AbstractThe formation of valuable ferrite in one step by adding ferrous sulfate to chromium-containing wastewater without adjusting to acidic conditions was studied under simple mechanical equipment. The process can not only solve the problem of heavy metals removal from wastewater, but also achieve the purpose of chromium resource recovery. Increasing temperature and pH can further promote the formation of chromium ferrite while prolonging the reaction time has no significant effect on the product’s performance. The response surface method (RSM) was utilized to optimize the process of fC2+/Cr6+ mole ratio of 7, pH value of 10.5, and temperature of 100°C, on which formed ferrite has a high saturation magnetization of 41.28 emu/g with Cr(VI) removal ratio around 100%. TCLP (Toxicity Characteristic Leaching Procedure) tests showed that the chromium concentrations leached from the solid were below the limit of 5 mg/L by USEPA. The molecular formula of chromium ferrite can be expressed as Fe3-xCrxO4 (where x is about 0.45), which has the potential to be recycled as a favorable material. The ferrite process has a low activation energy of 20.8 kJ/mol, which is an economical and environmentally friendly method for synthesizing ferrite from wastewater.
Graphical Abstract
1. IntroductionWith the rapid development of the economy, population growth and industrialization have caused a continuous deterioration in the quality of available water resources. The phenomenon is bothering many countries around the world, which causes people to be unable to access clean water [1]. Chromium (Cr) is a common contaminant in surface water and groundwater, which can be achieved by metal plating, metallurgy, dyeing, leather plating, weaving, and other artificial wastewater into the environment. The permissible concentration of total chromium in drinking water is 0.1 mg/L, issued by The United States Environmental Protection Agency (USEPA). Moreover, the limits have been set to 0.05 mg/L by European Union (EU) [2].
The valence state of chromium ranges from −2 to +6, while only the +3 and +6 stages are stable in the environment [3]. Hexavalent chromium is 100 times more toxic than trivalent chromium due to its high solubility, mobility, and oxidizability [4]. Trivalent and hexavalent chromium are listed individually as priority water pollutants by USEPA [5]. Cr(VI) has been characterized as a mutagenic and carcinogenic compound [2]. Studies of hexavalent chromium and its carcinogenicity focus on the rate of chromate passage through cell membranes and nuclear membranes. Trivalent chromium is inert due to its D3 electron configuration, and the ligand exchange process is prolonged [6]. After entering the cytoplasm, chromate can be reduced to trivalent chromium by the nuclear membrane or cytoplasm, then generated free radicals can bind to DNA. When touching skin, hexavalent chromium compounds may cause skin irritation, dermatitis, skin necrosis, and skin corrosion [7]. In addition, it may damage the human circulatory system and even lead to death in critical situations [8]. Therefore, it is necessary to analyze different valence states rather than total chromium levels to accurately evaluate chromium’s transformation, distribution, and transportation in the environment.
The removal of Cr(VI) has always been a hotspot in the purification of wastewater, including adsorption [9,10], reduction precipitation [11,12], ion exchange [13,14], and reverse osmosis membrane [15,16]. The adsorption method is regarded as one of the most studied methods because of its simple operation. Recently, efficient adsorbents, such as nanoparticle zero-valent iron (nFe0) or silty clay-supported for nFe0 (SC-nFe0) [17,18], SBA-15 [19,20], MCM-41 [21,22], and MCM-48 [23,24] have been intensively investigated. When applied in high-concentration chromium-containing wastewater, considerable amounts of adsorbents will be consumed due to the limited specific surface area, and the most difficult challenge is to effectively separate the particles from the aqueous solution. Moreover, solid waste is usually chosen to be landfilled or solidified and still poses a potential threat to the environment in the long term [25]. The ability of adsorbents to operate on a large scale is determined by the cost of their disposal or regeneration [26]. The reduction precipitation method is traditionally used in practical industrial wastewater with simple operation and low cost [27]. Unfortunately, excessive sludge production causes the increasing cost of sludge disposal [28]. At the same time, the toxicity test of the leaching process confirmed that hydroxide sludge might have negative influences on the environment [29]. Ion exchange and reverse osmosis membranes can almost completely remove metal ions from wastewater, but expensive treatment costs limit their large-scale application. However, the ferrite process can remove heavy metal ions from wastewater by immobilizing metal ions in the spinel structure. Heavy metals are less mobile and more stable than hydroxides in the environment [30]. Moreover, ferrite has received extensive attention due to its recognized magnetic [31,32], catalytic [33–35], and adsorbent properties [36]. There have already been many studies on the preparation of ferrites, including the sol-gel method [37–39], the precipitate method [25,30,40–42], the hydrothermal method [43–45], the solid-state reaction method [46] and ceramic method [47,48]. However, these methods suffer from the same shortcomings. On the one hand, they have strict requirements on the acidity of wastewater, and Cr(VI) must be first reduced to Cr(III) by a reducing agent. On the other hand, the temperature required for the preparation of ferrite is up to 200~1000°C, which leads to a considerable loss of energy.
Considering the above factors, we proposed a completely new technological process which is suitable for treating chromium-containing wastewater produced in industrial manufacturing. Higher-performance products can be attained near the theoretical value of Fe/Cr without considering the acidity of the wastewater. Meanwhile, the study will minimize energy consumption by controlling the temperature. Ferrite sludges are formed in a dense structure, easily separated from water by magnetism. Compared with other experiments [6,25], our study assesses the effects of synthetic parameters (molar ratio of Fe2+/Cr6+, pH, reaction temperature, and reaction time) for the whole reaction system. The optimum conditions can be investigated by using response surface methodology (RSM). In addition, ferrites formed in the process were chemically characterized, and their dissolution properties in the leaching solution were determined. It will be significant to apply low-cost methods to the treatment of high-concentration chromium-containing wastewater. Meanwhile, synthetic ferrite was widely investigated and used in various commercial fields, demonstrating excellent environmental friendliness.
2. Materials and Methods2.1. MaterialsChemical reagents, including potassium dichromate (K2Cr2O7, ≥ 99 wt. %), ferrous sulfate heptahydrate (FeSO4·7H2O, ≥ 99 wt. %), sulfuric acid (H2SO4, ≥ 98 wt. %), phosphoric acid (H3PO4, ≥ 85 wt. %), 1,5 diphenylcarbazide (C13H14N4O, > 98.0 wt. %), acetone (C3H6O, ≥ 99.5 wt. %), and sodium hydroxide (NaOH, ≥ 97 wt. %) were purchased from Sinopharm Group Chemical Reagent Co Ltd., China.
2.2. Mechanism InvestigationWith regard to Cr2O72−, it had to be reduced to obtain Cr3+ cation before ferrite transformation, as shown in Eq. (1). It has been reported that the amorphous colloid formed at the reduction stage is Fe3+ and Cr3+ co-precipitation, whose molecular formula can be expressed as CrxFe1−x(OH)3(Eq. (2–5)) [29]. Cr3+ can theoretically substitute all the Fe3+ into the crystal lattice of ferrite. When Cr(VI) is reduced, the Fe-Cr co-precipitation production will react immediately with the remaining Fe2+ to form the chromium ferrite. The reaction equation can be expressed as follows:
It can be seen from the above equations that the Fe2+ dosage is composed of two parts: The Fe2+ for reducing Cr6+ and the Fe2+ for generating ferrite. The reduction of 1 mol Cr2O72− requires 6 mol Fe2+, and the formation of 2 mol Cr3+ and 6 mol Fe3+, ferrites requires 1 mol Fe2+ and 3 mol Fe2+, respectively. Therefore, n (Cr2O72−):n (Fe2+) = 1:(6 + 1 + 3) = 1:10. Therefore, the theoretical dosage of Fe2+ is 5 times that of Cr6+(Eq. (6–7)).
A cube unit cell of spinel ferrite AB2O4 contains 8 formula units with 56 ions, including 32 oxygen anions, 8 cations A (Mg2+, Fe2+, Zn2+, Co2+, etc.), and 16 cations B (Al3+, Fe3+, Cr3+, etc.). Large-sized oxygen ions form a closely packed face-centered structure, and the smaller divalent metal cations occupy the interstitial positions with space group Fd-3m [49]. The crystal structure type, chemical bond type, and ion charge balance remain unchanged when other atoms or molecules occupy some atoms or molecules in the crystal structure with the same properties [50]. Yang et al. (2007) reported that the incorporation of metal ions into the lattice is mainly related to their ionic radii. It might enter the spinel lattice easier for heavy metal ion, which has high hydroxides solubility product constant [51]. Fig. 1(A) was drawn by the Vesta software to present the three-dimensional structure. The spatial group, Fd-3m of FeCr2O4 crystal structure (9007325. cif), was obtained by the Crystallography Open Database (COD) with lattice parameters a = b = c = 8.3765 Å and α = β = γ = 90°. It can be seen that Cr(III) mainly occupies the octahedral sites, which is correlated to their strong preference for large octahedral site energy [52], while Fe(II) predominates in tetrahedral sites. By improving the reaction conditions, unit cells continue to shrink due to Cr(III) with a radius of 0.63 Å, gradually replacing Fe(III) with a radius of 0.67 Å [52]. Sharma et al. (2002) reported that the bond lengths of both tetrahedrons and octahedrons decreased to accommodate various chemical compositions and cation distribution with increased Cr3+ ions [53,54]. Although the Cr-O bond distances are constant in the octahedra, the octahedra are inclined but do not affect the structural stability of the cubic spinel due to the internal angles are not consistent. Meanwhile, both bond distances and internal angles always keep constant in Fe-O tetrahedra (Fig. 1(B)) [55].
