AbstractThe degradation of methyl orange (MO) and neutral red (NR) was studied by the direct electrooxidation using nitrogen and sulfur co-doped graphene (NSG) supported antimony doped tin oxide (SnO2-Sb). Sol-gel and microwave technics were used to prepare the material (Ti/SnO2-Sb-NSG). Raman, XRD, FTIR and TGA/DSC analyses help to confirm the coating of titanium substrate with the SnO2-Sb-NSG film. Linear sweet voltammetry results show that Ti/SnO2-Sb-NSG material possesses high oxygen overvoltage. Three independent variables including electrolysis time, current density and dye concentration on the performance of the anodic oxidation system was modeled using the Box-Behnken Design. The optimum conditions for MO and NR degradation were for current density 18 mA/cm2 and 58 mA/cm2, electrolysis time 6 h and 6 h and dye concentration 29 mg/L and 82 mg/L, respectively. However, based on these optimums, MO was degraded at 98.71 % while NR was just degraded at 82.7%. Based on the intermediate compounds, the degradation mechanism of MO at the Ti/SnO2-Sb-NSG anode was proposed. Ti/SnO2-Sb-NSG electrode showed a higher efficient electrocatalytic performance for NR and MO degradation than that of Ti/Sb-SnO2 or Ti/Sb-SnO2-NG electrodes. Furthermore, the lifetime of Ti/SnO2-Sb-NSG was about 31.6 h while Ti/SnO2-Sb electrode was 9.0 h.
Graphical Abstract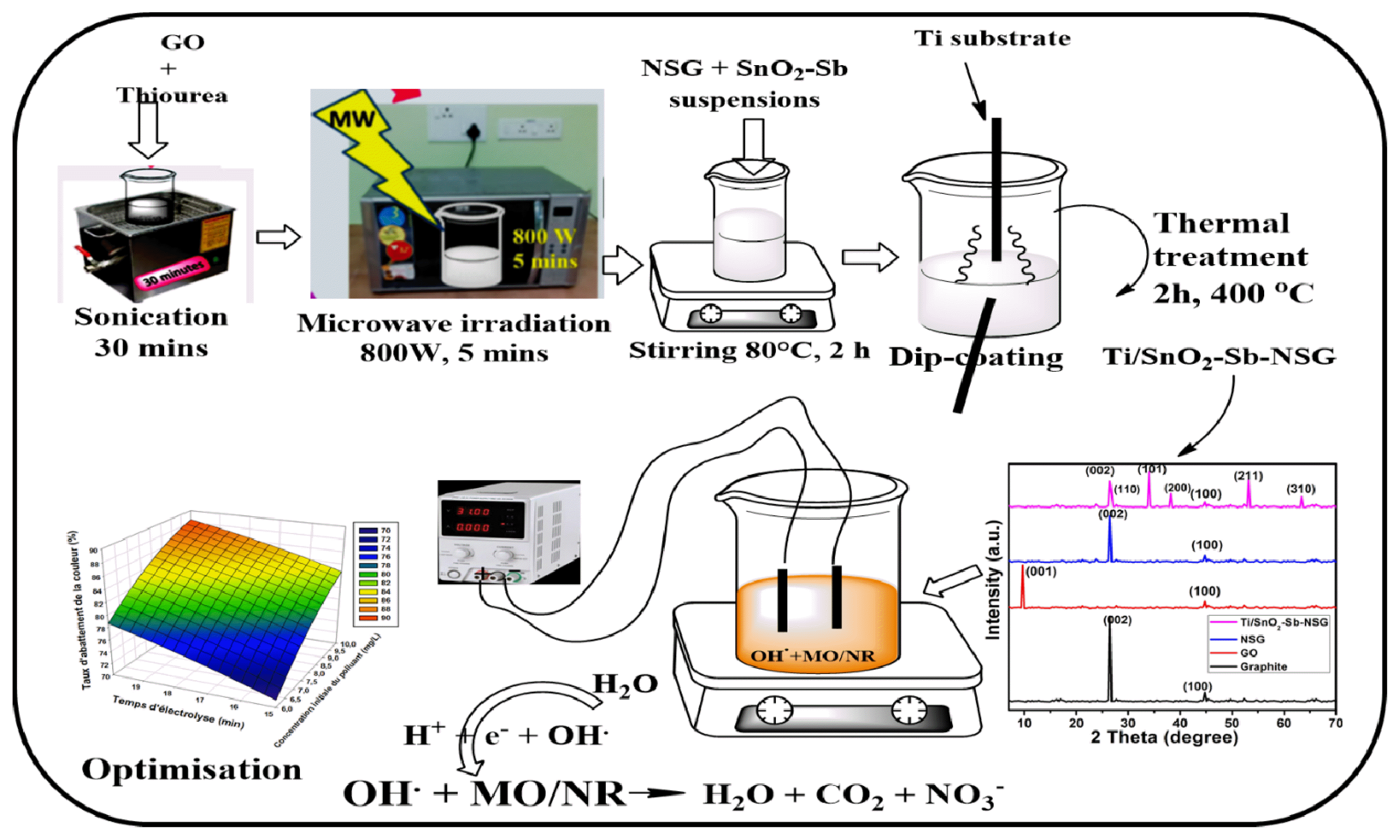
1. IntroductionVarious industries use some dyes as coloring agents. Serious environmental and health problems arise due to the discharge of synthetic dyes as effluent. Methyl orange is classified in the Anionic dye group or in the class of Azo dye meanwhile, neutral red (NR) is classified as a Cationic dye. They are being used in various applications including biology, chemistry, medical sciences and dyeing process. Some dyes like MO and NR release Hydrogen chlorides, Nitrogen oxide, Carbon monoxide, and Carbon dioxide which are toxic and can cause severe health problems for humans and animals [1]. Long-term exposure to these dyes leads to hypertension, nausea, anemia, and vomiting [2]. In order to destroy these organic dyes, various treatment technologies have been proposed such as nanofiltration [3], adsorption [4,5], biological [6,7], oxidation with chlorine [8,9], electrocoagulation [10]. However, these methods are not efficient for the mineralization of organic pollutants because they lead to a secondary pollution which needs further separation or treatment [11]. Characterized by high efficiency, low energy consumption, simplicity and environmental friendliness, electrochemical advanced oxidation processes (EAOPs) of wastewater attracted much attention [12]. In EAOPs, the main oxidant is the hydroxyl radical (•OH) which can be generated by direct electrochemistry also called direct anodic oxidation (DAO) or indirectly through electrochemically generation also called indirect anodic oxidation or electro-Fenton process. Among these processes, DAO is the main EAOPs. The DAO is usually used for the incineration of organic pollutants in wastewater. Recently, many studies have been carried out on both real and synthetic wastewater, being proved its robustness and effectiveness [13–15].
Both process efficiency and selectivity are highly influenced by the nature of the electrode material. However, in the literature it is reported that several type anodes favored complete combustion to CO2, while others favored selective and partial degradation of organic pollutants. In order to interpret these observations, they proposed a comprehensive model for the oxidation of organic pollutants at the metal oxide electrodes with simultaneous oxygen evolution [16,17]. As shown in Eq. (1), the first step in oxygen transfer reaction is the discharge of water molecules to form adsorbed hydroxyl radicals:
According to the next steps, we distinguish two classes of electrodes commonly called as non-active and active anodes:
At the “active” electrodes e.g. RuO2, platinum, IrO2, graphite, where higher oxidation states are available on the electrode surface, adsorbed hydroxyl radicals may interact with the anode, forming the so-called higher oxide [18].
At the “non-active” electrodes e.g. antimony-doped tin oxide, lead dioxide, or boron-doped diamond, where the formation of a higher oxide is excluded, hydroxyl radicals, called physisorbed “active oxygen”, may assist the non-selective oxidation of organic compounds, which may result in complete combustion to CO2.
