AbstractThe building blocks of biofilm are called extracellular polymeric substances (EPSs), and they are composed of biopolymers. The optimal properties of EPSs include chemical interactions, resistance to dehydration, and resilience in the face of hazardous shock loads. It is necessary to have an understanding of EPSs extraction, composition, and the biomatrix’s reaction to contaminants. This research aims to conduct chemical extraction to determine the amounts of EPSs found in unspiked-metaldehyde and spiked-metaldehyde rubber aerobic granular sludge (rAGS) and newly invented molasses aerobic granular sludge (mAGS). The rAGS was produced by feeding synthetic wastewater into sequencing batch reactors (SBRs). The mAGS was developed by adding the rAGS with different volumes of molasses wastewater. The EPSs were successfully extracted and identified by quantifying the number of proteins and carbohydrates present in each rAGS and mAGS condition. Hydroxyl, carboxyl, and amino groups showed that rAGS and mAGS EPSs contained carbohydrates and proteins. The compositions of the EPSs showed a strong correlation with the concentrations of metaldehyde in the spiked-metaldehyde rAGS and mAGS samples. When the total EPSs are taken into consideration, both rAGS and mAGS that are formed are effective treatments for metaldehyde treatment in wastewater.
Graphical Abstract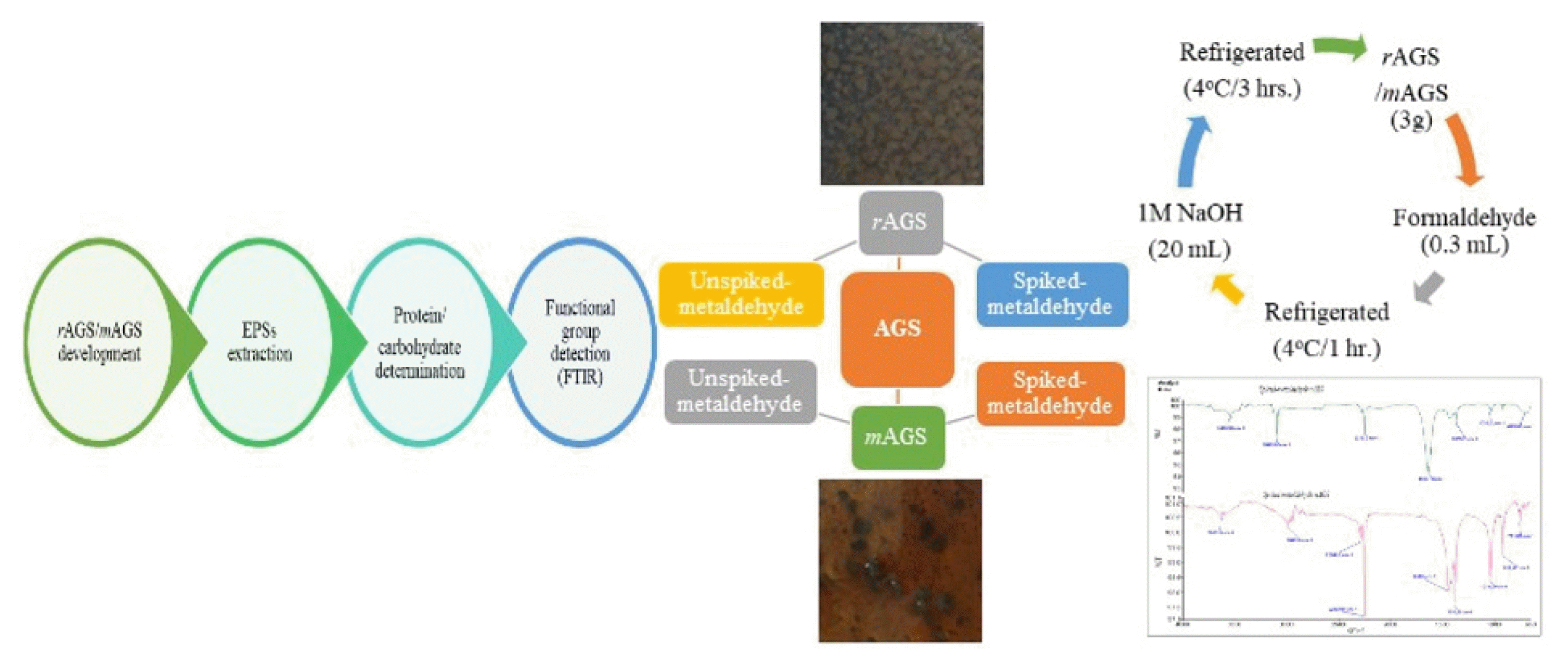
1. IntroductionExtracellular polymeric substances (EPSs) are natural organic biopolymers with a large molecular weight that are derived from microorganisms and are used by bacteria to interact with their surroundings [1–2]. Microorganisms are responsible for the biosynthesis of EPSs and then secreting them into their surrounding environment. Indicators of the surrounding environment are the most significant contributors to the formation of these matrix slimes [3]. Polysaccharides, proteins, DNA, lipids, and humic components make up most of the EPSs [4]. Generally, polysaccharides and proteins predominate in EPSs [5]. The percentage of these two macromolecules, however, differs depending on how the biomass basis is extracted [6]. The EPSs’ contents were also correlated with the bacteria’s capacity to excrete these substances [7]. The physiochemical characteristics of a biofilm are tightly regulated by the EPSs [8]. The structural integrity of biofilms was contingent on the presence of EPSs. EPSs can shield bacteria from toxic and stressful surrounding environments such as dryness and dehydration by generating a gel-like connection with the slimy feature that holds bacteria unruffled in the biofilms and sticks the biofilms to surfaces [9–10]. The presence of EPSs is not only advantageous for cell adhesion and aggregation, but it is also significant for biosorption, flocculation, and the establishment of biofilm [11–12]. Bacteria that are contained within a biofilm have several benefits concerning the degrading of hazardous pollutants, one of which is greater protection within the biofilm to withstand the effects of toxic pollutants [13–14]. The ability of bacteria contained within a biofilm to degrade harmful chemicals is facilitated by the bacteria’s improved ability to resist toxic pollutants, the bacteria’s production of EPSs, which helps in the removal of pollutants, the bacteria’s advanced cell density, which gives them access to a large pool of genetic properties, and the bacteria’s capability to adhere to a variety of substrates [15–16]. Because they include a high number of functional groups and binding sites, EPSs have proven to be an effective adsorbent for the removal of heavy metals, hydrocarbons, and pesticides from wastewater [17–19]. This has led to their widespread use in this capacity. The presence of EPSs in aerobic granules is advantageous for the removal of other newly discovered pollutants such as the antibiotic tetracycline [20]. Polysaccharides, which were one of the primary components in EPSs, were crucial in making the removal process go more smoothly [21]. There are two primary structural groups of EPSs, which are soluble EPSs and bound EPSs [22–23]. Soluble EPSs are the more common of the two. With surface-bound EPSs, there are two layers: the loosely bound (LB-EPSs) layer and the tightly bound (TB-EPSs) layer (TB-EPSs) [24]. The bacterial cell was sheltered by TB-EPSs, which also served as an outer layer [5], and TB-EPSs themselves were protected by LB-EPSs, which also served in this capacity. These EPSs layers interact with harmful pollutants including pesticides in the environment by emulsifying, dissolving, binding, precipitating, forming complexes, and exchanging ions [25]. In the context of aerobic granulation technology, EPSs are particularly important because they serve a variety of purposes, including enhancing the physicochemical properties of generated aerobic granules [26]. These properties include structural stability, settling time, flocculation effectiveness, and deposition [27]. The composition of EPSs, which includes a wide variety of functional groups and binding sites, makes it possible to use this substance as an adsorbent and a catalyst in the process of removing various contaminants, such as heavy metals and pesticides. This is made possible by the fact that the composition of EPSs includes a range of functional groups and binding sites. The matrix of EPSs in a bacterial biofilm is generally credited with the biofilm’s enhanced tolerance to hazardous contaminants and greater breakdown capabilities. The EPSs produced by bacteria aid in the adsorption and solubilization of organic chemical compounds such as pesticides, making them more bioavailable to the cells of the organism, which in turn improves the efficiency with which the cells’ enzymatic processes convert them to harmless by-products like water (H2O) and carbon dioxide (CO2). As a result, bioremediation mediated by biofilm-EPSs is a long-term, low-impact, and reasonably priced strategy for cleaning up polluted areas [25]. The presence of EPSs in aerobic granules is favorable for the transit and removal of heavy metals through a complex course of action [28]. Yet, to the best of our knowledge, very little is known regarding the composition and extraction of EPSs from aerobic granules, those that were produced from rubber and molasses effluent that had been subjected to pesticides, most notably metaldehyde. This is the first time that rubber aerobic granular sludge (rAGS) and molasses aerobic granular sludge (mAGS) developed from local wastewater have been used in an attempt to treat metaldehyde waste. Metaldehyde is a molluscicide that is used in agriculture, particularly in rice cultivation areas, to kill the golden apple snail. The new thing or innovation and the contribution that this study has to offer is the fact that it is the first attempt to use these two types of aerobic granules (rAGS and mAGS). As a result of this, it is essential to conduct an in-depth investigation of the process of extracting EPSs from the aerobic granular sludge that was employed in this study and determining their composition. This investigation focuses on the removal of EPSs from two distinct types of aerobic granular sludge (AGS), namely rAGS and mAGS, through the application of a chemical process involving a mixture of formaldehyde (CH2O) and sodium hydroxide (NaOH) treatment, both with and without exposure to the toxic compound known as metaldehyde. The chemical extraction method, which combines the two primary chemical types of CH2O and NaOH, has successfully minimized cell lysis in comparison to other extraction techniques, such as heating and ultrasonic methods [29]. In addition, in comparison to other methods, the chemical extraction methodology that was utilized was able to extract a greater quantity of EPSs from the aerobic granule samples. It is assumed that the flocculation process in the aerobic granules will benefit from the chosen chemical extraction technique to improve its effectiveness. The selection