2.3. Experiment ProcedureIn this study, the simulated electroplating wastewater containing Cr(VI) in the concentration of 1000 mg/L was prepared by dissolving K2Cr2O7 (Merck-1.04862) in deionized water. It has been calculated that the theoretical dosage of Fe2+ is 5 times that of Cr6+ in order to get the ferrite. According to the designed mole ratio, FeSO4·7H2O was added to the chromium-containing wastewater. When mixed thoroughly, it was adjusted between 8 and 12 by appending 5 M NaOH [6]. The mixture was stirred at a specific temperature. When the desired reaction time was reached, solid-liquid separation was carried out at 3000 r/min until the precipitation and supernatant were obtained [30]. The residual heavy metals in the filtrate were analyzed by ICP-AES, and the precipitate was dried at 80°C for 24 h. Finally, the solid product was pulverized to a fine powder for further characterization.
2.4. RSM Experimental DesignRSM is a collection of mathematical and statistical methods in which the designed experimental results are fitted by mathematical models (linear functions, square polynomial functions, etc.), and the models are verified by statistical methods [56]. It is beneficial for designing experiments by considering the interaction of parameters to develop models and optimize the process [57].
Generally, the saturation magnetization of the synthetical products mainly depends on several operating parameters. The RSM was availed to optimize the process to obtain better performance of products by analyzing the impact of preparation parameters on the magnetization of ferrite. All the experimental data were compiled using the Box-Behnken design (Design-Expert Software, Version 10.0). Box-Behnken design is a factorial combination of at least three parameters in an incomplete block design [58]. In this study, the influence of reaction time on magnetism is insignificant. The molar ratio of Fe2+/Cr6+ (X1), pH (X2), and reaction temperature (X3) are considered to be essential factors. The experimental design involves three parameters (X1, X2, X3) at three levels. Codes −1, 0, and 1 represent the low, medium, and high levels, respectively (Table 1). Therefore, the total number of experiments to be calculated as follows [59]:
where k is the number of factors and C0 is the number of center points.
The polynomial regression model is devoted to analyzing the causality between dependent variables and independent variables. The generalized second-order polynomial model used in the response surface (Y) analysis is:
where Y is the response variable, β0 represents a set of regression coefficients (as constants), βi is the linear effect, βii is the quadratic effect, βij is the bidirectional linear interaction with the linear effect, xi, xj is the coding value of the independent variable, and k is the number of exogenous variables (independent variables). The statistical significance of the model was confirmed by analysis of variance (ANOVA) for the polynomial model at a 95% confidence level, and residual plots examined the goodness of the model fit. Finally, the optimal value of each factor in the process was obtained by the RSM program [57].
2.5. TCLP TestIn this study, the USEPA TCLP was followed to determine the chemical stability of the prepared ferrite [60]. A leaching agent was utilized to adjust the pH of the product to carry out the vibrated extraction experiment. In this experiment, acetic acid buffer solution was devoted as a leaching agent to simulate the leaching process of industrial waste under the influence of leachate from landfills. According to the standard procedure, distilled water with adjusted pH (4.93 ± 0.05) was chosen as the extraction fluid. The volume ratio of solid to liquid was 1:20. The mixed samples were vibrated at the rate of (30 ±2) rpm for 18 h and then were filtered to collect the filtrate [61]. Finally, the leached metal concentrations were analyzed with ICP-AES in the filtrate.
2.6. Materials CharacterizationThe inductively coupled plasma atomic emission spectrometer (ICP-AES, Prodigy, Leeman Co.) was used to analyze the concentration of heavy metals in the solution. The Cr(VI) concentration was determined through the 1,5 diphenylcarbazide spectrophotometric method by using UV–Vis spectrum (U-3010, Hitachi Ltd., Japan) at 540 nm [62]. X-ray diffraction (XRD, MAX-2200X, Japan) patterns were collected on a D/max RBX diffractometer with Cu Kα radiation at 40 kV and 100 mA at a scanning rate of 8°/min from 5° to 80°. A vibrating sample magnetometer (VSM, 7407 type, Lake Shore Company, America) was applied to measure the magnetic properties of synthesized materials at room temperature. The sample was directly bonded to the conductive adhesive, and then Pt was sprayed by the Oxford Quorum SC7620 sputtering coating instrument. The morphology and the corresponding elemental mappings were examined using a scanning electron microscope (ZEISS Gemini SEM 300) along with an energy-dispersive X-ray spectroscopy analyzer (EDS). The acceleration voltage of the morphology shooting was 3 kV. The acceleration voltage for spectral mapping was 15 kV. X-ray photoelectron spectrum (XPS) was recorded on Thermo Scientific K-Alpha (USA) using the Al Kα source and the C1s spectral line at 284.4 eV as reference.
3. Results and Discussion3.1. The Effect of Different Variables on the Ferritic Transformation of Chromium3.1.1. Effect of Fe2+/Cr6+ mole ratioA dose of ferrous sulfate near the theoretical value was added into Cr(VI) solution for reduction and magnetic conversion reaction to figure out the optimum conditions for the removal and recovery of Cr(VI). When the mole ratio of Fe2+/Cr6+ was 3, the chromium was almost completely removed, and the chromium-containing wastewater relevantly changed from orange-yellow to transparent. As shown in Fig. 2(A), the corresponding precipitate will generate compounds containing amorphous or weak crystals. The phenomenon indicated that only Cr(VI) reduction was achieved under the circumstances. Therefore, the ability and efficiency of doping ions to enter the lattice could not be obtained only by the chromium removal efficiency, which was attributed to the fact metal ions might be trapped in the co-precipitates or compounds (Eq. (2–4) [51]. The obvious diffraction peaks of FeOOH reveal increasingly when Fe2+/Cr6+ mole ratio is 5 (Eq. (5)). With the progress of the Fe2+/Cr6+ mole ratio, total chromium was not detected in the liquid supernatant. Meanwhile, the intensity of the X-ray diffraction peak of chromite was enhanced. According to the hysteresis loop (Fig. 3(A)), it displayed that the product with a Fe2+/Cr6+ mole ratio of 7 had stronger magnetism than a Fe2+/Cr6+ mole ratio of 5. This may be ascribed to the increasing concentration of heavy metal ions, which authorizes more cations to access the lattice. Therefore, the peak intensity of XRD patterns is the strongest when the Fe2+/Cr6+ mole ratio is 7.
3.1.2. Effect of temperatureTemperature always plays a critical role in the process of magnetic conversion. The solution generates a non-magnetic or weak magnetic gelatinous precipitate at 60°C and below, which can be made up of a small portion of ferrite mixed with metal hydroxides that mainly cover FeOOH (Fig. 2(B)). As the temperature rose, the lattice vibration of FeOOH tended to become more frequent, which caused H+ or OH− ions to move in the lattice. Water molecules could be considered to escape from their original positions in the lattice to the surface of the particles, forming a water membrane that evaporated quickly to accelerate the dehydration process [63,64] (Eq. (10)). Therefore, vacancies were generated and merged into a huge hollow in the crystal [65,66]. The behavior is a flaw in improving the performance of the final product. However, as the temperature continued to rise, the number of hollows was gradually reduced or eliminated due to the migration of ions to the hollows [6]. The lattice started to transform when the ion diffusion concentration exceeded the solubility. FeOOH peak gradually disappeared, and the crystal lattice of the material microstructure changed. When the temperature reached 100°C, there was no obvious impurity peak in XRD patterns. It was discovered that the XRD diffraction peak position of the sample was consistent with the standard spectrum of chromite.
In summary, the rise in temperature increased the nucleation probability and the diffusion rate of ions by promoting the collision probability between ions. On the other hand, it made products gradually modify the defects of the lattice to improve the compactness of the product [67].
3.1.3. Effect of pHAccording to the experimental evidence, Cr(III), Fe(II), and Fe(III) in wastewater were required to combine with OH− in the solution to shape into an intermediate precipitate. This intermediate compound required additional oxidation of ferrous entities under alkaline conditions for its conversion into magnetite [64]. Subsequently, FeOOH dehydrates to accelerate ferrite growth as time goes on. As demonstrated in Fig. 2(C), there is no XRD diffraction peak of FeCr2O4, and only non-magnetism or poorly magnetism compounds were created in such a case. Walter et al. reported a close relation between precipitation pH and the kinetics of conversion of intermediate green rust-II into FeOOH and Fe2O3 [67].