During electrolysis, the main operational parameters are current density, electrolysis time, type and concentration of electrolyte, pH, electrode material, etc. Among these parameters, anode material plays the most important role in the performance of DAO process [19]. However, it is important to note that, when active anodes are employed the pollutants are incomplete oxidized due to its low oxygen evolution potential while the non-active anodes lead to the complete mineralization of pollutants to CO2, H2O and minerals due to its high oxidation potential. Among non-active anodes, SnO2 has presented much attention due of its physicochemical stability, good electrical conductivity, high natural abundance, and lack of toxicity [20]. However, due to its wide bandgap (3.6eV) SnO2 exhibited low electrocatalytic activity. In order to reduce the band gap and to improve its ability to degrade organic pollutants, some transition metals have been employed such as Pb, Sb, Ir, Ru etc. [21,22]. Among them, antimony doped tin oxide (SnO2-Sb) has gained much attention and represented a good choice due to its high ability to produce hydroxyl radical, its chemical inertness, high oxygen evolution potential, high electrical conductivity, low cost and environmental friendly [23]. Nevertheless, the relative short life service prevents its practical applications [24]. Recently, the intensive researches have demonstrated that Ti/SnO2 doping with antimony has a high efficiency for the elimination of organic compounds in aqueous solution [25]. Several researchers have done the modification of tin oxide anodes. Electrochemical deposition method was employed by Dalia to develop tin oxide anode by adding platinum (Ti/Pt/SnO2-Sb2O4). The material was used to degrade real effluents, sanitary landfill leachates, and humic acid in aqueous milieu. She showed that compared to other anode materials like BDD and Ti/Pt/PbO2, Ti/Pt/SnO2-Sb2O4 electrode proved to be an excellent alternative, although they were not so efficient in the removal of the organic matter [26]. Dusmant et al. [24] have studied the electrochemical degradation of triclosan with Ti/SnO2-Sb/Ce-PbO2. They have showed that the degradation efficiency attained 99.9% during 5 min of electrolysis and this material can be employed preliminary for rapid degradation of triclosan in wastewater. Thus, the incorporation of Pt, Ir in the tin oxide anodes can greatly increase the lifetime service [27], but the addition of Ir leads to the decrease of the oxygen evolution potential and also reduces the efficiency for wastewater treatment [28]. Furthermore, in some electronic devices such as solar cells, fuel cells, sensors, supercapacitors, and other optoelectronic devices, SnO2-SbxOy coatings are also used [29].
Nowadays, doping carbon nanomaterials into oxide layers of tin oxide during their formation has become a new highlight for the modification of SnO2 electrodes. Feng et al. [30] used carbon nanotube modified Ti/SnO2-Sb electrode and showed that the introduction of carbon nanotubes gives rise to the formation of compact active layer, increases the stability of the electrode and the active sites for electrocatalytic oxidation. Duan et al. [31] have reported that nitrogen doped graphene nanosheet modified Ti/SnO2-Sb electrode possesses improved electrocatalytic activity and stability.
Recently, graphene as carbon-based material has drawn an amount of attention as a promising candidate for wide applications in catalysis. This is may be due to its physical and chemical properties and a unique two-dimensional monolayer structure [31–33]. Furthermore, some works reported that graphene possesses numerous advantages such as superior conductivity, good mechanical durability and high surface area. These properties make it particularly feasible to be use as support for enhancing electrocatalytic properties of electrode materials used in EAOPs [34–37]. Graphene doped with non-metal elements such as Nitrogen, Phosphorous, Sulfur, Boron or Selenium atoms have been identified as the most popular choice, due to its excellent electrocatalytic performance and relatively low costs [38]. Mono-heteroatom doped carbon, especially nitrogen doped graphene carried out enhancement of electrocatalytic activity, enlarged surface area and enriched high nitrogen doping content [39]. Additionally, sulfur-doped graphene prepared by hydrothermal method also exhibited excellent electrocatalytic properties [40]. Recently, graphene doped with dual or multi-heteroatoms have also been developed. Graphene co-doped with two different heteroatoms presents good properties because, doping elements can create new-electron-neutral sites, change the distribution of electron density, and improve the electron spin density due to their carbon system [38,41]. For heteroatoms doping, multiple heteroatoms may take synergistic effect to improve chemical properties of carbon materials [42]. In literature, some technics have been reported for the synthesis of graphene-based materials. The widely used are the chemical vapor deposition, thermal pyrolysis, hydrothermal, plasma and supercritical fluid processing [43–45]. Despite their large utilization, it has been reported that these methods lead to the generation of toxic compounds such as carbon disulfide and nitro benzylamine [46]. Recently, some works report the use of microwave irradiation as new method which present a fast speed, superior efficiency, low cost and environmentally friendly technic for the synthesis of co-doped graphene [47–49]. This method is simple and can simultaneously accomplish reduction and co-doping of nitrogen and sulfur [34].
