphate especially HPO42− is a well-known Lewis base, the involved 1O2 should be generated via general acid/base and nucleophilic mechanism. As shown in Eq. (1), HPO42− reacts with HSO5− and produces some intermediates such as nucleophilic HPO42−—OH (Nu-OH) by breaking the peroxide O-O bond. Nu-OH further reacts with HSO5− and facilitates the PMS self-decomposition to generate 1O2 as demonstrated in Eq. (2). Since there was no HO2˙ or O2˙− detected, the contribution of HO2˙ and O2˙− conversion to 1O2 can be neglected. As far SO4˙− radical, it should be generated PMS decomposition by electrons from OFX in Eq. (4). Hence, the efficient OFX degradation should come from the contribution of 1O2 and SO4˙− as depicted in (Eq. (3) and Eq. (5).
3.3. Implications for Groundwater Remediation via In-Situ Chemical OxidationGenerally, ISCO processes can be divided into two phases: the delivery phase and the remediation phase [36]. It may take a few days for the oxidants to reach the entire area, while in some low-permeability zones, it may even take one month [36]. Moreover, the remediation phase takes several days in some cases where either heat, minerals, or base are usually used as an activation method [12, 37–39]. Especially, base activation of persulfate has been used at approximately 60% of sites where persulfate-based ISCO has been employed [39]. However, heat relies on specific equipment and the excess base can lead to secondary pollution in the treated water. Our results proved that HPO42− in solution can activate PMS into 1O2 and SO4˙− for the efficient degradation of OFX. Since the phosphate and solution pH level range from 8.5–10.0 widely exist in natural water environments, which is beneficial to HPO42− generation and efficient PMS activation, as demonstrated in this study. In other words, the in-situ remediation of groundwater by PMS oxidation is inevitably a combined process of HPO42− and PMS oxidation.
Moreover, as mentioned above, the unactivated PMS is relatively inert to most contaminants. Hence, the interaction between PMS and HPO42− also brings advantages to the ISCO process. On the one side, 1O2 and SO4˙− from the PMS activation by HPO42− have a much higher oxidation potential than PMS (PMS: 2.01 V, 1O2: 2.22 V, SO4˙−: 3.1 V), which possesses an excellent performance towards the OFX degradation. On the other side, the in-situ conversion of PMS to ROS by HPO42− can significantly reduce and even avoid the demand for PMS activators, while at the same time preventing secondary pollution induced by these activators. All these data can provide valuable guidance for designing a suitable ISCO process using PMS as oxidant for the groundwater remediation.
4. ConclusionsThis study has investigated the synergetic effect of phosphate and PMS on the OFX degradation in detail and evaluated the application potential of PMS in ISCO process of contaminated groundwater. Phosphate was proven to have a great effect on the PMS activation towards the OFX removal. Results showed that at a PMS concentration of 0.8 g/L, HPO42− concentration of 0.3 mol/L and solution pH of 9.0, above 80% of OFX was degraded in PMS/HPO42− system, while PO43− and H2PO4− showed almost no reactivity. The optimal parameters for OFX removal are solution pH of 9.0, higher concentration of PMS and HPO42−. The efficient OFX degradation can be assigned to the production of SO4˙− and 1O2 from the interaction of PMS and HPO42−. Hence, HPO42− is the most effective phosphate species in groundwater for PMS activation and SO4˙− and 1O2 generation. Given the alkaline pH and phosphate concentration in groundwater, much more HPO42− can be generated, which is favorable to the in-situ PMS activation. Generally, this study signals that engineers should pay more attention to the contribution of homogeneous PMS activation by phosphate to groundwater remediation in ISCO process.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 51878633, 41773126, 41807200, 42107102) and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 41521001) and the “Fundamental Research Funds for the Central Universities”.