The peak of FeOOH almost disappeared, and the peak of FeCr2O4 emerged with a pH value adjusted to 11. The phenomenon can be illustrated that alkaline surroundings could facilitate the composition of a chromite skeleton [68]. As pH continued to rise, the diffraction peak gradually weakened, indicating that an excessively alkaline imposes restrictions on ferrite formation. We deduce that more OH− anions produce a hydroxyl complex with good water solubility in the solution [68]. In this case, the primary precipitates covering Cr(OH)3 and Fe(OH)3 will dissolve again [25]. Correspondingly, the weight of the precipitate declined (Fig. 3(B)). In this work, the optimized condition for ferrite was obtained when the pH ranged from 10 to 11, as shown in the XRD pattern. In summary, it was confirmed that the ferrite was shaped in the pH range of 9–11 [6,25,69,70]. Mandaokar et al. (1994) explored that the optimal pH for ferrite formation also depended on the concentration of heavy metal ions. The higher the concentration of heavy metal ions in the solution, the higher the pH required for ferrite formation [71].
Moreover, the total iron concentration in the supernatant dwindled from 224.1 to 0.23mg/L with the progress of pH value. Meanwhile, the removal ratio of total iron approximately increased to 100% (Fig. 3(C)). The results demonstrated that the increase in pH is favorable for removing total iron from wastewater. This may be attributed to intermediate products generated, which Fe(II) was required to combine with OH− in the solution.
3.1.4. Effect of reaction timeReaction time is an essential factor affecting the apparent size of chromite which has a tremendous promoting effect on the growth of primary chromite particles. As time went on, the XRD pattern did not change significantly. The diffraction peak of FeCr2O4 gradually appeared until the reaction time reached 1 h, and the product was mainly composed of FeOOH and FeCr2O4 (Fig. 2(D)). Correspondingly, although the magnetic properties of the product showed an upward trend as a whole, the rising speed was slow. When the reaction time reached 1 h, the magnetization only reached 7.32 emu/g (Fig. 3(D)). From the perspective of grain growth, the sample grain size was calculated using the Scherrer formula (Eq. (11)), taking into account the most intense peak (311):
where D is the grain size, k is a constant equal to 0.94, λ is the X-ray wavelength equal to 0.1542 nm, and β is the half-peak width. As reaction time extends from 50 to 60 min, the size grows from 22.6 to 23.7 nm, increasing by 5%. When the particle size is smaller and the specific surface area is larger, which leads to the spin angle of the grain surface increases, and the saturation magnetization decreases [72]. It is speculated that a short-time effect on the sediment’s magnetism was not significant because of the slow particle collision rate [48,72]. Previous research had exhibited that it required at least 24 h at a lower temperature to achieve its establishment [68]. The initial concentration of the metal ion also determines the ferrite to take shape, while a higher concentration of metal ions requires more contact time [71]. Considering its practical operation and low cost, one hour was selected for the following experiments.
3.2. Kinetic Equation and Activation EnergyThe behavior of particles growth was investigated by fitting the kinetic equation, and the activation energy of grain growth can be calculated by the Arrhenius equation (Eq. (12)) [73]:
where k is the specific reaction rate constant, Q is the activation energy, T is the absolute temperature, and R is the ideal gas constant. The value of k is related to grain size directly. Integral can be turned into:
where D is the grain size and A is the intercept.
A plot of log D versus the reciprocal of absolute temperature (1/T) is shown in Fig. 3(E). The plot gives a good correlation with a correlation coefficient of R2 0.924. The slope of the resulting Arrhenius plot is -Q/2.303R, and the activation energy of growth can be obtained from the value of Q to be about 20.8 kJ/mol. However, the activation energy of ferrite synthesized by a high energy-consuming sinter process is 71.14 kJ/mol [74], while that of ferrite synthesized by the traditional hydrothermal method is 56.2 kJ/mol [75]. It is evident that the hydrothermal method and high energy-consuming sinter process need much higher energy. Thus, it is the main reason for obtaining spinel ferrite at a lower cost in the study.
3.3. Response Surface Methodology3.3.1. Development of regression model equationResponse surface methodology was employed to quantitatively evaluate the impact of three factors (mole ratio of Fe2+/Cr6+, pH, temperature) on the response value (magnetization). Multiple regression analysis was applied to investigate the results, as shown in Table 2. Design-expert software was utilized to determine and evaluate the polynomial equation in the coded form, as shown in Eq. (14).
where E is the response expressed as the predicted magnetization, and X1, X2, and X3 are the coded terms for three independent variables. It indicates that a wide range of magnetization from 17.20 to 37.94 emu/g was strongly related to the selected variables in this experiment.
3.3.2. Analysis of variance (ANOVA) analysisThe statistical significance of the model was appraised by the analysis of variance, including the sum of squares, DF, mean square, F-values, and p-values. As expressed in Table S1, the probability value (prob>F) was lower than 0.05, which implies that the model is significant. The sum of the square was 563.35 for the mole ratio of Fe2+/Cr6+, 6.08 for pH, and 72.26 for temperature, which advocated a powerful effect on the Fe2+/Cr6+ mole ratio of ferrite magnetism, followed by temperature and pH. Furthermore, the “Lack of fit F-value” of 2.20 > 0.05 ((prob > F) = 0.2311) exhibited that the lack of fit was non-significant, which adequately represented that the difference between the model and the experimental fitting is favorable to the model [76].
3.3.3. Three-dimensional (3D) response surfacesThe influence of the interaction among mole ratio of Fe2+/Cr6+ (X1), pH (X2), and reaction temperature (X3) on the magnetization of ferrites (R) could be directly expressed by using the 3D stereograph of the response surface (Fig. 4). As demonstrated in Fig. 4(A–C), when the mole ratio of Fe2+/Cr6+ remained constant, the magnetism of ferrite elevated initially and then diminished with the increase of pH. The range of optimal conditions for pH was between 10 and 11. In contrast, the higher temperature can dramatically enhance the magnetization of the generated ferrite due to the formation of superior properties of spinel ferrite. The response value R, along with Fe2+/Cr6+ mole ratio, ascends when the temperature remains unchanged. On the whole, the increased Fe2+/Cr6+ mole ratio promoted more metal ions to enter the spinel structure. Increasing temperature and pH can further promote the formation of chromium ferrite. However, the condition of high alkalinity makes it against forming chromite. These conclusions were consistent with previous experiments [6,68].
The adequacy of the model was assessed by the residuals (the difference between actual and predicted values), and the normal probability plot was exploited to judge the normality of the residuals. To make the model reliable, it required to ensure that the linear distribution of data was obvious and there was not an abnormal data point [57]. As described in Fig. 4(D), the data of experimental points distributed nearby the simulated line phenomenon indicated that the prediction results of the saturation magnetization model were highly consistent with the experimental data. Therefore, this model was able to analyze and predict the relationship between the saturation magnetization and various experimental parameters, as well as the optimal experimental conditions.
3.4. Characterization and Magnetic Properties of the Synthetic ProductsTo further explain the apparent characteristics of the synthesized samples, we performed a morphological analysis. As demonstrated in Fig. 5(A), the multiple particles with the rough surface had a tight structure, and the chromium-ferrite was spherical particles. Due to the strong magnetic properties of the product, the particles gather together. At the same time, rod-like morphology was found, which might be attributed to the doped goethite (FeOOH) nanorods. Goethite was the primary intermediate controlling ferrite transformation and particle growth. The presences of FeOOH were consistent with the previous analysis (3.1) [6]. The results of EDX analysis (Fig. 5(B)) indicated that Cr, Fe, and O elements were uniformly distributed in the powder sample during the magnetic conversion process (Fig. 5(C–E)). The molecular formula of the synthesized product was Fe3−xCrxO4, where x was about 0.45. The consequence confirmed that the ferrite process could transform Cr(VI) into a stable and reusable solid product to solve the problem of Cr(VI) removal from wastewater.
The XRD patterns of products under optimal conditions (Fig. 6(A)) showed obvious diffraction peaks of chromium ferrite (Fe3−xCrxO4), which were identical to the XRD patterns of products in the references [6,25]. The diffraction intensity of the crystallographic plane (311) of chromium ferrite reached 1300 counts, which was higher than that of 1013 counts with the highest diffraction intensity in the previous experiment (Fe2+/Cr6+ mole ratio of 7). We inferred that more chromium ferrite is formed under the optimum condition. As demonstrated in Fig. 6(B), The XPS spectra of Cr 2p in chromium ferrite exhibited two peaks at the binding energies of 587.02 eV (Cr 2p1/2) and 577.25 eV (Cr 2p3/2), respectively. The binding energies are lower than those of Cr 2p1/2 (589.00 eV) and Cr 2p3/2 (579.70 eV) in K2Cr2O4 [77], indicating the Cr(VI) state in the wastewater is reduced and converted into Cr(III) state into the sediment [6]. In general, we concluded that Cr(VI) could be transformed into magnetic chromium ferrite under optimal conditions. In the meanwhile, chromium was not detected in the supernatant, which conformed to the environmental quality standards for surface water (Cr(VI) ≤ 0.05 mg/L) (Table S2).