of the optimal extraction procedure is of the utmost importance since it plays a role in determining the fundamental structure and content of the EPSs that are extracted. After the extraction process, the isolated EPSs were analyzed to determine the number of proteins and carbohydrates they contained. This was done since proteins and carbohydrates are the macromolecules that are found in EPSs in the greatest quantity. In addition, the association between the varied compositions of EPSs and the presence of varying quantities of metaldehyde was noticed during this research. Therefore, the extraction result that was obtained could provide a better understanding and comparison facts for the responses of EPSs in rAGS and mAGS in these two distinct circumstances, with and without the occurrence of metaldehyde, a substance that is commonly used as a molluscicide to protect paddy plants from the destruction caused by golden apple snails. This article discusses the significance of doing a study in which there is a discernible contrast between AGS that were exposed to metaldehyde and those that were not exposed to the chemical. This research may provide advanced perspectives on how aerobic granules technology, which is made of several types of microorganisms, works to treat wastewater efficiently including pesticide substances even when there are EPSs present in the biofilm systems. The management of wastewater in a more targeted, environmentally friendly, and cost-effective manner that is more beneficial for all stakeholders is made possible by having a more comprehensive understanding of EPSs based on the composition and distribution of the primary components included in them. This is the consequence of the study’s commendable attempt to ensure the long-term reliability of the strategy applied to treat pesticide waste in wastewater by utilizing rubber and molasses wastewater. This has come about as a result of the study. The first section of the article serves as the foundation for the entire piece (Introduction). The materials used and the procedures followed are outlined in Section 2. The findings and discussion are elaborated on in more detail in Section 3. The conclusions are discussed in detail in Section 4.
2. Materials and Methods2.1. Development of Rubber Aerobic Granular Sludge (rAGS) and Molasses Aerobic Granular Sludge (mAGS)Rubber aerobic granular sludge (rAGS) was developed from rubber seed sludge wastewater in the laboratory-scale sequencing batch reactor (SBR), which was operated for more than one month and fed with synthetic wastewater containing sodium acetate as the sole carbon source for the granulation process. To form a new aerobic granule recognized as molasses aerobic granular sludge (mAGS), the rAGS was augmented with molasses wastewater. The molasses wastewater was diluted by 50% (50% molasses wastewater and 50% parts of water), as this dilution value previously demonstrated the best growth of biomass. Furthermore, in this investigation, aerobic granules that have not been subjected to metaldehyde are specifically referred to as unspiked-metaldehyde rAGS and unspiked-metaldehyde mAGS. However, metaldehydeexposed aerobic granules were recognized as spiked-metaldehyde rAGS and spiked-metaldehyde mAGS. Unspiked-metaldehyde and spiked-metaldehyde of both rAGS and mAGS were employed in this present study for the extraction and quantification of extracellular polymeric substances (EPSs).
2.2. Spiked-Metaldehyde ExperimentsThe rubber aerobic granular sludge (rAGS) and molasses aerobic granular sludge (mAGS) used in the spiked-metaldehyde experiments had an average particle size of about >0.6 mm. The aerobic granular sludge was collected at the closing stages of the aeration process and rinsed three times with deionized water before being exposed to metaldehyde. The spiked-metaldehyde experiments were taken place in the orbital shaker at 150 rpm for two weeks to maximize the homogenization process. The metaldehyde doses utilized in this investigation, ranging from 180 to 330 mg/L based on the ecotoxicological effects of molluscicide metaldehyde on amphibious freshwater fish famous as climbing perch (Anabas testudineus) [30].
2.3. EPSs ExtractionExtracellular polymeric substances (EPSs) were recovered from unspiked-metaldehyde and spiked-metaldehyde of both aerobic granules’ samples (rAGS and mAGS) by exploiting a chemical process [31–33]. In this extraction method, two main types of chemicals were used: formaldehyde (CH2O) and sodium hydroxide (NaOH). In addition, by adding the total protein and total carbohydrate of the soluble EPSs, loosely bound EPSs, and tightly bound EPSs, the sum of protein and carbohydrate contents in the extracted EPSs of rAGS and mAGS were discovered. The existence of proteins in EPSs of rAGS and mAGS was scrutinized by using the Lowry method [34–35]. For the determination of total carbohydrates in EPSs-rAGS and EPSs-mAGS of unspiked-metaldehyde and spiked-metaldehyde samples, the phenol sulfuric acid method was used to measure glucose concentration [36–37]. The extraction process of EPSs started with, approximately 3 g/wet weight rAGS and mAGS from unspiked-metaldehyde and spiked-metaldehyde samples deposited in two separate 100 mL sealed vials. Demineralized water was added to the vials for up to 50 mL. After that, each vial received 0.3 mL of 37 percent formaldehyde. Subsequently, the vials were gently shaken to combine the solution. The vials were then placed in the refrigerator for 1 hour at 4°C. After one hour, both unspiked-metaldehyde and spiked-metaldehyde of rAGS and mAGS suspensions received 20 mL of 1M NaOH. The vials were gently shaken before being placed in the refrigerator for 3 hours at 4°C. The samples were then transferred to a centrifuge tube. and centrifuged at 4,000 x g for 20 minutes before being filtered through nylon membranes with diameters of 0.2 μm. After the extraction, the samples were then tested for the presence of protein and carbohydrates.
2.4. Determination of Protein in Unspiked-Metaldehyde and Spiked-Metaldehyde of EPSs-rAGS and EPSs-mAGS ExtractionProtein components in unspiked-metaldehyde and spiked-metaldehyde of EPSs-rAGS and EPSs-mAGS were determined using the Lowry technique [34–35]. The protein standard used is bovine serum albumin (BSA) (Sigma-Aldrich; Cat#A-3608) [38]. The BSA concentrations of 0 mg/L, 20 mg/L, 40 mg/L, 60 mg/L, 80 mg/L, and 100 mg/L were prepared from a stock solution with a preliminary concentration of 100 mg/L (0 mg/L, 20 mg/L, 40 mg/L, 60 mg/L, 80 mg/L, and 100 mg/L). This approach requires the use of four different types of reagents. Reagent P (dilute alkaline solution) consists of 2% sodium carbonate (Na2CO3) in a 0.10 N sodium hydroxide (NaOH) Milli-Q water solution. Reagent Q is a 0.5 percent copper sulfate pentahydrate solution (CuSO4. 5H2O). Reagent R was made up of 1% sodium tartrate (Na2C4H4O6). Reagent S (i.e., Lowry solution) was made up of a combination of three reagents: Reagent P, Reagent Q, and Reagent R, with a volume-to-volume ratio of 3:1. (100:1:1). A 0.2 mL aliquot of extracted EPSs samples was inserted into the test tubes to assess the quantity of protein. After that, 1 mL of Reagent S was introduced to each of the test tubes’ samples. The EPSs samples were carefully combined with a vortex and left at room temperature for almost 20 minutes. Following that, each test tube received 0.10 mL of Folin-Ciocalteu phenol reagent. Following the addition of the Folin-Ciocalteu phenol reagent, the test tubes were instantly mixed to homogenize the samples. The test tubes were left at room temperature and in dark conditions for half an hour. The absorbance at 750 nm was then measured using a spectrophotometer [39]. To plot the standard calibration curve of protein, a spectrophotometer was used to read the various BSA standards and all the reagents. Following the plotting of the standard calibration curve, all samples were measured using the same approach to determine the protein content.