Recent studies, researches involving the removal of OM and/or NR from water by direct anodic oxidation with Nb/PbO2 [50], with Ti/IrO2-SnO2-Sb2O5 [51] with RuOx-MnOx-CoOx [52], Fenton process [53] and adsorption with activated carbon [54] are reported. At the limit of our knowledge, no work has been found reporting the use of antimony doped tin oxide-based materials to the mineralization and optimization of MO and NR. In our previous work, we reported the degradation of MO dye on graphite electrode and manganese oxide coated on graphite as electrode using sodium chloride as electrolyte then we obtained a maximum rate of degradation up to 77,0% [55,56]. Thus, MO was partially removed from aqueous solution this can be due to the fact that graphite has shown low stability in acidic medium which led to the low degradation of MO. Herein, SnO2-Sb electrodes were modified by using nitrogen and sulfur co-doped graphene (NSG). However, in the environmental field especially electrochemical wastewater treatment, the application of graphene is not widely reported.
The optimization of operating parameters of EAOPs for the treatment of organic pollutants could be done by the mean of numerous statistically designed experimental models. Thus, response surface methodology (RSM) is a very useful tool that reduces the number of experimental runs needed to evaluate multiple parameters and their interaction. RSM using Box-Behnken design (BBD) can provide the improvement or optimization process. BBD is a quadratic model which contains statistical and mathematical techniques useful for modeling and analysis of problems in which a response is influenced by several independent variables. BBD has been applied to optimize and model different treatment methods for dyeing effluents such as photocatalysis [57], membrane process [58], electrochemical oxidation [59].
In this manuscript, we report an easy synthesis of antimony doped tin oxide supported with nitrogen and sulfur co-doped graphene (Ti/SnO2-Sb-NSG) electrode material via microwave and sol-gel approaches and the optimization of the degradation of MO and NR by direct anodic oxidation.
2. Materials and Methods2.1. Chemical ReagentsTitanium plates (99.6%) were purchased from BaoTi Co. Ltd, China. All the other chemicals including tin chloride (SnCl4.2H2O, 99%), antimony potassium tartrate (98%), graphite (99%), oxalic acid (99%), sodium hydroxide (99%), sulfuric acid (99%), hydrochloric acid (99%), thiourea (99%), phosphoric acid (99%), potassium permanganate (99%) and hydrochloric acid (99%) were purchased from Merck. All the reagents were analytical grade and used without further purification. The chemical used as model pollutant and electrolytes were methyl orange (99.9%) and neutral red (99.9%) from Aldrich, sodium sulfate, 99% and sodium chloride, 99% from Merck. Deionized water was used during the experimental process.
2.2. Electrode Preparation2.2.1. Titanium pretreatmentTitanium plates (1mm thickness, 99.6% purity) were used as substrate to prepare the electrode materials. Titanium plates with 20 mm wide and 20 mm long were previously polished mechanically with a silicon carbide paper, degreased in a 10 % NaOH solution at 85 for 1 h, and etching in a 10 % oxalic acid solution at 85 for 1 h. Then pretreated Ti substrates were washed with deionized water, dried with a hot air blown and then stored in a vacuum sealed desiccator before being coated with the NG or NSG supported antimony doped tin oxide film.
2.2.2. Graphene oxide (GO) preparationGO was prepared by the oxidation of synthetic graphite following Hummers process with a slight modification [39]. An amount of KMnO4 (18.0 g) was measured and grind into fine powder using a mortar and pestle. It was mixed with graphite powder (3.0 g). Then 360 mL of H2SO4 and 40 mL of H3PO4 were mixed and stirred for 15 minutes in another beaker. The mixture of KMnO4 and graphite was added to the latter solution in small volumes while stirring. Then, the mixture was kept for stirring at 70 for 16 hours to give a brown solution. In the next step, the reaction was continued by placing the solution in an ice-cold bath and 14 mL of H2O2 was added drop-wise to the solution which turns to yellow. Then, 500 mL of distilled water was added in the mixture which was transferred into a separating funnel. The upper layer was decanted after 2 hours and 500 mL of distilled water was added again. This process was repeated thrice and the solution was subsequently washed with 5% HCl (10 times), centrifuged with distilled water (22 times) and washed with ethanol (10 times) at 3500–4000 rpm. After these washings, the precipitate was dried in an oven at 105 for 10 h. The obtained black colored graphene oxide material was crushed and ground into fine powder.