In industrial production, solid-liquid separation is usually arduous and expensive. Compared with traditional solid-liquid separation methods such as precipitation filtration and centrifugation. Magnetic separation is a practical and direct method. The method separates the product from the wastewater under the action of an external magnetic field. The hysteresis loop of the synthesized product is revealed in Fig. 6(C). When Fe2+/Cr6+ mole ratio is 7, pH of 10, and temperature of 80°C, the saturation magnetization value was 37.94 emu/g, and both remanence and coercivity were almost zero, which are typical characteristics of spinel ferrite materials [78]. It can be seen that the curve exhibited a normal narrow hysteresis loop. S-shaped curves indicated chromium ferrite nanoparticles were soft magnetic materials with easy magnetization and demagnetization [79]. Yang [6] et al. removed and recovered Cr(VI) from alkaline wastewater to produce chromite ferrite with saturation magnetization of 15~25 emu/g. It is obvious that this study has obtained a product with better performance through a straightforward process. The superparamagnetic properties help to separate a solid from a liquid via external magnets while improving product reusability.
3.5. Analysis of the Product StabilityTo investigate the stability under the optimized conditions, we further carried out a toxic leaching experiment. The concentration of chromium in the leaching solution was lower than 5.0 mg/L, which reached the toxic leaching limit set by The United States Environmental Protection Agency (USEPA) and GB5085.3-1996 (Chinese standard) [30]. In Table S2, the leaching concentration of chromium was 2.78 mg/L. The consequence illustrated that the heavy metals were not easily dissolved, which could be fastened in the stable and compact ferrite lattice.
4. ConclusionThis study indicates that the one step ferrite process is a feasible method to remove chromium by conversion of chromium into ferrite spinel compounds. With the increasing Fe2+/Cr6+ mole ratio, Cr(III) with a radius of 0.63 Å, gradually replacing Fe(III) with a radius of 0.67 Å. The chromium removal rate in the supernatant can be up to 100%, and the concentration in the leaching solution is less than 5.0 mg/L, which accords with the wastewater discharge standard. Meanwhile, the process can create valuable crystalline ferrite with high stability. From the consequence of the RSM, 41.28 emu/g of the saturation magnetization of the ferrite is acquired at Fe2+/Cr6+ mole ratio of 7, pH of 10.5, and temperature of 100°C. The molecular formula of the product is Fe3−xCrxO4, where x is about 0.45. Compared with the conventional ferrite process, the new ferrite method exhibits a more straightforward reaction process and higher performance of ferrite products. Not only do we get the ferrite through a one-step process with the addition of ferrous sulfate solution, but also there is no need to create a strong acid environment for the initial solution. The activation energy of the process is 20.8 kJ/mol, indicating that the operation can be carried out easily and economically. In summary, this low-cost process can obtain superior magnetic spinel minerals by exceedingly simplifying the flow of the process. Furthermore, treatment costs can be alleviated by adopting industrial wastes, such as picking liquor from metal finishing plants as a supplementary source of ferrous ions.
AcknowledgmentsThis work was supported by the National Nature Science Foundation of China Nos. (51274138,50974086, and 50704023) and the Program for stabilization and harmless treatment technology and equipment for solid waste incineration residues (2019YFC1906900). We thank Shanghai University for the support of this work. The sponsor was not involved in the study design; the collection, analysis, or interpretation of the data; the preparation of the manuscript, or the decision where to submit the manuscript for publication.
NotesConflict-of-Interest Statement The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Author Contributions D.C. (Professor) performed all experiments and analyses along with data collection and discussion of the results and wrote the manuscript. W.S. (M.S. student) worked on chemical analysis and data plotting. D.W. (Associate Researcher) revised and edited the manuscript. Y.M. (M.S. student) worked on chemical analysis and data plotting. H.Y. (M.S. student) worked on chemical analysis and data plotting. Y.L. (M.S. student) worked on chemical analysis and data plotting. G.Q. (Professor) revised and edited the manuscript. References1. Hsini A, Benafqir M, Naciri Y, et al. Synthesis of an arginine-functionalized polyaniline@FeOOH composite with high removal performance of hexavalent chromium ions from water: Adsorption behavior, regeneration and process capability studies. Colloids Surf. A Physicochem. Eng. Asp. 2021;617:126274. https://doi.org/10.1016/j.colsurfa.2021.126274
2. Vaiopoulou E, Gikas P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere. 2020;254:126876. https://doi.org/10.1016/j.chemosphere.2020.126876
3. Kotaś J, Stasicka ZJ. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000;107:263–283. https://doi.org/10.1016/S0269-7491(99)00168-2
4. Xie YQ, Lin J, Liang J, et al. Hypercrosslinked mesoporous poly (ionic liquid)s with high density of ion pairs: Efficient adsorbents for Cr(VI) removal via ion-exchange. Chem. Eng. J. 2019;378:https://ARTN12210710.1016/j.cej.2019.122107
5. EPA, Office of Water, Office of ScienceTechnology. National recommended water quality criteria. 2009. Available from: https://www.epa.gov/ground-water-and-drinking-water