2.5. Determination of Carbohydrates in Unspiked-Metaldehyde and Spiked-Metaldehyde of EPSs-rAGS and EPSs-mAGS extractionIn this study, the carbohydrate content of extracted EPSs of unspiked-metaldehyde and spiked-metaldehyde rAGS and mAGS was determined using the phenol-sulfuric acid technique [40]. This colorimetric approach is widely used to assess carbohydrates in aerobic granular sludge [36–37]. This approach has been shown to identify a variety of carbohydrate groups, including monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Complex carbohydrates such as polysaccharides, oligosaccharides, and disaccharides are broken down into simple monosaccharides by concentrated sulfuric acid. Pentoses containing five-carbon molecules are dehydrated to furfural derivatives during the procedure. Hexoses containing six-carbon molecules are also dehydrated to hydroxymethyl furfural at the same time. These chemicals reacted with phenol to generate a yellow-gold hue. Glucose was used to create the standard curve. At 490 nm, the absorption is observed. Under ideal conditions, the color of this reaction remains steady for several hours, and the method’s accuracy is within 2% [41]. A five percent aqueous phenol solution was combined with a 2 mL aliquot of extracted EPSs in glass test tubes for this process. After that, 5 mL of concentrated sulfuric acid is added to the mixture immediately. The test tubes were then left to stand for 10 minutes. The samples were then vortexed for 30 seconds before being placed in a water bath at room temperature for 20 minutes to build up color. The samples were examined using a spectrophotometer with a 490 nm absorption wavelength. Based on a standard curve prepared with glucose, the amount of total carbohydrate present in the sample solution was determined [42]. The reference solutions are made in the same way as the samples, with the exception that the 2 mL aliquot of samples is replaced with distilled water. The five percent phenol in the water (w/w) solution was made right before the tests [43].
2.6. Fourier Transform Infrared Detection of Functional Groups (Protein and Carbohydrate)The analytical method of Fourier transform infrared spectroscopy (FTIR) is used to distinguish between organic, polymeric, and occasionally inorganic materials. Infrared light is used to scan test materials and examine chemical characteristics using the FTIR analysis method. In this investigation, FTIR was employed to detect the functional groups of protein and carbohydrate in the unspiked-metaldehyde and spiked-metaldehyde rAGS and mAGS samples on a qualitative level. The background spectrum was meticulously monitored before the samples were tested. The samples were transported to the lab and stored in vials. On the FTIR detector, one drop of each sample must be deposited and analyzed. The FTIR results are displayed in the form of a spectrum. FTIR analysis reveals the functional group name and characterizes the covalent bonding, according to the spectrum discovered in this study. The spectrum is a fingerprint of the sample, with absorption peaks corresponding to the frequency of vibrations between the atoms’ bonds that make up the molecule [44–45].
3. Results and Discussion3.1. Rubber Aerobic Granular rAGS and Molasses Aerobic Granular Sludge (mAGS)After a month of cultivation in the sequencing batch reactor (SBR), rubber aerobic granular sludge (rAGS) reached a stable state with larger size and compact structure forms than the seed sludge utilized before. As well, on Day-35 of the granulation process, the biomass concentration for rAGS was found to be at its optimal value. On the other hand, the biomass concentrations significantly decreased in value after rAGS was added to the molasses wastewater. This circumstance, which did not affect the biomass weight and density, may have resulted from molasses aerobic granular sludge (mAGS) adaptability and adjustment to the new enrichment mediums. However, the biomass value in mAGS kept increasing after two weeks exposed to molasses wastewater.
3.2. Extracellular Polymeric Substances of Unspiked-Metaldehyde rAGS and mAGSThe overall concentration of EPSs for unspiked-metaldehyde rAGS and mAGS increased dramatically throughout the granulation days, according to the findings. On Day-60, the maximum EPSs concentration was discovered. For both unspiked-metaldehyde rAGS and mAGS samples, the highest concentrations of EPSs were 288.87 mg/g MLVSS and 341.60 mg/g MLVSS, respectively. The preliminary concentration of EPSs from the seed sludge was found at 93.50 mg/g MLVSS during the early granulation process of rAGS. On Day-5 and Day-12, there was a quick rise to 138.5 mg/g MLVSS and 205.5 mg/g MLVSS, respectively. The protein concentration rose from 65.3 mg/g MLVSS on Day-0 to 130.3 mg/g MLVSS on Day-12. Additionally, this study found that the concentration of carbohydrates substantial rise from 28.2 mg/g MLVSS on Day-0 to 45.7 mg/g MLVSS on Day-12. The total concentrations of EPSs, protein, and carbohydrates in unspiked-metaldehyde rAGS are displayed in Fig. 1.
The unspiked-metaldehyde mAGS showed a similar pattern to unspiked-metaldehyde rAGS. Since mAGS were derived from rAGS, a higher total EPSs value (230.4 mg/g MLVSS) was discovered during the preliminary phase (Day-35) as compared to rAGS. The EPSs of unspiked-metaldehyde mAGS increased throughout time, from 230.40 mg/g MLVSS on Day-0 to 250.80 mg/g MLVSS on Day-5, and 265.20 mg/g MLVSS on Day-12. At the same time, protein concentration also increased significantly from 72.40 mg/g MLVSS on Day-0 to 78.30 mg/g MLVSS on Day-12. In this investigation, a considerable increase in carbohydrate concentration was also detected, ranging from 158.00 mg/g MLVSS on Day-0 to 186.90 mg/g MLVSS on Day-12. Fig. 2 exhibits the overall concentrations of EPSs, protein, and carbs in unspiked-metaldehyde mAGS. The main factor of the rise of EPSs secretion in unspiked-metaldehyde mAGS, notably protein and carbohydrate levels, was attributable to microorganisms’ normal reaction to being exposed to a changing environment situation since the rAGS was exposed to molasses wastewater for the augmentation process to develop mAGS in this study.
EPSs secretion, which takes place in a variety of environmental situations, is now acknowledged as a key microbial adaptation for the survival process [46–47]. On the other hand, when resources are scarce, bacteria are more likely to restrict EPSs production level, which is generally associated with carbohydrate content and to form larger clusters to endure [48]. When the bioreactor was fed with an abundance of food, however, EPSs production increased. The availability of sufficient food is one of the factors that stimulated microbial metabolism, resulting in the production of a large number of EPSs in rAGS and mAGS, which aided in the formation of aerobic granules. The results showed that the carbohydrate/protein ratio of EPSs in unspiked-metaldehyde rAGS and mAGS dramatically rose during the granulation days. The carbohydrate/protein ratio of EPSs in unspiked-metaldehyde rAGS was increased from 0.43 (Day-0) to 0.58 (Day-10) (Day-12). The carbohydrate/protein ratio from EPSs samples displayed a similar increasing trend in unspiked-metaldehyde mAGS. As we know, a significant portion of a bacterial cell is protein. Protein was produced in the early stages to protect cells from the effects of the new and different environment, with such conditions as nutrition shortage and medium toxicity. The protein was also produced by the bacteria as a source of internal energy [49–50]. From the observation, the proportion of carbohydrate/protein ratio heightened from 2.18 (Day-0) to 2.39 (Day-12). Thus, the rise in carbohydrate/protein ratio aided the smoothness looks of the outer surface and the compact formation of the aerobic granular sludge (AGS) [51–52]. As a result, adequate food resources were required to provide enough nutrition for microbial growth and propagation in the aerobic granules. Therefore, the microorganism may produce EPSs, primarily protein, and carbohydrates, easing the formation of AGS in addition to conveying and storing food and energy sources [53–54]. According to the statistical results in this study, there was a strong positive association between total EPSs concentrations and granulation days for unspiked-metaldehyde rAGS, with an r-value of 0.94 and a p-value of 0.01. Statistical analysis results for the unspiked-metaldehyde mAGS sample revealed a strong positive association between total EPSs and augmentation days of mAGS itself, with an r-value of 0.98 and a very significant p-value of 0.01. The presence of EPSs during the granulation process of AGS aids the development and firmness of AGS in terms of form and density [55]. The EPSs, in particular, have been shown to cause cell contacts to agglomerate and, as a result, lead to microbial development in aerobic granulation systems. EPSs not only act as a key player in biofilm growth and cohesion but are also important in releasing energy through catabolic reactions [56–58]. The statistical findings of the correlation between total EPSs concentrations and granulation days of rAGS and mAGS are tabulated in Table 1.