NotesAuthor Contributions M.T. (PhD student) conducted the experiments and wrote the original draft. Y.L.H. (Master’s graduate) conducted part of the experiments and contributed to the wring-review & editing. N.T. (Post-doctor) wrote and revised the manuscript. Y.L.N. (Professor) played a role of supervision, funding acquisition. References1. Postigo C, Barcelo D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015;503–504:32–47.
https://doi.org/10.1016/j.scitotenv.2014.06.019
2. Kong L, Kadokami K, Duong H, Chau H. Screening of 1300 organic micro-pollutants in groundwater from Beijing and Tianjin, North China. Chemosphere. 2016;165:221–230.
https://doi.org/10.1016/j.chemosphere.2016.08.084
3. O’Connor D, Hou D, Ok Y, et al. Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: A review. J. Control. Release. 2018;283:200–213.
https://doi.org/10.1016/j.jconrel.2018.06.007
4. Devi P, Das U, Dalai A. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016;571:643–657.
https://doi.org/10.1016/j.scitotenv.2016.07.032
5. Tsitonaki A, Petri B, Crimi M, Mosbaek H, Siegrist R, Bjerg P. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Env. Sci. Tech. 2010;40:55–91. 10.1080/10643380802039303
6. Yang Y, Banerjee G, Brudvig G, Kim J, Pignatello J. Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ. Sci. Technol. 2018;52:5911–5919. 10.1021/acs.est.8b00735
7. Zhou Y, Jiang J, Gao Y, et al. Activation of peroxymonosulfate by phenols: Important role of quinone intermediates and involvement of singlet oxygen. Water Res. 2017;125:209–218. 10.1016/j.watres.2017.08.049
8. Shukla P, Wang S, Sun H, Ang H, Tadé M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Appl. Catal. B: Environ. 2010;100:529–534. 10.1016/j.apcatb.2010.09.006
9. Ghanbari F, Moradi M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017;310:41–62. 10.1016/j.cej.2016.10.064
10. Guan Y, Ma J, Li X, Fang J, Chen L. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011;45:9308–9314. 10.1021/es2017363
11. Yang Q, Choi H, Chen Y, Dionysiou D. Heterogeneous activation of peroxymonosulfate by supported cobalt catalysts for the degradation of 2,4-dichlorophenol in water: The effect of support, cobalt precursor, and UV radiation. Appl. Catal. B: Environ. 2008;77:300–307. 10.1016/j.apcatb.2007.07.020
12. Yang S, Wang P, Yang X, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010;179:552–558. 10.1016/j.jhazmat.2010.03.039
13. Cai C, Zhang H, Zhong X, Hou L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015;283:70–79. 10.1016/j.jhazmat.2014.08.053
14. Pang Y, Lei H. Degradation of p-nitrophenol through microwave- assisted heterogeneous activation of peroxymonosulfate by manganese ferrite. Chem. Eng. J. 2016;287:585–592. 10.1016/j.cej.2015.11.076
15. Qi C, Liu X, Lin C, Zhang H, Li X, Ma J. Activation of peroxymonosulfate by microwave irradiation for degradation of organic contaminants. Chem. Eng. J. 2017;315:201–209. 10.1016/j.cej.2017.01.012
16. Yan S, Geng J, Guo R, Du Y, Zhang H. Hydronium jarosite activation of peroxymonosulfate for the oxidation of organic contaminant in an electrochemical reactor driven by microbial fuel cell. J. Hazard. Mater. 2017;333:358–368. 10.1016/j.jhazmat.2017.03.043
17. Zeng T, Zhang X, Wang S, Niu H, Cai Y. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ. Sci. Technol. 2015;49:2350–2357. 10.1021/es505014z
18. Yao Y, Cai Y, Wu G, et al. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water. J. Hazard. Mater. 2015;296:128–137. 10.1016/j.jhazmat.2015.04.014
19. Ren Y, Lin L, Ma J, Yang J, Feng J, Fan Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M=Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B: Environ. 2015;165:572–578. 10.1016/j.apcatb.2014.10.051
20. Anipsitakis G, Dionysiou D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004;38:3705–3712. 10.1021/es035121o
21. Qi C, Liu X, Ma J, Lin C, Li X, Zhang H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere. 2016;151:280–288. 10.1016/j.chemosphere.2016.02.089
22. Khan S, He X, Ali Khan J, Hasan M, Boccelli D, Dionysiou D. Kinetics and mechanism of sulfate radical-and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017;318:135–142. 10.1016/j.cej.2016.05.150
23. Ali Khan J, Shah N, Khan H. Decomposition of atrazine by ionizing radiation: Kinetics, degradation pathways and influence of radical scavengers. Sep. Purif. Technol. 2015;156:140–147. 10.1016/j.seppur.2015.09.064
24. Duan P, Liu X, Liu B, et al. Effect of phosphate on peroxymonosulfate activation: Accelerating generation of sulfate radical and underlying mechanism. Appl. Catal. B: Environ. 2021;298:120532. 10.1016/j.apcatb.2021120532
25. Yang F, Huang Y, Fang C, et al. Peroxymonosulfate/base process in saline wastewater treatment: The fight between alkalinity and chloride ions. Chemosphere. 2018;199:84–88.