6. Yang J, Wang R, Cheng Z, Chen Y, Li L, Wang X. Removal and recycling of hexavalent chromium from alkaline wastewater via a new ferrite process to produce the valuable chromium ferrite. J. Colloid Interface Sci. 2022;608:3059–3068. https://doi.org/10.1016/j.jcis.2021.11.035
7. Dinari M, Haghighi A. Ultrasound-assisted synthesis of nanocomposites based on aromatic polyamide and modified ZnO nanoparticle for removal of toxic Cr(VI) from water. Ultrason. Sonochem. 2018;41:75–84. https://doi.org/10.1016/j.ultsonch.2017.09.023
8. Wang YT, Chirwa EM, Shen HJ. Cr(VI) reduction in continuous-flow coculture bioreactor. J. Environ. Eng. 2000;126:300–306. https://doi.org/10.1061/(ASCE)0733-9372(2000)126:4(300)
9. Lakhanpal S, Dhulia A, Ganguly R. Magnetite coated sand adsorbent for Cr(VI) removal from synthetic and pharmaceutical wastewater: adsorption isotherms and kinetics. Arab. J. Geosci. 2021;14(12)1–15. https://doi.org/10.1007/s12517-021-07559-5
10. Xiao F, Cheng J, Cao W, Yang C, Chen J, Luo Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019;540:579–584. https://doi.org/10.1016/j.jcis.2019.01.068
11. Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012;223:1–12. https://doi.org/10.1016/j.jhazmat.2012.04.054
12. Xie B, Shan C, Xu Z, et al. One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: Reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J. 2017;308:791–797. https://doi.org/10.1016/j.cej.2016.09.123
13. Huang Y, Li C, Lin Z. EDTA-Induced Self-Assembly of 3D Graphene and Its Superior Adsorption Ability for Paraquat Using a Teabag. ACS Appl. Mater. Interfaces. 2014;6:19766–19773. https://doi.org/10.1021/am504922v
14. Lanigan KC, Pidsosny K. Reflectance FTIR spectroscopic analysis of metal complexation to EDTA and EDDS. Vib. Spectrosc. 2007;45:2–9. https://doi.org/10.1016/j.vibspec.2007.03.003
15. George JS, Ramos A, Shipley HJ. Tanning facility wastewater treatment: Analysis of physical–chemical and reverse osmosis methods. J. Environ. Chem. Eng. 2015;3:969–976. https://doi.org/10.1016/j.jece.2015.03.011
16. Alardhi SM, Alrubaye JM, Albayati TM. Removal of methyl green dye from simulated waste water using hollow fiber ultra-filtration membrane. IOP Conf. Ser.: Mater. Sci. Eng. 2020;928(5)052020. https://doi.org/10.1088/1757-899X/928/5/052020
17. Kadhum ST, Alkindi GY, Albayati TM. Determination of chemical oxygen demand for phenolic compounds from oil refinery wastewater implementing different methods. Desalin. Water Treat. 2021;231:44–53. https://10.5004/dwt.2021.27443
18. Kadhum ST, Alkindi GY, Albayati TM. Remediation of phenolic wastewater implementing nano zerovalent iron as a granular third electrode in an electrochemical reactor. Int. J. Environ. Sci. Technol. 2022;19(3)1383–1392. https://doi.org/10.1007/s13762-021-03205-5
19. Ali NS, Alismaeel ZT, Majdi HS, et al. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon. 2022;8(6)e09737. https://doi.org/10.1016/j.heliyon.2022.e09737
20. Atiyah NA, Albayati TM, Atiya MA. Interaction behavior of curcumin encapsulated onto functionalized SBA-15 as an efficient carrier and release in drug delivery. J. Mol. Struct. 2022;1260:132879. https://doi.org/10.1016/j.molstruc.2022.132879
21. Khadim AT, Albayati TM, Saady NMC. Desulfurization of actual diesel fuel onto modified mesoporous material Co/MCM-41. Environ. Nanotechnol. Monit. Manag. 2022;17:100635. https://doi.org/10.1016/j.enmm.2021.100635
22. Atiyah NA, Albayati TM, Atiya MA. Functionalization of mesoporous MCM-41 for the delivery of curcumin as an anti-inflammatory therapy. Adv. Powder Technol. 2022;33(2)103417. https://doi.org/10.1016/j.apt.2021.103417
23. Taheri R, Bahramifar N, Zarghami MR, Javadian H, Mehraban Z. Nanospace engineering and functionalization of MCM-48 mesoporous silica with dendrimer amines based on [1,3,5]-triazines for selective and pH-independent sorption of silver ions from aqueous solution and electroplating industry wastewater. Powder Technol. 2017;321:44–54. https://doi.org/10.1016/j.powtec.2017.08.022
24. Han Y, Chen J, Gu X, Chen J. Adsorption of multi-bivalent heavy metal ions in aqueous solution onto aminopropyl-functionalized MCM-48 preparation by co-condensation. Sep. Sci. Technol. 2021;56(11)1819–1829. https://doi.org/10.1080/01496395.2020.1799009
25. Lv JF, Quan YC, Tong X, Peng Y, Zheng YX. The Crystallization Behavior and Stability of Chromite Synthesized in Chromium-containing Wastewater at Room Temperature. Engineering. 2022;9:67–76. https://doi.org/10.1016/j.eng.2020.12.018
26. Singh NB, Nagpal G, Agrawal S. Water purification by using adsorbents: a review. Environ. Technol. Innov. 2018;11:187–240. https://doi.org/10.1016/j.eti.2018.05.006
27. Kurniawan TA, Chan GY, Lo WH, Babel S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006;118:83–98. https://doi.org/10.1016/j.cej.2006.01.015
28. Malaviya P, Singh A. Physicochemical technologies for remediation of chromium-containing waters and wastewaters. Crit. Rev. Environ. Sci. Technol. 2011;41:1111–1172. https://doi.org/10.1080/10643380903392817
29. Erdem M, Tümen F. A study on dissolution properties of the sludges from Cr(VI) reduction–precipitation processes. J. Environ. Sci. Health A. 2004;39:253. https://doi.org/10.1081/ESE-120027382
30. Erdem M, Tumen F. Chromium removal from aqueous solution by the ferrite process. J. Hazard. Mater. 2004;109:71–77. https://doi.org/10.1016/j.jhazmat.2004.02.031
31. Benhalima C, Amari S, Beldi L, Bouhafs B. First-Principles Study of Ferromagnetism in Iron Chromite Spinels: FeCr2O4 and CrFe2O4. Spin. 2019;9(3)1950014. https://doi.org/10.1142/S2010324719500140
32. Younis M, Saleem M, Atiq S, Naseem S. Magnetic phase transition and magneto-dielectric analysis of spinel chromites: MCr2O4 (M = Fe, Co and Ni). Ceram. Int. 2018;44:10229–10235. https://doi.org/10.1016/j.ceramint.2018.03.024