The EPSs have a variety of roles that help bacteria survive during the granulation process [59–60]. Their main role is to assemble microorganisms into flocs or biofilms. Micro consortia expand the number of sorts of bacteria that may have a symbiotic interaction, microorganisms thrive in groups [61]. They may also act as a protective barrier, allowing microorganisms to live in hostile environments, such as biocides, dry conditions, and malnutrition [62–63]. The EPSs is a biopolymer matrix that contains proteins, polysaccharides, lipids, uronic acids, humic-like compounds, nucleic acids/DNA, and other micromolecules [64–65]. EPSs also contained dissociated functional groups such as carboxyl, phosphoric, and hydroxyl [7]. The results for the aerobic granular sludge membrane bioreactor (AGMBR) demonstrated that EPSs had a high protein content, particularly at high concentrations of total organic carbon (TOC) [66]. In addition, the TOC value has a substantial primary influence on both the protein and carbohydrate components. This demonstrates the significance of organic carbon sources in the generation of EPSs during the development of biofilm. Several strains of microbes generate and release these biopolymers throughout the wastewater treatment process [10]. In another example, the secretion of EPSs during the traditional wastewater treatment technique especially the activated sludge process prompted the flocculation process that formed sludge flocs [67]. The EPSs play a vital part in the biogranulation of aerobic granular sludge from seed sludge [68]. In particular, the EPSs improved microbe aggregation, granule formation, and structural strength. Microscope examination (in situ) and chemical analysis (ex-situ) identified the differences in concentrations and compositions of EPSs constituents such as polysaccharides and proteins between the activated sludge and AGS [69–70]. As in the EPSs formation case, adhesion is the earliest stage in the development of biofilms. Adhesin-receptor contacts are engaged in the early bacterial adherence to surfaces [1–2]. The EPSs include the matrix’s lipids, proteins, and some polysaccharides that serve as the adhesive agents during the adhesion process. The EPSs are secreted to enhance the aggregation of cells. Finally, the continuous secretion and production of EPSs will strengthen the bacterial aggregation colonies formed [11–12].
3.3. Extracellular Polymeric Substances of Spiked-Metaldehyde rAGS and mAGSTo measure the amounts of extracellular polymeric substances (EPSs), different metaldehyde concentrations ranging from 180 mg/L to 330 mg/L were spiked into rAGS and mAGS samples. From the observation, the concentrations of EPSs in spiked-metaldehyde rAGS (rAGS-MTD) and spiked-metaldehyde mAGS (mAGS-MTD) considerably rose with an increase in metaldehyde concentration. The maximum EPSs amount (320.40 mg/g MLVSS and 343.00 mg/g MLVSS for spiked-metaldehyde rAGS and spiked-metaldehyde mAGS, respectively) was found at the highest metaldehyde concentration (330 mg/L). In order to shield the bacterial cells from the inhibitory effects of pesticides, more EPSs were released when culture bacteria were exposed to higher pesticide concentrations [71]. The lowest EPSs levels, conversely, were found at 180 mg/L which was the lowest metaldehyde concentration tested, with spiked-metaldehyde rAGS and spiked-metaldehyde mAGS, respectively, having MLVSS values of 120.30 mg/g and 252.40 mg/g. As a result, in this investigation, higher metaldehyde concentrations led to greater EPSs secretion in comparison to lower amounts of metaldehyde concentrations. This proves that the addition of metaldehyde has become one of the factors that increase the production of EPSs in rAGS and mAGS. This occurs when harmful chemicals, such as metaldehyde, are detected by the biofilm. Therefore, when cultivated bacteria are exposed to larger quantities of metaldehyde, more EPSs are generated to protect bacterial cells from the inhibitory effects of the metaldehyde. Compared to spiked-metaldehyde mAGS, was produced higher overall EPSs than spiked-metaldehyde rAGS. In this study, spiked-metaldehyde mAGS showed a more significant response than spiked-metaldehyde rAGS against metaldehyde shock load. This is due to the different compositions of EPSs between these two types of aerobic granules. The extraction results showed that the content of EPSs in spiked-metaldehyde mAGS was higher than the content of EPSs in spiked-metaldehyde rAGS at all metaldehyde concentrations (200 – 330 mg/L). Due to this condition, spiked-metaldehyde mAGS received double protection against metaldehyde toxicity compared to spiked-metaldehyde rAGS. Specifically, the excreted EPSs act as the aerobic granules’ outer layer of defense against any toxicity brought on by dangerous compounds, in this case, metaldehyde. Fig. 3 presents the amounts of EPSs, protein, and carbohydrates in spiked-metaldehyde rAGS and spiked-metaldehyde mAGS that were extracted in this study.
The diverse environmental factors present during the aerobic granules’ development may have an impact on how microorganisms react to metaldehyde exposure. Phenols can also be utilized to examine microorganisms’ capacity to endure harmful chemicals [72]. Bacteria released more EPSs to shield the cell from the harmful effects of phenols. In this present study, from the lowest to the highest metaldehyde concentrations, spiked-metaldehyde rAGS protein and carbohydrate concentrations considerably increased. At all metaldehyde concentrations, it was shown that protein concentration in spiked-metaldehyde rAGS was higher than carbohydrate concentration. This occurred because more protein was required during this phase to create aerobic granules. Consequently, spiked-metaldehyde rAGS had more protein than carbohydrate. Aerobic granule formation and stability depend heavily on protein for their development process [73–74]. A large amount of this biopolymer was located in the center of the aerobic granules. On the other hand, it was discovered that in aerobic granular sludge, carbohydrates provided an intermolecular force and this polymer was positioned at the granules’ outer border [75–76]. The visualization of EPSs components and microorganisms in the aerobic granular sludge was utilized by specific fluorophores and confocal laser scanning microscope (CLSM) [77–79]. In this research, the lowest protein value (85.2 mg/g MLVSS) was discovered at a metaldehyde concentration of 180 mg/L, which was also the lowest. While the metaldehyde concentration was found to be at its maximum (330 mg/L), the protein content was found to be at its highest (172.4 mg/g MLVSS). On the other hand, it was also found that spiked-metaldehyde rAGS had a considerable rise in carbohydrate concentration. From 35.10 mg/g MLVSS at 180 mg/L metaldehyde concentration to 148.00 mg/g MLVSS at 330 mg/L metaldehyde concentration, the carbohydrate concentration increased. A different situation was detected in spiked-metaldehyde mAGS as compared to spiked-metaldehyde rAGS. At all metaldehyde concentrations in spiked-metaldehyde mAGS, it was found that the concentration of carbohydrates was higher than the protein. Due to a lack of resources, the cells had to excrete carbohydrates to provide them with the energy they needed to survive in the presence of metaldehyde. The lowest levels of protein and carbohydrates were found to be 94.40 mg/g MLVSS and 158.00 mg/g MLVSS, respectively, at the lowest metaldehyde concentration (180 mg/L). The highest levels of protein and carbohydrates, which were 128.00 mg/g MLVSS and 215.00 mg/g MLVSS, respectively, were discovered at the highest metaldehyde concentration (330 mg/L). According to Fig. 4, EPSs were generally more prevalent in spiked-metaldehyde rAGS and spiked-metaldehyde mAGS compared to unspiked-metaldehyde rAGS and unspiked-metaldehyde mAGS. Rapid initial cell attachment and long-term microalgal biofilm stability are promoted by extreme conditions occurring on the membrane surface of the cells [80].
In that case, biofilm was created when a considerable number of EPSs which are essential components of biofilms were secreted under difficult environmental circumstances and collected on the cell surface [81–82]. Indirectly, the biofilm’s capacity to endure nutrient shortages or harmful conditions might increase and simultaneously encourage persistent metabolism under unusual circumstances. The EPSs are built from various biopolymer materials that make them a strong and stable defense against any situations that happen in the biofilms ambient [83]. The presence of EPSs protects the biofilm and prevents it from any harmful conditions [84]. Bacteria, therefore, tended to adhere to the bio-carrier when EPSs were present. Among the proofs, EPSs also prevent the loss of water from the cells to the inhabited environment [85]. High toxicity in wastewater caused seed sludge to secrete more EPSs compared to seed sludge placed in wastewater containing lower toxicity [86]. It is possible to conclude that the creation or secretion of EPSs is not based on chance but rather on certain aspects of the surrounding environment. This proves the efficiency of this process. Under the findings of the statistical study, there is a strong positive connection between total EPSs concentrations and metaldehyde concentrations in spiked-metaldehyde rAGS, with an r-value of 0.98 and a very significant p-value of 0.0006. Simultaneously, the spiked-metaldehyde mAGS sample statistical findings also showed a positive association (r-value = 0.94) between total EPSs and metaldehyde pesticide concentrations and a very significant (p-value = 0.005). The statistical findings of the correlation between total EPSs concentrations and spiked-metaldehyde rAGS and spiked-metaldehyde mAGS are presented in Table 2.