2.2.3. Nitrogen doped graphene and Nitrogen-Sulfur co-doped graphene preparationsNitrogen-sulfur co-doped graphene (NSG) was prepared by one-pot assisted microwave method. Distilled water (10 mL) was used to disperse GO (0.1 g). This solution was sonicated for 30 minutes. Simultaneously, 0.03 g of thiourea was also dispersed in 10 mL of distilled water, followed by 30 minutes sonication. Afterwards, the above two solutions were mixed together and kept for sonication for about 30 minutes. The solution was then irradiated in a microwave oven (IFB, 800W) for 4 minutes. It was cooled to room temperature and then centrifuged with distilled water for 3 times at 3500–4000 rpm. The remaining precipitate was collected and dried in a vacuum oven at 105 for 10 h. The Nitrogen doped graphene was prepared as reported above, except the addition urea instead thiourea with the same amount [34].
2.2.4. Preparation of SnO2-Sb-NSG solThe antimony doped tin oxide (SnO2-Sb) coating, which ought to be deposited onto pretreated titanium substrate, was prepared by sol-gel method following the dip-coating and thermal treatment of the outer layer. The tin precursor solution was prepared by dissolving 3.4 g SnCl2·4H2O into 75 mL ethyl alcohol and reflowing at 80 for 2 h. Antimony precursor solution was prepared by dissolving antimony potassium tartrate (0.889 g) into 50 mL ethyl alcohol and reflowing at 80 for 1 hour. Then, both precursor solutions were mixed uniformly and reflowed at 80 for 2 h. Finally, the resulting solution was aged in a 40 water bath to obtain a flavescent sol precursor. For the preparation of Sb-SnO2-NSG solution, 17.3 mg as-prepared NSG was mixed into 20 mL methyl glycol until a homogeneous suspension was obtained. Then the resulted solution was added into the above prepared solution and the mixture was stirred for 4 h at room temperature. Then Sb-SnO2-NSG solution obtained and used for dip-coating. The Sb-SnO2-NG solution was prepared as reported above, except the addition NSG instead NG. The solutions were evaporated to 50 mL at 83 and were ultrasonically dispersed for 10 min. The gel was dried in an oven at 150 for 5 h [60].
2.2.5. Obtention of Ti/SnO2-Sb-NSGTi/SnO2-Sb-NSG electrodes were prepared using sol-gel route followed dip coating method. Before dip coating, the above sol was stirred for 10 min. Dip coater was used to performed the electrode materials. Thus, the pretreated Ti plates were dipped and coated in the sol precursor solution using a dip coater. In addition, the coated Ti plate was dried at ambient temperature for 10 min, then dried at 105 for 10 min, and precalcined at 400 for 5 min in a muffle furnace. These procedures were repeated eight, twelve, sixteen, and twenty times. Finally, the coated Ti plate was calcined at 400 for 2 h to obtain Ti/SnO2-Sb-NSG. The procedure of the preparation of Ti/Sb-SnO2-NG was the same as mentioned above, except the addition of NG instead of NSG, while Ti/Sb-SnO2 material was prepared through the above process without adding NG.
2.3. Characterization of the Electrodes2.3.1. Physicochemical characterizationsPowder X-ray diffraction (XRD) characterization has been used to determine the grain size, phase purity and, lattice space increment and decrease, before and after doping and reduction of carbon materials. It is also often used to study the crystal defect of graphite, GO, NS-co-doped graphene and Ti/SnO2-Sb-NSG materials. The XRD patterns of all the samples were measured using Bruker D8 Advance X-ray Diffractometer with Cu Kα (λ=1.5405 Å) radiation in the 2θ range from 5 to 70°. Fourier Transform infrared (FT-IR), Bruker spectrometer (Model no TENSOR 27) was used to determine the functional groups located in GO and N-doped graphene. Potassium bromide (KBr) was mixed with the sample to make the pellet and the spectra were recorded from 400 to 4000 cm−1. Raman Laser microscope (LabRam HR, Horiba) with 633 nm wavelength was used to carry out Raman measurements. It was used to ascertain the defects band and the heteroatom doping. The spectra were recorded from 100 to 3000 cm−1. To determine the best calcination temperature, the gel was characterized by Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). TGA (TGA, Q600) and DSC (Setaram, DSC 131 evo, caluire) were performed under a nitrogen atmosphere from 50 to 700 at a heating rate of 10/min. Atomic force microscopy was also used to characterize the samples (AFM, Agilent technologies 5500 AFM instrument using the tapping mode at a scanning rate of 0.5 Hz) and field emission scanning electron microscopy (FE-SEM, Carl Zeiss Supra 55VP microscope equipped with an accelerating voltage of 0.1 – 30 kV) for determining the state of the surface morphology and acquiring the morphology of the as-prepared material.