33. Boudjemaa A, Bouarab R, Saadi S, Trari M. Photoelectrochemical H2-generation over Spinel FeCr2O4 in X2− solutions (X2− = S2− and SO32−). Appl. Energy. 2009;86(7–8)1080–1086. https://doi.org/10.1016/j.apenergy.2008.06.007
34. Abbasi A, Keihan AH, Golsefidi MA, Rahimi-Nasrabadi M, Khojasteh H. Synthesis, characterization and photocatalytic activity of FeCr2O4 and FeCr2O4/Ag nanocomposites. J. Nanostruct. 2020;10(3)518–530. https://doi.org/10.22052/jns.2020.03.008
35. Ruszel M, Grzybowska B, Samson K, Gressel I, Klisinska A. MIICr2O4-spinels as supports for Au nanoparticles in oxidation of CO. Catal. Today. 2006;112(1–4)126–129. https://doi.org/10.1016/j.cattod.2005.11.055
36. Gamal R, Rizk SE, El-Hefny NE. The adsorptive removal of Mo(VI) from aqueous solution by a synthetic magnetic chromium ferrite nanocomposite using a nonionic surfactant. J. Alloys Compd. 2021;853:157039. https://doi.org/10.1016/j.jallcom.2020.157039
37. Ashour A, El-Batal AI, Maksoud MA, et al. Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology. 2018;40:141–151. https://doi.org/10.1016/j.partic.2017.12.001
38. Goyal A, Bansal S, Singhal S. Facile reduction of nitrophenols: Comparative catalytic efficiency of MFe2O4 (M = Ni, Cu, Zn) nano ferrites. Int. J. Hydrog. Energy. 2014;39:4895–4908. https://doi.org/10.1016/j.ijhydene.2014.01.050
39. Shafqat MB, Ali M, Atiq S, Ramay SM, Shaikh HM, Naseem S. Structural, morphological and dielectric investigation of spinel chromite (XCr2O4, X = Zn, Mn, Cu & Fe) nanoparticles. J. Mater. Sci. Mater. Electron. 2019;30:17623–17629. https://doi.org/10.1007/s10854-019-02111-4
40. Petcharoen K, Sirivat A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B. 2012;177:421–427. https://doi.org/10.1016/j.mseb.2012.01.003
41. Yang S, Wang C, Li J, Yan N, Ma L, Chang H. Low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B. 2011;110:71–80. https://doi.org/10.1016/j.apcatb.2011.08.027
42. Xiang Y, Yang X, Xu Z, et al. Fabrication of sustainable manganese ferrite modified biochar from vinasse for enhanced adsorption of fluoroquinolone antibiotics: Effects and mechanisms. Sci. Total Environ. 2020;709:136079. https://doi.org/10.1016/j.scitotenv.2019.136079
43. Wang L, Li J, Wang Y, Zhao L, Jiang Q. Adsorption capability for Congo red on nanocrystalline MFe2O4 (M = Mn, Fe, Co, Ni) spinel ferrites. Chem. Eng. J. 2012;181–182:72–79. https://doi.org/10.1016/j.cej.2011.10.088
44. Zhao X, Wang W, Zhang Y, Wu S, Li F, Liu JP. Synthesis and characterization of gadolinium doped cobalt ferrite nanoparticles with enhanced adsorption capability for Congo Red. Chem. Eng. J. 2014;250:164–174. https://doi.org/10.1016/j.cej.2014.03.113
45. Chang Q, Liang H, Shi B, et al. Ethylenediamine-assisted hydrothermal synthesis of NiCo2O4 absorber with controlled morphology and excellent absorbing performance. J. Colloid Interface Sci. 2021;588:336–345. https://doi.org/10.1016/j.jcis.2020.12.099
46. Sun K, Lan Z, Yu Z, Jiang X, Huang J. Materials M. Phase formation, grain growth and magnetic properties of NiCuZn ferrites. J. Magn. Magn. Mater. 2011;323:927–932. https://doi.org/10.1016/j.jmmm.2010.11.071
47. Domenichini B, Amilain-Basset K, Bourgeois S. Dynamic segregation during ferrite oxidation revealed by XPS. Surf. Interface Anal. 2002;34:527–530. https://doi.org/10.1002/sia.1353
48. Kawano K, Hachiya M, Iijima Y, Sato N, Mizuno Y. The grain size effect on the magnetic properties in NiZn ferrite and the quality factor of the inductor. J. Magn. Magn. Mater. 2009;321:2488–2493. https://doi.org/10.1016/j.jmmm.2009.03.015
49. Narang SB, Pubby K. Nickel Spinel Ferrites: A review. J. Magn. Magn. Mater. 2021;519:167163. https://doi.org/10.1016/j.jmmm.2020.167163
50. Korovushkin V, Trukhanov A, Kostishin V, et al. Correlation between the Chemical Composition, Crystal Structure, and Magnetic Properties of Hexagonal Barium Ferrite with Zn2+ Heterovalent Substitution. Inorg. Mater. 2020;56:707–715. https://link.springer.com/article/10.1134/S0020168520070080
51. Yang J, Peng J, Liu K, Guo R, Xu D, Jia J. Synthesis of ferrites obtained from heavy metal solutions using wet method. J. Hazard. Mater. 2007;143:379–385. https://doi.org/10.1016/j.jhazmat.2006.09.038
52. More SS, Kadam RH, Kadam AB, Shite AR, Mane DR, Jadhav KM. Cation distribution in nanocrystalline Al3+ and Cr3+ co-substituted CoFe2O4. J. Alloys Compd. 2010;502:477–479. https://doi.org/10.1016/j.jallcom.2010.04.201
53. Lavina B, Salviulo G, Giusta AD. Cation distribution and structure modelling of spinel solid solutions. Phys. Chem. Miner. 2002;29:10–18. https://link.springer.com/article/10.1007/s002690100198
54. Sharma A, Batoo KM, Raslan EH, Kumar G. Effect of chromium ions on the structural, magnetic, and optical properties of manganese–zinc ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2020;31:16959–16967. https://doi.org/10.1007/s10854-020-04252-3
55. Cerón JA, Téllez DA, Roa-Rojas J. Weak Ferromagnetism in the FeCr2O4 Semiconductor Spinel with Half-Metallic Feature in the Ground State. J. Electron. Mater. 2022;51:822–830. https://doi.org/10.1080/10643380903392817
56. Witek-Krowiak A, Chojnacka K, Podstawczyk D, Dawiec A, Pokomeda K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014;160:150–160. https://doi.org/10.1016/j.biortech.2014.01.021
57. Behbahani M, Moghaddam MR, AArami M. Techno-economical evaluation of fluoride removal by electrocoagulation process: Optimization through response surface methodology. Desalination. 2011;271:209–218. https://doi.org/10.1016/j.desal.2010.12.033
58. Chaker H, Ameur N, Saidi-Bendahou K, Djennas M, Fourmentin S. Modeling and Box-Behnken design optimization of photocatalytic parameters for efficient removal of dye by lanthanum-doped mesoporous TiO2. J. Environ. Chem. Eng. 2021;9:104584. https://doi.org/10.1016/j.jece.2020.104584