3.4. Fourier Transform Infrared Spectroscopy for the Verification of Extracellular Polymeric SubstancesThe presence of EPSs in rAGS, mAGS, spiked-metaldehyde rAGS, and spiked-metaldehyde mAGS was verified using Fourier transform infrared spectroscopy (FTIR). As a result, the FTIR spectrum was used to analyze the functional groups of EPSs in the rAGS, mAGS, rAGS-MTD, and mAGS-MTD samples. Three significant absorption bands were found in the rAGS and mAGS samples based on the findings. The broadest peak was observed at wavelength 3399.91 cm−1, and 3370.559 cm−1 in rAGS and mAGS were identified as hydroxyl groups (O-H) [87]. The peak at wavelengths 1641.47 cm−1 and 1641.99 cm−1 in rAGS and mAGS, respectively, were simultaneously categorized as carboxyl groups (COOH). Due to the presence of the amino groups (O-C-N) and (C-O) in the samples, the other bands with values of 711.24 and 683.68 cm−1 were found in both rAGS and mAGS, respectively. In summary, as those polymers are the primary constituent in EPSs, the discovery of hydroxyl, carboxyl, and amino groups demonstrated the presence of carbohydrates and proteins in the EPSs of rAGS and mAGS, respectively. These hydroxyl, carboxyl, and amino groups determined how bacterial aggregation led to the creation of biofilms. The functional groups like hydroxyl and carboxyl, as well as aliphatic and aromatic groups, influence a variety of activities during wastewater treatment, such as the development of biofilms and the accumulation of bacteria to form stable and denser aerobic granules [88]. Hydroxyl bonds connect the carbohydrate molecules in their structure. These bonds facilitate interactions between molecules of carbohydrates and other substances. The carboxyl group for proteins was found in the rAGS and mAGS, respectively. The carboxyl group is typically present in the amino acids that serve as the building blocks of proteins. Additionally, the carboxyl group is crucial for creating peptide bonds when it interacts with an additional amino group from another molecule. The FTIR spectrum of rAGS and mAGS with (a) hydroxyl groups, (b) carboxyl groups, and (c) amino groups is presented in Fig. 5.
It is disclosed that the two primary functional groups hydroxyl and amino constitute the EPSs of granular activated carbon linked biofilm and the biofilm formation during fermentation that produces hydrogen [89–91]. In addition, sulfate-reducing bacteria (SRB) also play an important role in the wastewater treatment system. There was also a high availability of adsorption sites and a high binding strength between EPSs and ciprofloxacin [92]. The EPSs of SRB sludge had a high ratio of proteins to polysaccharides, which contributed to its increased hydrophobicity. Even though they can only survive in anaerobic environments, SRB have been found in a variety of aerobic environments, such as aerobic fixed film and activated sludge, and was used for heavy metals removal [93–94]. Earlier, comparable functional groups in the EPSs of marine bacteria were also discovered [95–96]. Recently, it is demonstrated that the inclusion of microphytes not only stimulated the content of overall EPSs but simultaneously enhanced both varieties and contents of functional groups in the EPSs in their observed samples during the wastewater treatment process [97]. Different bands in rAGS-MTD and mAGS-MTD were likely caused by the samples’ inclusion of metaldehyde. As a result, in addition to bands that indicate the presence of EPSs, other bands indicate the presence of functional groups in metaldehyde and its derivatives in the FTIR spectrum. Since more bands were determined from the spectrum absorption, it makes sense that the addition of molasses wastewater during the enrichment process of mAGS enhanced the number of functional groups in mAGS. For rAGS-MTD and mAGS-MTD, respectively, hydroxyl was found at spectra 3410.50 and 3620.30 cm−1. At bands 2990.65 and 2995.60 cm−1 in rAGS-MTD and mAGS-MTD, respectively, the aliphatic group was identified. The band in rAGS-MTD (2258.04 and 1637.45 cm−1) and mAGS-MTD (2253.64 cm−1) was used to determine the aromatic group. In rAGS-MTD and mAGS-MTD, the ether group was found to be 1375.70 and 1443.0 cm−1. The amino group in rAGS-MTD and mAGS-MTD was identified at 1038.90 and 1039.06 cm−1, respectively. For rAGS-MTD and mAGS-MTD, the FTIR spectrum is shown in Fig. 6.
In summary, this study’s FTIR examination verified the presence of a variety of functional groups in rAGS-MTD and mAGS-MTD, including hydroxyl and carboxyl, as well as aliphatics and aromatic with polar groupings. Their presence was connected to the presence of proteins and carbohydrates in EPSs. The way the group look improves EPSs’capacity to shield biofilms from any dangerous substances while the wastewater is being treated. Since these functional and polar groups connect to proteins and carbohydrates, all the components including carboxyl, hydroxyl, aliphatic, and aromatic in EPSs samples are recorded when FTIR is applied [98–100].
4. ConclusionThe basic objectives of this study have been accomplished, on the whole. First and foremost, contributions of this study, the extracellular polymerase substances (EPSs) of rubber aerobic granular sludge (rAGS) and molasses aerobic granular sludge (mAGS) were effectively chemically extracted, both with and without exposure to metaldehyde. The second contribution is proteins and carbohydrates are the primary biopolymer constituents that were successfully quantified in EPSs for all situations of rAGS and mAGS in our current investigation. In detail, the finding of hydroxyl, carboxyl, and amino groups in the EPSs of rAGS and mAGS, respectively, provided evidence that carbohydrates and proteins were present in those EPSs. These conditions included both the presence and absence of metaldehyde. In addition, the link between the different compositions of EPSs and the presence of various quantities of metaldehyde in the rAGS and mAGS was investigated in depth during this investigation. In conclusion, the results of the research show that produced aerobic granules, such as rAGS and mAGS, are effective in treating metaldehyde when considering the overall number of EPSs that were found. However, this study does have certain limitations. For the process that makes reactivated and modified activated sludge (rAGS and mAGS, respectively), a sample of activated sludge with a good amount of biomass is needed. Nonetheless, the efforts that we put in paid off in the end because we were able to effectively get the necessary samples of activated sludge. When developing aerobic granules, this study is taking into consideration the utilization of wastewater such as rubber wastewater and molasses wastewater. The fact that this research was the first to make use of aerobic granules that were supplemented with molasses is what gives it its unique importance. In addition, demonstrating the presence of EPSs in both the rAGS and the mAGS samples is one of the critical aspects of our study. This study is given additional weight by the demonstration that the presence of metaldehyde led to an increase in the number of EPSs that were produced. Considering that EPSs are chemicals that protect rAGS and mAGS from the damaging effects of pollutants. It is to be hoped that this study will reveal cutting-edge insights into the operation of aerobic granular technology for the treatment of wastewater containing dangerous chemicals, particularly pesticides. This would be an improvement that is very much desired. The total amount of EPSs secreted in rAGS and mAGS in the presence of other detrimental pollutants such as heavy metals and hydrocarbons will be the subject of future studies that will be carried out to ascertain this quantity. In addition, it is recommended that more research be carried out using a variety of synthetic wastewater inputs to feed biomass in a sequencing batch reactor (SBR) to determine the effect that this has on the EPSs that are secreted by the biofilms in the aerobic granules.
AcknowledgmentThe authors would like to extend their sincere gratitude to the Government of Malaysia and the Ministry of Education Malaysia for the Fundamental Research Grant Scheme (FRGS) No. 9003-00386, as well as to Universiti Malaysia Perlis for the financial assistance that was provided through the Postgraduate Academic Activities Fund (PAAF).