2.3.2. Electrochemical measurementsDuring electrochemical experiments, the stability of the electrode material is one of the most important factors considered for the choice of an anode material. The accelerated lifetime tests can be used to measure this property. A 0.5 M H2SO4 solution was used as the electrolyte. The as-prepared electrode (effective exposed area of 4 cm2) served as the working electrode and one copper plate was employed as the counter electrode. A constant anodic current density of 100 mA/cm2 was applied. Thus, when the cell voltage reached to 5 V the working electrode was considered deactivated. Linear sweet voltammetry tests using a standard three-electrode cell with 1M Na2SO4 as the electrolyte were carried out. The counter electrode was platinum plate (1 cm2), the reference electrode was Ag/AgCl/saturated KCl electrode and the prepared materials served as the working electrode. All electrochemical tests were conducted on the CHI 660D electrochemical workstation (Shanghai CH Instrument Company, China). Linear sweep voltammograms were acquired with the simultaneous iR compensation provided using an electrochemical work station at a sweep rate of 5 mVs−1.
2.4. Apparatus and Analytical ProceduresElectrochemical degradation experiments were performed in 100 mL of synthetic solutions containing MO or NR with 0.05 M Na2SO4. The electrochemical oxidation of MO or NR was performed in a bath reactor equipped with two electrodes that is Ti/SnO2-Sb-NSG as anode and carbon felt as cathode in conjunction with an adjustable power supply unit LW LONGWEI LK-K30100 (Fig. 1). The inter-electrode spacing was fixed at 10 mm. The duration of all the electrolysis experiments was varying. The reactor was placed on a magnetic stirrer (MIVAR MAGNETIC STIRRER) mixing its content during the electrolysis in order to maximize mass transport. All the experiments were performed at pH 3 (using a PCE-PHD pH meter) because some studies have been carried out to optimize the pH during DAO of reactive dyes, and the results have shown that low pH lead to the high oxidation of organic matter [61,62]. In addition, low acidic medium favors the generation of hydroxyl radicals and hydrogen peroxide.
The closed reflux titrimetric method with hexahydrate ferrous ammonium sulfate as the titrant was used to evaluate the chemical oxygen demand (COD) (mgO2/L) of the synthetic effluent. The samples were oxidized by dichromate in acidic solution with the heater apparatus (Hachi, USA) at 150°C for 2 h [63]. The COD removal was calculated using Eq. (5):
Where COD0 and CODt are the COD solution values at initial and t time of the electrolysis, respectively.
2.5. Experimental Design and Process OptimizationResponse surface methodology (RSM) is one of the most widely used methods in the design of experiments for wastewater treatment processes [64]. By using Box-Behnken design (BBD) method, 18 experiments (including 6 repetitions at the central point) were designed. The factors were current density (X1), electrolysis time (X2) and initial dye concentration (X3). For each factor, 3 levels were defined, designed by the codes: −1, 0, and +1 and given in Table S.1. The optimization of parameters was designed for the determination of the COD removal percentage (%) for each pollutant. In order to study the correlation between the responses and the variables, the second-order polynomial model (Eq. (6)) was applied [65]. Analysis of variance (ANOVA) was done using the statistically software MINITAT 18.1.
Where, Y is the predicted response, β0 the offset term (Intercept process effect), β1, β2, β3, and β4 are linear effects; β12, β13, β14, β23, β24 and β34 are cross product effects, and β11, β22, β33 and β44 are squared effects. The interactions are represented by X1X2, X1X3 and X2X3.