59. Karimifard S, Moghaddam MRA. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018;640–641:772–797. https://doi.org/10.1016/j.scitotenv.2018.05.355
60. Sophia AC, Swaminathan K. Assessment of the mechanical stability and chemical leachability of immobilized electroplating waste. Chemosphere. 2005;58:75–82. https://doi.org/10.1016/j.chemosphere.2004.09.006
61. Chen D, Hou J, Yao LH, Jin HM, Qian GR, Xu ZP. Ferrite materials prepared from two industrial wastes: Electroplating sludge and spent pickle liquor. Sep. Purif. Technol. 2010;75:210–217. https://doi.org/10.1016/j.seppur.2010.07.009
62. Mills CT, Bern CR, Wolf RE, Foster AL, Morrison JM, Benzel WM. Modifications to EPA Method 3060A to Improve Extraction of Cr(VI) from Chromium Ore Processing Residue-Contaminated Soils. Environ Sci Technol. 2017;51:11235–11243. https://doi.org/10.1021/acs.est.7b01719
63. Hao H, Wang Y, Shi B, et al. Strong enhancement of methylene blue removal from binary wastewater by in-situ ferrite process. J. Environ. Sci. 2018;73:107–116. https://doi.org/10.1016/j.jes.2018.01.019
64. Perez OP, Umetsu Y. ORP-monitored magnetite formation from aqueous solutions at low temperatures. Hydrometallurgy. 2000;55:35–56. https://doi.org/10.1016/S0304-386X(99)00078-X
65. González G, Sagarzazu A, Villalba R. Study of the mechano-chemical transformation of goethite to hematite by TEM and XRD. Mater. Res. Bull. 2000;35:2295–2308. https://doi.org/10.1016/S0025-5408(00)00434-7
66. Fan H, Song B, Li Q. Thermal behavior of goethite during transformation to hematite. Mater. Chem. Phys. 2006;98:148–153. https://doi.org/10.1016/j.matchemphys.2005.09.005
67. Walter D, Buxbaum G, Laqua W. The mechanism of the thermal transformation from goethite to hematite. J. Therm. Anal. Calorim. 2001;63:733–748. https://doi.org/10.1023/A:1010187921227
68. Perez OP, Umetsu Y, Sasaki H. Precipitation and densification of magnetic iron compounds from aqueous solutions at room temperature. Hydrometallurgy. 1998;50:223–242. https://doi.org/10.1016/S0304-386X(98)00054-1
69. Barrado E, Prieto F, Medina J, López FA. Characterisation of solid residues obtained on removal of Cr from waste water. J. Alloys Compd. 2002;335:203–209. https://doi.org/10.1016/S0925-8388(01)01803-5
70. Tu CH, Huang YJ, Tsai CH, Chin CJM. In situ XANES study of removal of heavy metals from laboratory wasteliquid by the ferrite process. J. Electron Spectrosc. Relat. Phenom. 2007;156–158:228–231. https://doi.org/10.1016/j.elspec.2006.12.033
71. Mandaokar SS, Dharmadhikari DM, Dara SS. Retrieval of heavy metal ions from solution via ferritisation. Environ. Pollut. 1994;83:277–282. https://doi.org/10.1016/0269-7491(94)90148-1
72. Chen D, Mei CY, Yao LH, Jin HM, Qian GR, Xu ZP. Flash fixation of heavy metals from two industrial wastes into ferrite by microwave hydrothermal co-treatment. J. Hazard. Mater. 2011;192(3)1675–1682. https://doi.org/10.1016/j.jhazmat.2011.06.091
73. Li X, Wang G. Low-temperature synthesis and growth of superparamagnetic Zn0.5Ni0.5Fe2O4 nanosized particles. J. Magn. Magn. Mater. 2009;321:1276–1279. https://doi.org/10.1016/j.jmmm.2008.11.006
74. Chunjing L, Xin W, Yu W, et al. Study on Crystallization Knetics Mechanism of Soft-Magnetic MnZn Ferrite. Rare Metal Mat. Eng. 2009;38:515–519. https://WOS:000276258900117
75. Wang HW, Kung SC. Crystallization of nanosized Ni–Zn ferrite powders prepared by hydrothermal method. J. Magn. Magn. Mater. 2004;270(1–2)230–236. https://doi.org/10.1016/j.jmmm.2003.09.019
76. Chen D, Li Y, Zhang J, et al. Efficient removal of dyes by a novel magnetic Fe3O4/ZnCr-layered double hydroxide adsorbent from heavy metal wastewater. J. Hazard. Mater. 2012;243:152–160. https://doi.org/10.1016/j.jhazmat.2011.06.091
77. Xu T, Nan F, Jiang X, et al. Effect of soil pH on the transport, fractionation, and oxidation of chromium (III). Ecotoxicol. Environ. Saf. 2020;195:110459. https://doi.org/10.1016/j.ecoenv.2020.110459
78. Kaur M, Kaur N, Jeet K, Kaur P. MgFe2O4 nanoparticles loaded on activated charcoal for effective removal of Cr(VI) – A novel approach. Ceram. Int. 2015;41:13739–13750. https://doi.org/10.1016/j.ceramint.2015.08.040
79. Jung KW, Lee S, Lee YJ. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour. Technol. 2017;245:751–759. https://doi.org/10.1016/j.biortech.2017.09.035
Fig. 1(A) Schematic of a representative spinel ferrite structure, showing oxygen atoms(red), tetrahedral (blue), and octahedral (purple) units. The structure was drawn using the VESTA software and (B) bond angles in the Cr-O and Fe-O bonds. 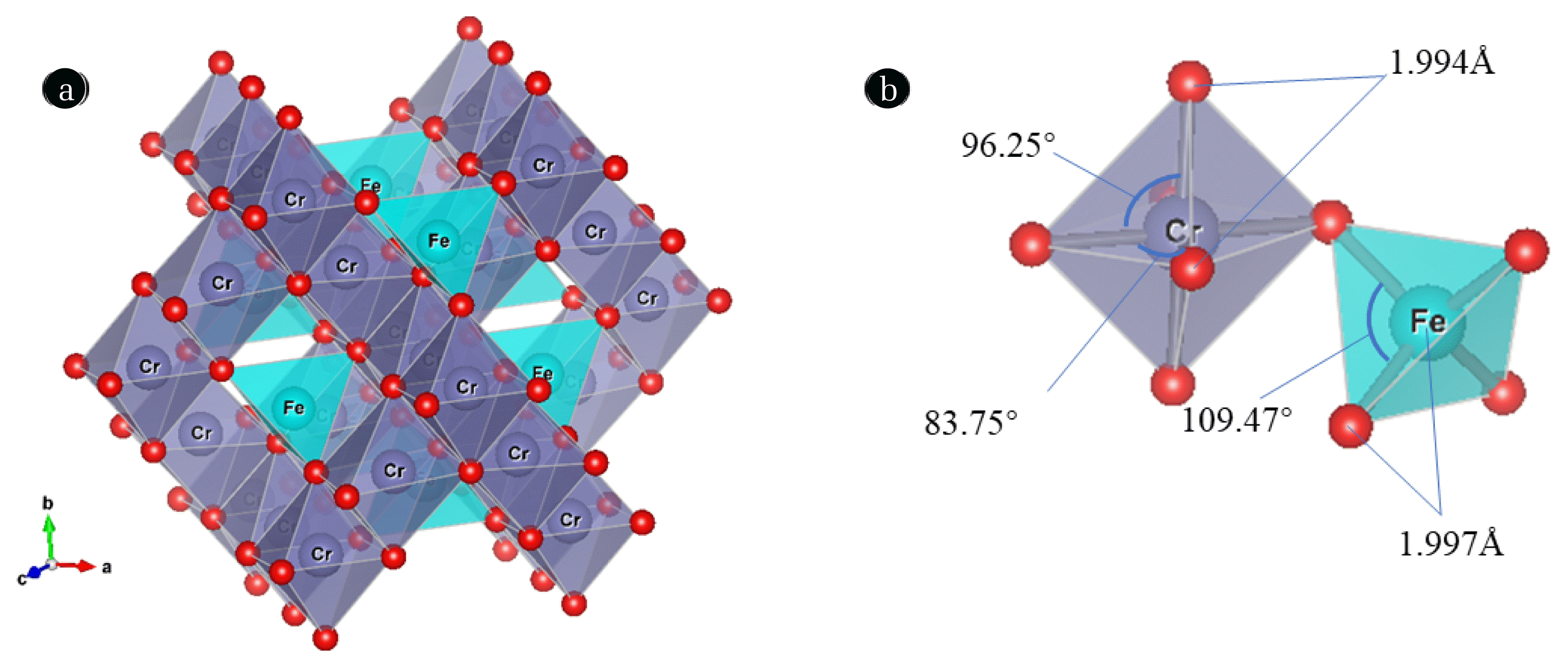
Fig. 2The XRD patterns of products were obtained with (A) different n (Fe2+/Cr6+) (a) 3, (b) 4, (c) 5, (d) 6, and (e) 7; (B) different temperatures (a) 20 °C, (b) 40 °C, (c) 60 °C, (d) 80 °C, and (e) 100 °C; (C) different pH(a) 8, (b) 9, (c) 10, (d) 11, and (e) 12; (D) different reaction time (a) 20min, (b) 30min, (c) 40min, (d) 50min, and (e) 60min. 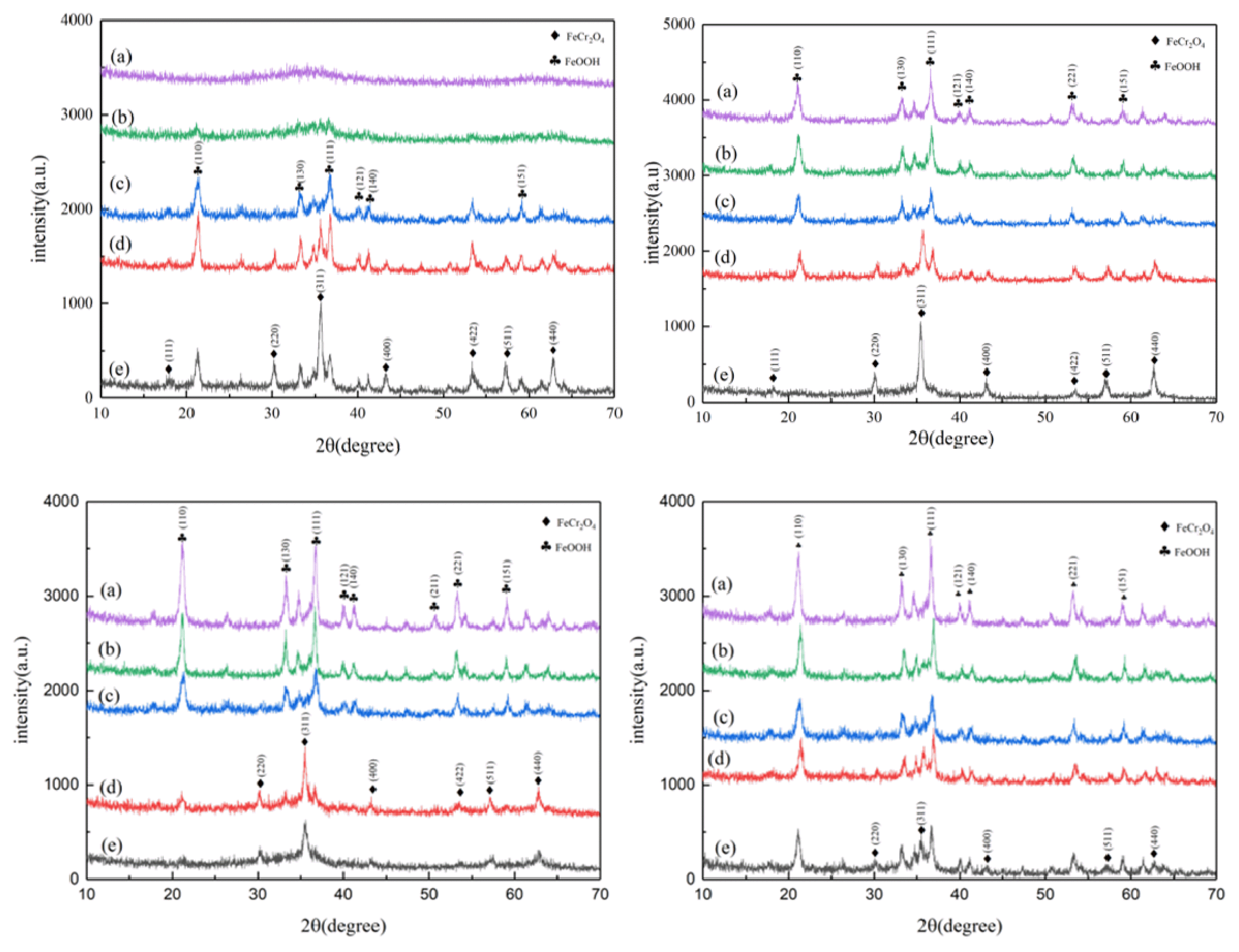
Fig. 3(A) The magnetic properties of the products obtained at n (Fe2+/Cr6+) of (a)7 and (b) 5; (B) The weight of the product was obtained at different pH; (C)Effects of pH on the total Fe in the supernatant; (D) Magnetizations with different reaction time; (E) Plots of log D versus the reciprocal of absolute temperature(1/T). 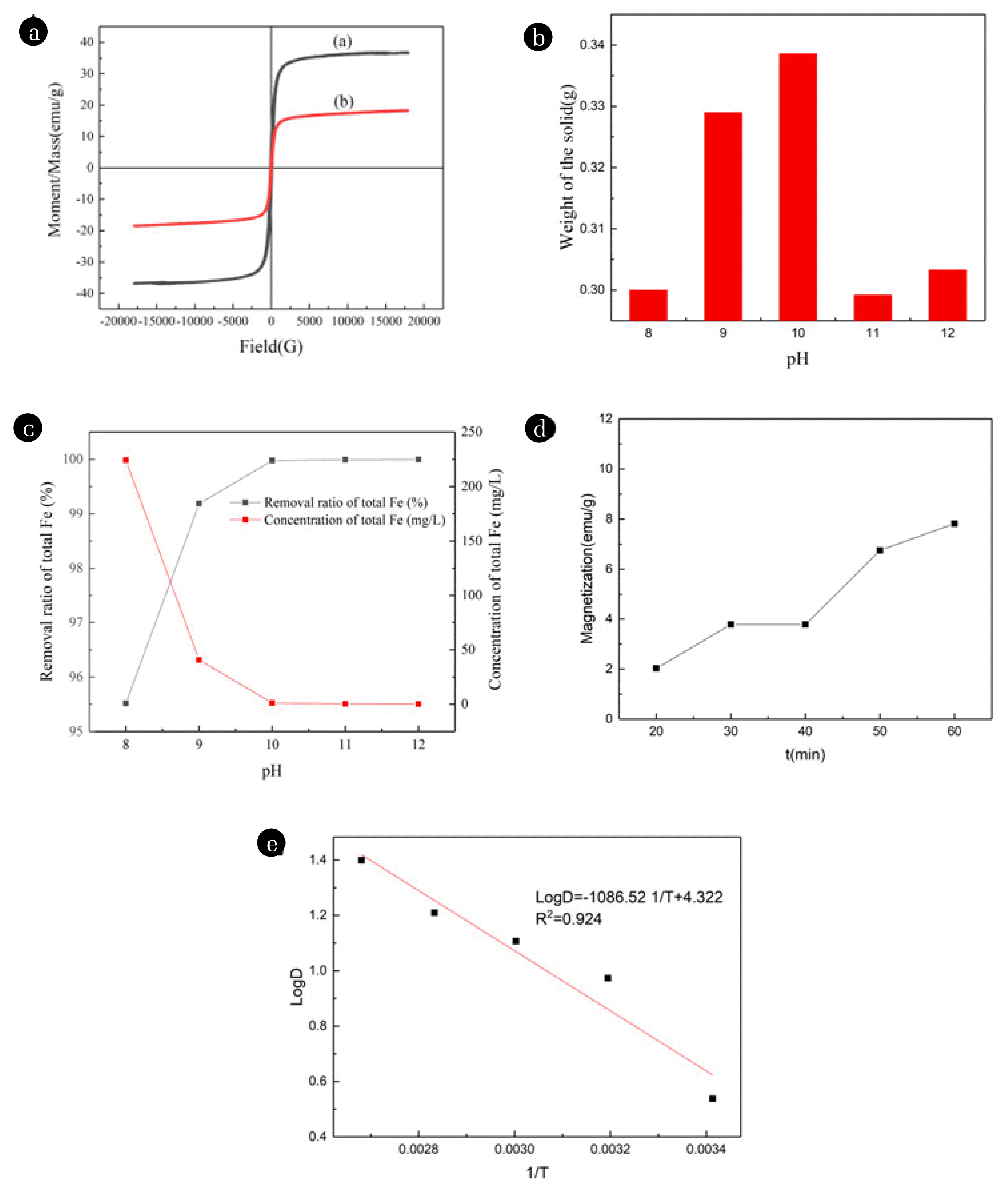
Fig. 4The three-dimensional response plot of the effects of (A) pH and mole ratio of Fe2+/Cr6+, (B) temperature and mole ratio of Fe2+/Cr6+ and (C) temperature and pH; (D)the plot of predicted value versus actual values for removal efficiency. 