https://doi.org/10.1016/j.chemosphere.2018.02.023
26. Sheng B, Huang Y, Wang Z, Yang F, Ai L, Liu J. On peroxymonosulfate-based treatment of saline wastewater: when phosphate and chloride co-exist. RSC Adv. 2018;8:13865–13870. 10.1039/C8RA00600H
27. Lou X, Fang C, Geng Z, et al. Significantly enhanced base activation of peroxymonosulfate by polyphosphates: Kinetics and mechanism. Chemosphere. 2017;173:529–534. 10.1016/j.chemosphere.2017.01.093
28. Lou X, Wu L, Guo Y, et al. Peroxymonosulfate activation by phosphate anion for organics degradation in water. Chemosphere. 2014;117:582–585. 10.1016/j.chemosphere.2014.09.046
29. Wang C, Wang Y, Yu Y, et al. Effect of phosphates on oxidative species generation and sulfamethoxazole degradation in a pig manure derived biochar activated peroxymonosulfate system. Sep. Purif. Technol. 2022;295:121255.
https://doi.org/10.1016/j.seppur.2022.121255
30. Kong X, Jiang J, Ma J, Yang Y, Liu W, Liu Y. Degradation of atrazine by UV/chlorine: Efficiency, influencing factors, and products. Water Res. 2016;90:15–23. 10.1016/j.watres.2015.11.068
31. Miserli K, Kogola D, Paraschoudi I, Konstantinou I. Activation of persulfate by biochar for the degradation of phenolic compounds in aqueous systems. Chem. Eng. J. Adv. 2022;9:100201.
https://doi.org/10.1016/j.ceja.2021.100201
32. Yang B, Pignatello J, Qu D, Xing B. Activation of hydrogen peroxide and solid peroxide reagents by phosphate ion in alkaline solution. Environ. Eng. Sci. 2016;33:193–199.
https://doi.org/10.1089/ees.2015.0460
33. Huang G, Liu C, Zhang Y, Chen Z. Groundwater is important for the geochemical cycling of phosphorus in rapidly urbanized areas: a case study in the Pearl River Delta. Environ. Pollut. 2020;260:114079. 10.1016/j.envpol.2020.114079
34. Isiuku B, Enyoh C. Pollution and health risks assessment of nitrate and phosphate concentrations in water bodies in South Eastern, Nigeria. Environ. Adv. 2020;2:100018.
https://doi.org/10.1016/j.envadv.2020.100018
35. Rao N, Prasad P. Phosphate pollution in the groundwater of lower Vamsadhara river basin, India. Environ. Geol. 1997;31:117–122. 10.1007/s002540050170
36. Chowdhury A, Gerhard J, Reynolds D, O’Carroll D. Low permeability zone remediation via oxidant delivered by electrokinetics and activated by electrical resistance heating: Proof of concept. Environ. Sci. Technol. 2017;51:13295–13303. 10.1021/acs.est.7b02231
37. Tian X, Gao P, Nie Y, et al. A novel singlet oxygen involved peroxymonosulfate activation mechanism for degradation of ofloxacin and phenol in water. Chem. Commun. 2017;53:6589–6592. 10.1039/C7CC02820B
38. Tian N, Tian X, Nie Y, Yang C, Zhou Z, Li Y. Enhanced 2, 4-dichlorophenol degradation at pH 3–11 by peroxymonosulfate via controlling the reactive oxygen species over Ce substituted 3D Mn2O3
. Chem. Eng. J. 2019;355:448–456.