3. Results and Discussion3.1. Electrode CharacterizationsThe functional groups present in the materials were elucidated by using Fourier transform infrared spectroscopy. Fig. 2a shows FTIR spectra of G, GO, NSG, and Ti/SnO2-Sb-NSG materials in the range of 4000–500 cm−1. FTIR graphite spectrum shows mainly three peaks at 625 cm−1, 1645 cm−1 and 3325 cm−1, ascribed to alkene (C-H), aromatic ring (C=C), and aromatic (C-H), respectively [66]. Characteristic absorption peaks at 3423, 1731, 1633, 1375, 1251 and 1064 cm−1 which can be associated to hydroxyl (O-H), carbonyl (C-O), aromatic ring (C-C), alkene (C=C), carboxy (C=O), and epoxy (C-O-C) stretching vibration peaks respectively are observed clearly in the GO spectrum. However, a large band at 3400 cm−1 ascribed to OH bond may be due to absorbed moisture which returns to (C-OH) carboxylic acid. FTIR NSG spectrum also shows three peaks at 815 cm−1, 1475 cm−1, and 1615 cm−1, assigned to N-H stretching, C-S stretching, and C=N stretching vibrations, respectively [34]. Furthermore, it is important to mention that after microwave irradiation, the intensity of the broad peak centered at 3423 cm−1, depicting hydroxyl (O-H) group of GO, dramatically reduced in nitrogen and sulfur co-doped sample, which can be adduced to the removal of hydroxyl (O-H) group. This result means that thiourea (reducing agent) has well reduced GO. FTIR SnO2-Sb-NSG material spectrum presents O-H, C=N, C-S, Sb-O and Sn-O-Sn bonds at 3401 cm−1, 1675cm−1, 1635 cm−1, 1025 cm−1 and 695 cm−1 respectively. SnO2-Sb-NSG material has a few organic elements number because of reducing graphene oxide. These characterizations results are in good agreement with the findings of Silwana and his collaborators [67]. Thus, it can be suggested that there is a new bond formed chemical interaction occurring within the nanomaterials.
Raman spectroscopy is a useful tool for investigating disorder in oxide and carbon materials. Fig. 2b shows a typical room temperature Raman graphite spectrum of as-prepared GO, NSG and SnO2-Sb-NSG samples. Firstly, it is observed three peaks at 1348 cm−1, 1575 cm−1 and 2668 cm−1 which are denominated D, G and 2D bands respectively in the Raman spectra of graphite. The main peak is the G band, which arises from the crystalline structure of graphitic samples and is associated to the symmetric E2g vibrational mode observed for graphitic materials. This peak is related to the sp2-bonded carbon atoms in a two-dimensional hexagonal lattice. Another dominant peak is the D band, related to the defects and disorder in the hexagonal graphitic layer. The latter peak is the 2D band, also attributed to the sp2-bonded carbon atoms in a two-dimensional lattice and connotes that the graphite sheet of the sample contains most layers. The low intensity of 2D bands observed in GO, NSG and Ti/SnO2-Sb-NSG spectra could imply that the graphene sheet of the sample contains few layers [68]. Raman spectra of GO, NSG and Ti/SnO2-Sb-NSG also present the peaks mentioned above. Additionally, the Raman spectrum of nitrogen sulfur co-doped graphene supported antimony doped tin oxide presents five new peaks. The peaks at 477, 629 and 775 cm−1 corresponding to three fundamental active Raman vibration modes Eg, A1g and B2g respectively. These results suggest that the typical feature of the rutile phase of SnO2 nanoparticles. The three peaks of the Raman shift at 241cm−1, 284 cm−1, and 691 cm−1 can be attributed to SnO2 nanoparticles doped with antimony [22]. Usually, in order to characterize the degree of defects and disorder in graphene-based material, the ratio intensity of D and G bands (ID/IG) is commonly used. After the preparation of electrode materials, the ID/IG ratios of GO (0.83), NSG (0.91) and SnO2-Sb-NSG (0.99) are larger than the ID/IG ratio of G (0.10). Showing that these samples contain more defects and disorder due to the insertion of oxygen groups in GO and their removal into graphene and also due to the substitution of carbon atom with heteroatom (nitrogen and sulfur) into graphene. The highest intensity ratio observed in SnO2-Sb-NSG material can suggest that new graphitic domains are formed and the sp2 cluster number is increased [66,69].
The size of in plane crystallites is also an important parameter for characterizing graphene related materials. Thus, the electrical resistivity is considered to partially arise from the hopping of charge carriers between the crystallite zones. The crystallite size La should be somehow related to the D peak in the Raman spectrum since it expresses disorder in a system. La is expressed by the following formula [69]:
Where IG and ID are the intensities of the G and D bands respectively.