NotesAuthor Contributions A.M.S. (A graduate Ph.D. student, now an Independent Scholar) has done the introduction, scientific literature review, methodology, results, discussion, and conclusion, and written the manuscript along with Figures and Tables. F.A.D. (Associate Professor) has reviewed the manuscript. N.I. and S.Y.Y. (Associate Professor) have been co-supervisors for the graduate Ph.D. student. References1. Cheah YT, Chan DJC. A methodological review on the characterization of microalgal biofilm and its extracellular polymeric substances. J. Appl. Microbiol. 2022;132(5)3490–3514. https://doi.org/10.1111/jam.15455
2. Martino PD. Extracellular polymeric substances are a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4(2)274. https://doi.org/10.3934%2Fmicrobiol.2018.2.274
3. Aljerf L, Dehmchi F, Pham VT, Choukaife AE. Polyurethane foam in a reliable method for electrophoretic separation of proteins. Am. Res. J. Chem. 2019;3(1)1–14. https://doi.org/10.21694/2577-5898.19002
4. Can HK, Kavlak S, Gurbuz F, Odabaşı M. Insights into the viscoelastic peculiarities of cyanobacterial extracellular polymeric substance (EPS). J. Polym. Environ. 2022;30(7)3055–3062. https://doi.org/10.1007/s10924-022-02399-0
5. Shao Y, Zhang H, Buchanan I, Mohammed A, Liu Y. Comparison of extracellular polymeric substance (EPS) in nitrification and nitritation bioreactors. Int. Biodeterior. Biodegrad. 2019;143:104713. https://doi.org/10.1016/j.ibiod.2019.06.001
6. Choi OK, Hendren Z, Kim GD, Dong D, Lee JW. Influence of activated sludge derived-extracellular polymeric substance (ASD-EPS) as bio-flocculation of microalgae for biofuel recovery. Algal Res. 2020;45:101736. https://doi.org/10.1016/j.algal.2019.101736
7. Tian X, Shen Z, Han Z, Zhou Y. The effect of extracellular polymeric substances on exogenous highly toxic compounds in biological wastewater treatment: An overview. Bioresour. Technol. Rep. 2019;5:28–42. https://doi.org/10.1016/j.biteb.2018.11.009
8. Fulaz S, Vitale S, Quinn L, Casey E. Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019;27(11)915–926. https://doi.org/10.1016/j.tim.2019.07.004
9. Lee K, Lee S, Lee J, Zhang X, Lee SH. Roles of soluble microbial products and extracellular polymeric substances in membrane fouling. Ng HY, Ng TCA, Ngo HH, Mannina G, Panday A, editorsCurrent developments in biotechnology and bioengineering. Elsevier; 2020. p. 45–79. https://doi.org/10.1016/B978-0-12-819809-4.00003-6
10. Costa OY, Raaijmakers JM, Kuramae EE. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018;9:1636. https://doi.org/10.3389/fmicb.2018.01636
11. Yadav S, Chandra R. Biofilm-mediated bioremediation of pollutants from the environment for sustainable development. Yadav MK, Singh B, editorsNew and future developments in microbial biotechnology and bioengineering: Microbial biofilms. Elsevier; 2020. p. 177–203. http://dx.doi.org/10.1016/B978-0-444-64279-0.00014-1
12. Srivastava A, Seo SH, Ko SR, Ahn CY, Oh HM. Bioflocculation in natural and engineered systems: Current perspectives. Crit. Rev. Biotechnol. 2018;38(8)176–1194. https://doi.org/10.1080/07388551.2018.1451984
13. Ismail WNW, Syah MIAI, Abdul MNH, Bakar NHA, Yusop HM, Samah NA. Adsorption behavior of heavy metal ions by hybrid inulin-teos for water treatment. Civ. Eng. J. 2022;8(9)1787–1798. https://doi.org/10.28991/CEJ-2022-08-09-03
14. Eissa ME, Rashed ER, Eissa DE. Dendrogram analysis and statistical examination for total microbiological mesophilic aerobic count of municipal water distribution network system. HighTech Innov. J. 2022;3(1)28–36. https://doi.org/10.28991/HIJ-2022-03-01-03
15. Jagaba AH, Kutty SRM, Isa MH, et al. Toxic effects of xenobiotic compounds on the microbial community of activated sludge. ChemBioEng. Rev. 2022;9(5)497–535. https://doi.org/10.1002/cben.202100055
16. Mahto KU, Kumari S, Das S. Unraveling the complex regulatory networks in biofilm formation in bacteria and relevance of biofilms in environmental remediation. Crit. Rev. Biochem. Mol. Biol. 2022;57(3)305–332. https://doi.org/10.1080/10409238.2021.2015747
17. Pagliaccia B, Carretti E, Severi M, Berti D, Lubello C, Lotti T. Heavy metal biosorption by extracellular polymeric substances (EPS) recovered from anammox granular sludge. J. Hazard. Mater. 2022;424:126661. https://doi.org/10.1016/j.jhazmat.2021.126661
18. Ma Z, Liu J, Dick RP, et al. Rhamnolipid influences biosorption and biodegradation of phenanthrene by phenanthrene-degrading strain Pseudomonas sp. Ph6. Environ. Pollut. 2018;240:359–367. https://doi.org/10.1016/j.envpol.2018.04.125
19. Timková I, Sedláková-Kaduková J, Pristaš P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Sep 2018;5(4)54. https://doi.org/10.3390/separations5040054
20. Tan Z, Chen J, Liu Y, et al. The survival and removal mechanism of Sphingobacterium changzhouense TC931 under tetracycline stress and its’ ecological safety after application. Bioresour. Technol. 2021;333:125067. https://doi.org/10.1016/j.biortech.2021.125067
21. Flemming HC, Wingender J. Relevance of microbial extracellular polymeric substances (EPSs) - Part II: Technical aspects. Water Sci. Technol. 2001;43(6)9–16. https://doi.org/10.2166/wst.2001.0328
22. Chen R, Sheng Q, Chen S, Dai X, Dong B. The three-stage effect of hydrothermal treatment on sludge physical-chemical properties: Evolution of polymeric substances and their interaction with physicochemical properties. Water Res. 2022;211:118043. https://doi.org/10.1016/j.watres.2022.118043
23. Wang H, Cai WW, Liu WZ, et al. Application of sulfate radicals from ultrasonic activation: Disintegration of extracellular polymeric substances for enhanced anaerobic fermentation of sulfate-containing waste-activated sludge. Chem. Eng. J. 2018;352:380–388. https://doi.org/10.1016/j.cej.2018.07.029
24. Teng J, Wu M, Chen J, Lin H, He Y. Different fouling propensities of loosely and tightly bound extracellular polymeric substances (EPSs) and the related fouling mechanisms in a membrane bioreactor. Chemosphere. 2020;255:126953. https://doi.org/10.1016/j.chemosphere.2020.126953
25. Mahto KU, Vandana , Priyadarshanee M, Samantaray DP, Das S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J. Clean. Prod. 2022;134759. https://doi.org/10.1016/j.jclepro.2022.134759
26. Sarma SJ, Tay JH, Chu A. Finding knowledge gaps in aerobic granulation technology. Trends Biotechnol. 2017;35(1)66–78. https://doi.org/10.1016/j.tibtech.2016.07.003
27. Shi Y, Huang J, Zeng G, et al. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere. 2017;180:396–411. https://doi.org/10.1016/j.chemosphere.2017.04.042
28. Huang L, Li M, Si G, et al. Assessment of microbial products in the biosorption process of Cu (II) onto aerobic granular sludge: Extracellular polymeric substances contribution and soluble microbial products release. J. Colloid Interface Sci. 2018;527:87–94. https://doi.org/10.1016/j.jcis.2018.05.032
29. Sun M, Li WW, Mu ZX, et al. Selection of effective methods for extracting extracellular polymeric substances (EPSs) from Bacillus megaterium TF10. Sep. Purif. Technol. 2012;95:216–221. https://doi.org/10.1016/j.seppur.2012.05.010
30. Ismail SWM, Dahalan FA, Zakaria A, et al. The acute toxicity of the metaldehyde on the climbing perch. E3S Web Conf. EDP Sciences. 2018;34:02031. https://doi.org/10.1051/e3sconf/20183402031
31. Felz S, Al-Zuhairy S, Aarstad OA, Van LMC, Lin YM. Extraction of structural extracellular polymeric substances from aerobic granular sludge. J. Vis. Exp. 2016;115:e54534. https://dx.doi.org/10.3791/54534
32. Adav SS, Lee DJ. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J. Hazard. Mater. 2008;154(1–3)1120–1126. https://doi.org/10.1016/j.jhazmat.2007.11.058
33. Liu H, Fang HH. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002;95(3)249–256. https://doi.org/10.1016/S0168-1656(02)00025-1
34. Frølund B, Palmgren R, Keiding K, Nielsen PH. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996;30(8)1749–1758. https://doi.org/10.1016/0043-1354(95)00323-1
35. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol Chem. 1951;193:265–275. http://dx.doi.org/10.1016/S0021-9258(19)52451-6
36. Masuko T, Minami A, Iwasaki N, Majima T, Nishimura SI, Lee YC. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005;339(1)69–72. https://doi.org/10.1016/j.ab.2004.12.001
37. Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956;28(3)350–356. https://doi.org/10.1021/ac60111a017
38. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2)248–254. https://doi.org/10.1016/0003-2697(76)90527-3
39. Tseng LY. Extracellular polymeric substances (EPS) and their interaction with anthropogenic pollutants during activated sludge wastewater treatment processes [dissertation]. Irvine: Univ. of California; 2012.