https://doi.org/10.1016/j.cej.2018.08.183
Fig. 1Effect of phosphate existing species on OFX removal by PMS activation: (a) 0.1mol/L PO43−, (b) 0.1mol/L H2PO4−, (c) 0.1mol/L HPO42−, (d) 0.3mol/L HPO42− and the corresponding pseudo- first-order kinetics. Reaction conditions: [OFX]0=10mg/L, [PMS]0=0.8 g/L, pH=9.0. 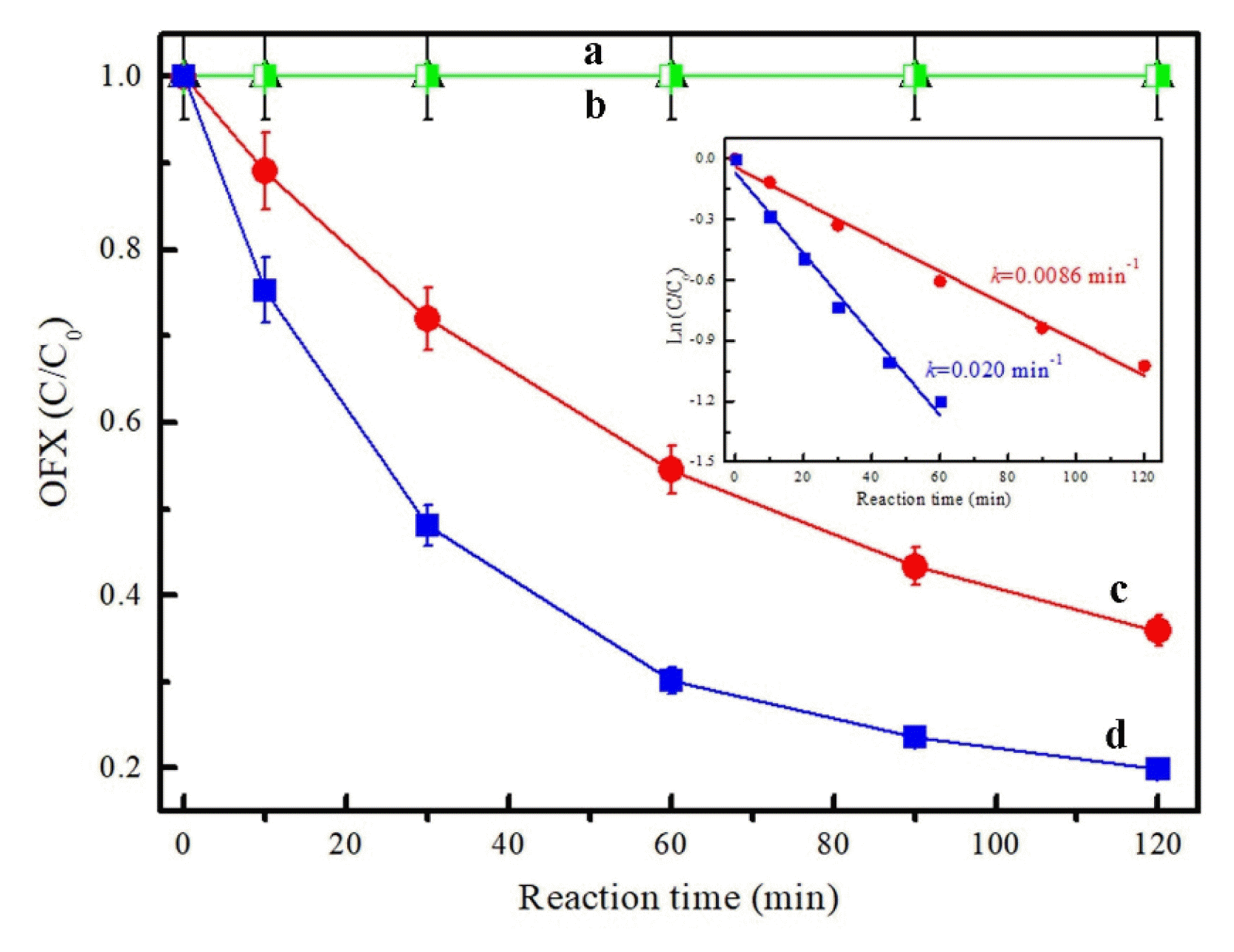
Fig. 2Comparison of the OFX degradation efficiency in different reaction systems: (a) PMS at pH 9, (b) HPO42− + PMS at pH 9 and the corresponding