Fig. 5SEM-EDS results of the products: (A) SEM image; (B) EDS at a specific position; and (C–E) Fe, Cr, and O distribution images. 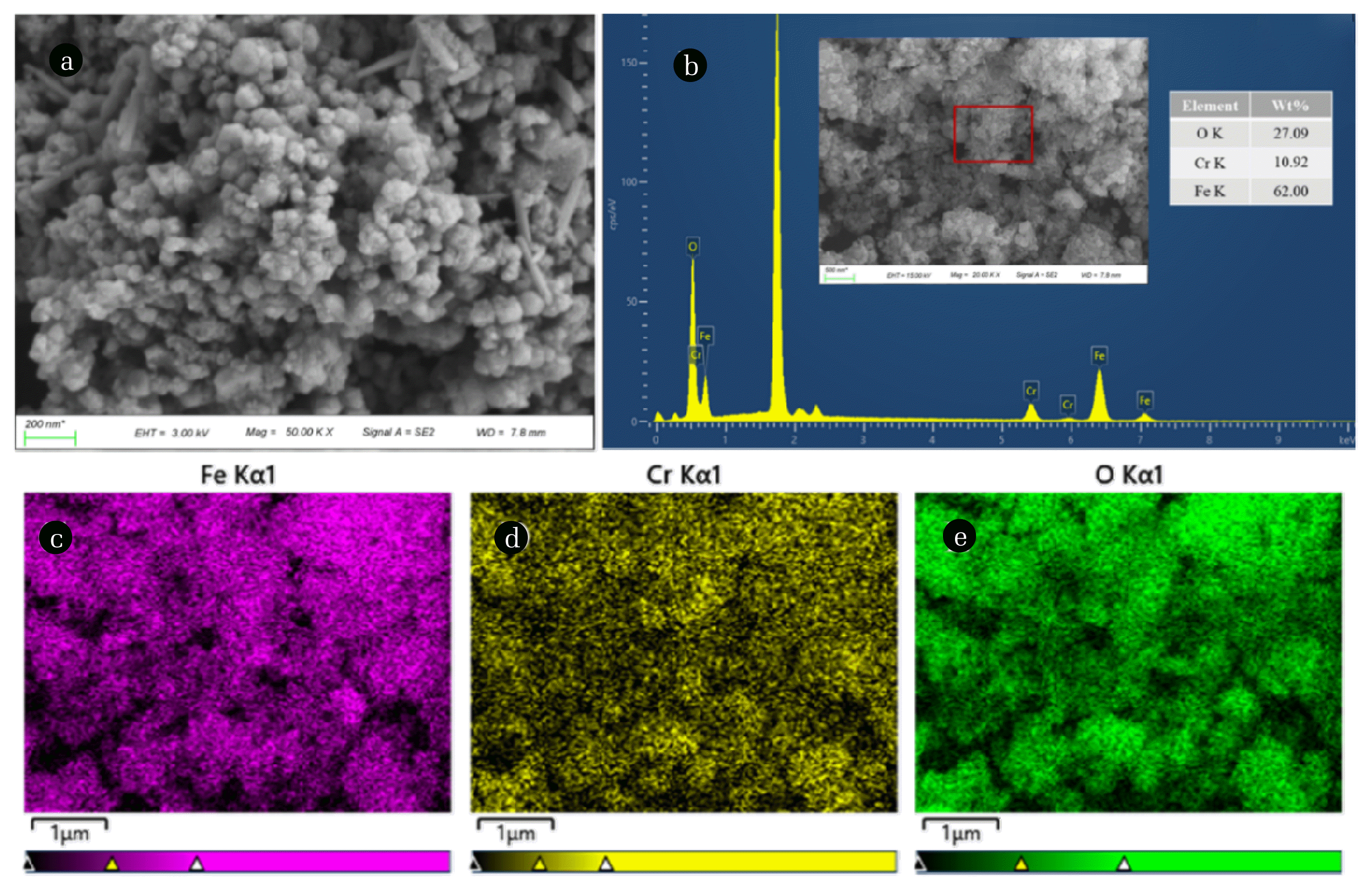
Fig. 6(A)The XRD patterns of products obtained under optimal conditions;(B) The XPS of Cr 2p in the magnetic product obtained under optimal conditions; (C) Magnetization curve of the product. 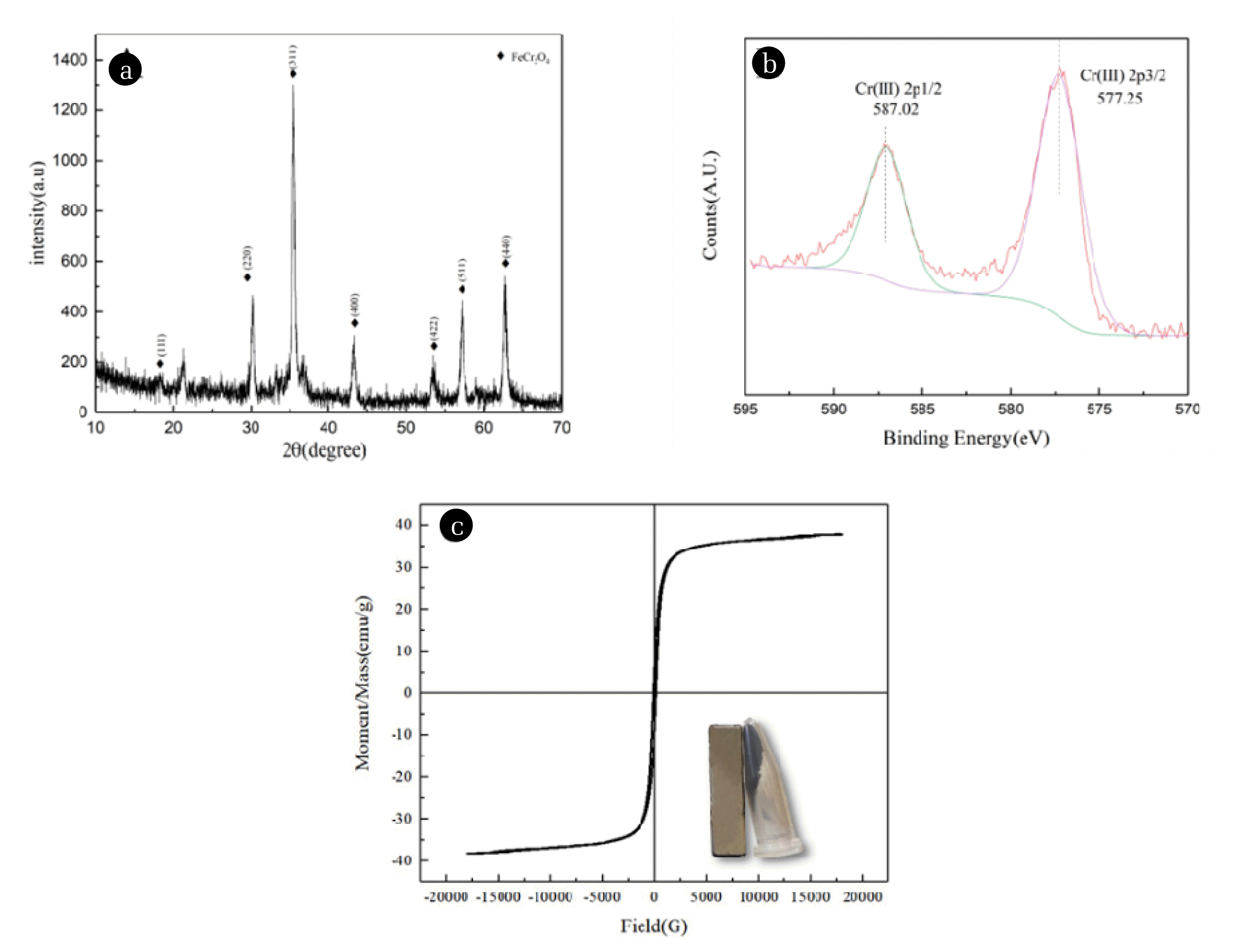
Table 1Experimental ranges and levels of independent variables
Table 2Experimental design matrix and magnetization values |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||