40. Nowotny A. Carbohydrate determination by phenol-sulfuric acid. Nowotny A, editorBasic exercises in immunochemistry. Heidelberg: Springer Berlin; 1979. p. 171–173. https://doi.org/10.1007/978-3-642-67356-6_52
41. Nielsen SS. Phenol-sulfuric acid method for total carbohydrates. Nielsen SS, editorFood analysis laboratory manual. Food science texts series. Boston MA: Springer; 2010. p. 47–53. https://doi.org/10.1007/978-1-4419-1463-7_6
42. Aljerf L, Alhaffar I. Salivary distinctiveness and modifications in males with diabetes and Behçet’s disease. Biochem. Res. Int. 2017;2017:1–12. https://doi.org/10.1155/2017/9596202
43. Albalasmeh AA, Berhe AA, Ghezzehei TA. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013;97(2)253–261. https://doi.org/10.1016/j.carbpol.2013.04.072
44. Mandal SM, Paul D. Spectroscopy: Principle, types and microbiological applications. Paul D, Mandal SM, editorsAutomation and basic techniques in medical microbiology. New York: Springer; 2022. p. 49–75. https://doi.org/10.1007/978-1-0716-2372-5_5
45. Akbal FÖ, Akdemir N, Onar AN. FT-IR spectroscopic detection of pesticide after sorption onto modified pumice. Talanta. 2000;53(1)131–135. https://doi.org/10.1016/S0039-9140(00)00380-5
46. Wang Y, Gong X, Huang D, Yan S, Zhang J. The binding effect and photooxidation on oxytetracycline with algal extracellular polymeric substances and natural organic matter. Chemosphere. 2022;307:135826. https://doi.org/10.1016/j.chemosphere.2022.135826
47. Sheng GP, Xu J, Li WH, Yu HQ. Quantification of the interactions between Ca2+, Hg2+ and extracellular polymeric substances (EPS) of sludge. Chemosphere. 2013;93(7)1436–1441. https://doi.org/10.1016/j.chemosphere.2022.135826
48. Zhu L, Zhou J, Lv M, Yu H, Zhao H, Xu X. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere. 2015;121:26–32. https://doi.org/10.1016/j.chemosphere.2014.10.053
49. Moradali MF, Rehm BH. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020;18(4)195–210. https://doi.org/10.1038/s41579-019-0313-3
50. Zhang L, Feng X, Zhu N, Chen J. Role of extracellular protein in the formation and stability of aerobic granules. Enzyme Microb. Technol. 2007;41(5)551–557. https://doi.org/10.1016/j.enzmictec.2007.05.001
51. Corsino SF, Campo R, Di BG, Torregrossa M, Viviani G. Aerobic granular sludge treating shipboard slop: Analysis of total petroleum hydrocarbons loading rates on performances and stability. Process Biochem. 2018;65:164–171. https://doi.org/10.1016/j.procbio.2017.11.005
52. Deng S, Wang L, Su H. Role and influence of extracellular polymeric substances on the preparation of aerobic granular sludge. J. Environ. Manage. 2016;173:49–54. https://doi.org/10.1016/j.jenvman.2016.03.008
53. Cui P, Ge J, Chen Y, Zhao Y, Wang S, Su H. The Fe3O4 nanoparticles-modified mycelium pellet-based anaerobic granular sludge enhanced anaerobic digestion of food waste with high salinity and organic load. Renew. Energ. 2022;185:376–385. https://doi.org/10.1016/j.renene.2021.12.050
54. Zhu L, Lv M, Dai X, Xu X, Qi H, Yu Y. Reaction kinetics of the degradation of chloroanilines and aniline by aerobic granule. Biochem. Eng. J. 2012;68:215–220. https://doi.org/10.1016/j.bej.2012.07.015
55. Sardar UR, Bhargavi E, Devi I, Bhunia B, Tiwari ON. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018;199:353–364. https://doi.org/10.1016/j.carbpol.2018.07.037
56. Song T, Zhang X, Li J. The formation and distinct characteristics of aerobic granular sludge with filamentous bacteria in low strength wastewater. Bioresour. Technol. 2022;127409. https://doi.org/10.1016/j.biortech.2022.127409
57. Jiang Y, Shang Y, Zhang W, Zhang X, Li J, Shao S. Assessing the effect of SiO2 and TiO2 nanoparticles on granule stability and microbial community shift in aerobic granular sludge process. Chemosphere. 2022;307:135677. https://doi.org/10.1016/j.chemosphere.2022.135677
58. Zhang Z, Cao R, Jin L, et al. The regulation of N-acyl-homoserine lactones (AHLs)-based quorum sensing on EPS secretion via ATP synthetic for the stability of aerobic granular sludge. Sci. Total Environ. 2019;673:83–91. https://doi.org/10.1016/j.scitotenv.2019.04.052
59. Flemming HC, Baveye P, Neu TR, et al. Who put the film in biofilm? The migration of a term from wastewater engineering to medicine and beyond. NPJ Biofilms and Microbiomes. 2021;7(1)1–5. https://doi.org/10.1038/s41522-020-00183-3
60. Yu HQ. Molecular insights into extracellular polymeric substances in activated sludge. Environ. Sci. Technol. 2020;54(13)7742–7750. https://doi.org/10.1021/acs.est.0c00850
61. Davey ME, O’toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64(4)847–867. https://doi.org/10.1128/MMBR.64.4.847-867.2000
62. Cheng Q, Jiang Y, Jin Z, et al. Enhanced excretion of extracellular polymeric substances associated with nonylphenol tolerance in Dictyosphaerium sp. J. Hazard. Mater. 2020;395:122644. https://doi.org/10.1016/j.jhazmat.2020.122644
63. Yin W, Wang Y, Liu L, He J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019;20(14)3423. https://doi.org/10.3390/ijms20143423
64. Lotti T, Carretti E, Berti D, Martina MR, Lubello C, Malpei F. Extraction, recovery and characterization of structural extracellular polymeric substances from anammox granular sludge. J. Environ. Manage. 2019;236:649–656. https://doi.org/10.1016/j.jenvman.2019.01.054
65. Miao L, Zhang Q, Wang S, et al. Characterization of EPS compositions and microbial community in an anammox SBBR system treating landfill leachate. Bioresour. Technol. 2018;249:108–116. https://doi.org/10.1016/j.biortech.2017.09.151
66. Iorhemen OT, Hamza RA, Zaghloul MS, Tay JH. Aerobic granular sludge membrane bioreactor (AGMBR): Extracellular polymeric substances (EPS) analysis. Water Res. 2019;156:305–314. https://doi.org/10.1016/j.watres.2019.03.020
67. Adav SS, Lee DJ, Lai JY. Effects of aeration intensity on formation of phenol-fed aerobic granules and extracellular polymeric substances. Appl. Microbiol. Biotechnol. 2007;77(1)175–182. https://doi.org/10.1007/s00253-007-1125-3
68. Nancharaiah YV, Reddy GKK. Aerobic granular sludge technology: Mechanisms of granulation and biotechnological applications. Bioresour. Technol. 2018;247:1128–1143. http://dx.doi.org/10.1016/j.biortech.2017.09.131
69. Seviour T, Derlon N, Dueholm MS, et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019;151:1–7. https://doi.org/10.1016/j.watres.2018.11.020
70. McSwain BS, Irvine RL, Hausner M, Wilderer PA. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ. Microbiol. 2005;71(2)1051–1057. https://doi.org/10.1128/AEM.71.2.1051-1057.2005
71. Dash DM, Osborne WJ. Rapid biodegradation and biofilm-mediated bioremoval of organophosphorus pesticides using an indigenous Kosakonia oryzae strain-VITPSCQ3 in a vertical-flow packed bed biofilm bioreactor. Ecotoxicol. Environ. Saf. 2020;192:110290. https://doi.org/10.1016/j.ecoenv.2020.110290
72. Wei D, Wang Y, Wang X, et al. Toxicity assessment of 4-chlorophenol to aerobic granular sludge and its interaction with extracellular polymeric substances. J. Hazard. Mater. 2015;289:101–107. https://doi.org/10.1016/j.jhazmat.2015.02.047