pseudo-first-order kinetics. Reaction conditions: [OFX]0=10 mg/L, [PMS]0=0.8 g/L, [HPO42−]0=0.3 mol/L. 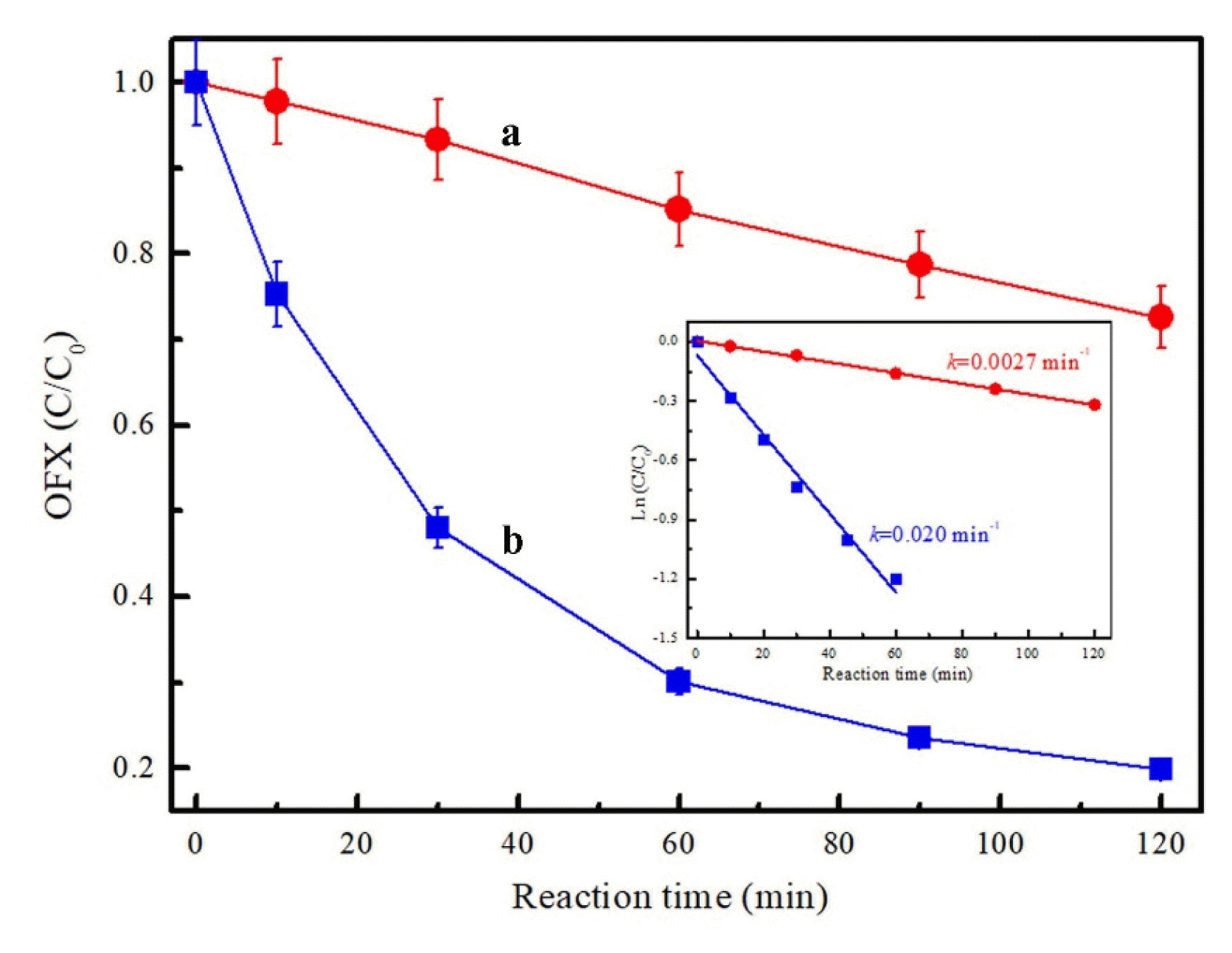
Fig. 3Effect of initial solution pH (a) and phosphate concentration (b) on the OFX degradation efficiency in HPO42− + PMS system (inset: the degradation efficiency of OFX in the presence of 0.1 mmol/L HPO42− during PMS oxidation process). Reaction conditions: [OFX]0=10 mg/L, [PMS]0=0.8 g/L, [HPO42−]0=0.3 mol/L (a), pH=9.0 (b). 
|
|
||||||||||||||||||||||||||||||||||