73. Guo H, van LJB, De KM. Digestibility of waste aerobic granular sludge from a full-scale municipal wastewater treatment system. Water Res. 2020;173:115617. https://doi.org/10.1016/j.watres.2020.115617
74. Meng F, Huang W, Liu D, et al. Application of aerobic granules-continuous flow reactor for saline wastewater treatment: Granular stability, lipid production and symbiotic relationship between bacteria and algae. Bioresour. Technol. 2020;295:122291. https://doi.org/10.1016/j.biortech.2019.122291
75. Gupta P, Pruthi PA, Pruthi V. Role of exopolysaccharides in biofilm formation. Rathinam NK, Sani RK, editorsIntroduction to biofilm engineering. ACS Publications; 2019. p. 17–57. 10.1021/bk-2019-1323.ch002
76. Wang XC, Chen ZL, Kang J, Zhao X, Shen JM, Yang L. The key role of inoculated sludge in fast start-up of sequencing batch reactor for the domestication of aerobic granular sludge. J. Environ. Sci. 2019;78:127–136. https://doi.org/10.1016/j.jes.2018.08.008
77. Nancharaiah YV, Sarvajith M, Mohan TK. Aerobic granular sludge: The future of wastewater treatment. Curr. Sci. 2019;117(3)395–404. https://doi.org/10.18520/cs%2Fv117%2Fi3%2F395-404
78. Hao L, Guo Y, Byrne JM, et al. Binding of heavy metal ions in aggregates of microbial cells, EPS and biogenic iron minerals measured in-situ using metal-and glycoconjugates-specific fluorophores. Geochim. Cosmochim. Acta. 2016;180:66–96. https://doi.org/10.1016/j.gca.2016.02.016
79. Szilveszter S, Ráduly B, Ábrahám B, Lányi S. In situ imaging of biopolymers and extracellular enzymes in activated sludge flocs of a municipal wastewater treatment plant. J. Chem. Technol. Biotechnol. 2013;88(7)1295–1304. https://doi.org/10.1002/jctb.3975
80. Tong CY, Derek CJC. Membrane surface roughness promotes rapid initial cell adhesion and long term microalgal biofilm stability. Environ. Res. 2022;206:112602. https://doi.org/10.1016/j.envres.2021.112602
81. Sonawane JM, Rai AK, Sharma M, Tripathi M, Prasad R. Microbial biofilms: Recent advances and progress in environmental bioremediation. Sci. Total Environ. 2022;153843. https://doi.org/10.1016/j.scitotenv.2022.153843
82. Shi Y, Huang J, Zeng G, et al. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere. 2017;180:396–411. https://doi.org/10.1016/j.chemosphere.2017.04.042
83. Karygianni L, Ren Z, Koo H, Thurnheer T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020;28(8)668–681. https://doi.org/10.1016/j.tim.2020.03.016
84. Alshaarani F, Alaisami RM, Aljerf L, Jamous IA, Elias K, Jaber A. An auxiliary factor for increasing the retention of short abutments. Heliyon. 2019;5(10)e02674. https://doi.org/10.1016/j.heliyon.2019.e02674
85. Nicolaus B, Kambourova M, Oner ET. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010;31(10)1145–1158. https://doi.org/10.1080/09593330903552094
86. Hu Q, Zhou N, Rene ER, Wu D, Sun D, Qiu B. Stimulation of anaerobic biofilm development in the presence of low concentrations of toxic aromatic pollutants. Bioresour. Technol. 2019;281:26–30. https://doi.org/10.1016/j.biortech.2019.02.076
87. Aljerf L, Nadra R. Developed greener method based on MW implementation in manufacturing CNFs. Int. J. Nanomanuf. 2019;15(3)269–289. https://doi.org/10.1504/IJNM.2019.100461
88. Maddela NR, Zhou Z, Yu Z, Zhao S, Meng F. Functional determinants of extracellular polymeric substances in membrane biofouling: Experimental evidence from pure-cultured sludge bacteria. Appl. Environ. Microbiol. 2018;84(15)1–17. https://doi.org/10.1128%2FAEM.00756-18
89. Gu W, Wang L, Liu Y, et al. Anammox bacteria enrichment and denitrification in moving bed biofilm reactors packed with different buoyant carriers: Performances and mechanisms. Sci. Total Environ. 2020;719:137277. https://doi.org/10.1016/j.scitotenv.2020.137277
90. Fung LW, Lutpi NA, Shian WY, Tengku ITN. Characteristics of biofilm formation from mixed microflora at mesophilic and thermophilic fermentative hydrogen production. ESTEEM Acad. J. 2017;13:24–34. https://ir.uitm.edu.my/id/eprint/28781/1/AJ_LAM%20WAI%20FUNG%20EAJ%2017.pdf
91. Lutpi NA, Jahim JM, Mumtaz T, Abdul PM, Nor MTM. Physicochemical characteristics of attached biofilm on granular activated carbon for thermophilic biohydrogen production. RSC Adv. 2015;5(25)19382–19392. http://dx.doi.org/10.1039/c4ra12730g
92. Zhang H, Jia Y, Khanal SK, Lu H, Fang H, Zhao Q. Understanding the role of extracellular polymeric substances on ciprofloxacin adsorption in aerobic sludge, anaerobic sludge, and sulfate-reducing bacteria sludge systems. J. Environ. Sci. Technol. 2018;52(11)6476–6486. https://doi.org/10.1021/acs.est.8b00568
93. Zhang Z, Zhang C, Yang Y, et al. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. J. Clean. Prod. 2022;134109. https://doi.org/10.1016/j.jclepro.2022.134109
94. Xu YN, Chen Y. Advances in heavy metal removal by sulfate-reducing bacteria. Water Sci. Technol. 2020;81(9)1797–1827. https://doi.org/10.2166/wst.2020.227
95. Kumar MA, Anandapandian KTK, Parthiban K. Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz. Arch. Biol. Technol. 2011;54(2)259–265. https://doi.org/10.1590/S1516-89132011000200006
96. Iyer A, Mody K, Jha B. Characterization of an exopolysaccharide produced by a marine Enterobacter cloacae. Indian J. Exp. Biol. 2005;43:467–471. https://nopr.niscpr.res.in/bitstream/123456789/23131/1/IJEB%2043(5)%20467-471.pdf
97. Tang CC, Wang R, Wang TY, He ZW, Tian Y, Wang XC. Characteristic identification of extracellular polymeric substances and sludge flocs affected by microalgae in microalgal-bacteria aggregates treating wastewater. J. Water Process. Eng. 2021;44:102418. https://doi.org/10.1016/j.jwpe.2021.102418
98. Wu B, Wang H, Li W, Dai X, Chai X. Influential mechanism of water occurrence states of waste-activated sludge: potential linkage between water-holding capacity and molecular compositions of EPS. Water Res. 2022;213:118169. https://doi.org/10.1016/j.watres.2022.118169
99. Wang L, Chen W, Song X, et al. Cultivation substrata differentiate the properties of river biofilm EPS and their binding of heavy metals: A spectroscopic insight. Environ. Res. 2020;182:109052. https://doi.org/10.1016/j.envres.2019.109052
100. Chen YP, Zhang P, Guo JS, Fang F, Gao X, Li C. Functional groups characteristics of EPS in biofilm growing on different carriers. Chemosphere. 2013;92(6)633–638. https://doi.org/10.1016/j.chemosphere.2013.01.059
Fig. 3Concentrations of EPSs, protein, and carbohydrates in spiked-metaldehyde rAGS and spiked-metaldehyde mAGS. 
Fig. 5Spectrum of FTIR for rAGS and mAGS with three different groups (a) hydroxyl groups, (b) carboxyl groups, and (c) amino group. 
Fig. 6Spectrum of FTIR for rAGS-MTD and mAGS-MTD with three different groups detected (a) hydroxyl groups, (b) carboxyl groups, and (c) amino group. 
Table 1Results of a Statistical Analysis Granulation/Augmentation Days vs Total EPSs Concentration of rAGS and mAGS
Table 2Statistical Results of the Relationship between Total EPSs Concentrations and Spiked-metaldehyde rAGS and Spiked-metaldehyde mAGS
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||