This relation leads to the determination of La from the Raman spectrum. The La 44.00, 5.30, 4.84, and 4.44 for graphite, graphene oxide, nitrogen-sulfur co-doped graphene and nitrogen-sulfur co-doped graphene supported antimony doped tin oxide nanoparticles, respectively were calculated which suggests larger crystalline zone in the graphene oxide and graphite. One may therefore consider nitrogen sulfur co-doped graphene supported antimony doped tin oxide to be the nanocomposite suited for further study of various chemical and electrochemical properties. These results agreed with those obtained by Silwana et al. [67] and Costa et al. [70]. Thus, the Raman spectra confirm the presence of nitrogen sulfur co-doped graphene, SnO2 and SnO2-Sb on the nanocomposite materials and the FTIR spectroscopy results.
For studying the crystallinity and crystal phases of the prepared material X-Ray diffraction is used. Fig. 3a shows the experimental XRD patterns of the graphite (G), graphene oxide (GO) nitrogen-sulfur co-doped graphene (NSG) and nitrogen-sulfur co-doped graphene supported antimony doped tin oxide (SnO2-Sb-NSG). The graphite diffraction peaks appear around 26.1° and 44.1°, which can be attributed to the (002) and (100) reflections in the graphitic structure plan respectively. While diffraction peak of graphene oxide appears around 9.71° and 43°, which can also be ascribed to (001) and (100) reflections in the graphite structure plan respectively. The calculated d spacing to the plane (001) is 0.939 nm, which is greater than that of graphite (0.339 nm), this can be due to the presence of oxygen functional groups formed during oxidation. For NSG XRD spectrum, it is seen a new peak around 26.21° after the disappearance of the diffraction peak of GO around 9.89° during microwave irradiation. This peak can be ascribed to the (002) reflection of graphitic structure and the calculated d spacing was 0.346 nm. Five other peaks around 26.8°, 34.0°, 38.2°, 53.2°, and 63.4° corresponding to (110), (101), (200), (211), and (310), tetragonal rutile SnO2 phase reflections respectively were observed to the XRD spectrum of SnO2-Sb-NSG material. The peaks corresponding to antimony oxide are not observed, this can be due to the connection with either the low content of antimony element or the doping antimony ions into the stannic oxide phase. These results were also reported by Costa et al. [70]. The results indicate that all of the Sb doped SnO2 films have rutile structure and are in a good agreement with JCPD: 41-1445.
Thermal stability of nitrogen and sulfur co-doped graphene supported antimony doped tin oxide was examined by TG, DSC and DTG analysis as shown in Fig. 3b. In the TG graph, it is observed that the precursor (SnO2-Sb-NSG) decomposes in mainly three steps of weight loss. Thus, the firstly, for increasing temperature from room temperature to approximately 180 is due to water removal; the secondly, from that temperature to approximately 325 is probably due to the pyrolysis of organic materials such as oxygen, nitrogen and sulfur-containing groups. The last one, associated to the sharp endothermic peak in the DTG curve, can be assigned to the decomposition of Sn(C2H5OH)5 + Sb(C2H5OH)3. The last step above 390 relates to an unstable carbon remaining in the structure and the antimony doped tin oxide functional groups in the main structure to yield CO, CO2, SnO, SnO2, SnO2-Sb. The formation of the main desired phase (SnO2-Sb-NSG) via the formation of SnO2-Sb2O5-NSG, the precursor is associated to the endothermic process which occurring around 372. The endothermic peak around 228 can be assigned to the decomposition of SnO4-Sb2O5. In the investigated temperature range, precursor exhibited nearly 23.31 % mass loss. Thus, the thermal stability of the as-prepared precursor has been affected by the sol-gel and irradiation microwave. The same conclusions were made from the DTG curves, where no thermal changes in the precursor could be observed.
Fig. 4a presents the LSV curves of Ti/SnO2-Sb, Ti/SnO2-Sb-NG and Ti/SnO2-Sb-NSG electrodes. All electrocatalysts have a wide electrochemical potential window from 0 V to about 1.85 V/ vs. SCE, and present high oxygen evolution potentials. From Fig. 4a, we can observe that the oxygen overvoltage of Ti/SnO2-Sb, Ti/SnO2-Sb-NG and Ti/SnO2-Sb-NSG electrodes are 1.85, 1.99 and 2.18 V/ vs. SCE respectively. Obviously, doping nitrogen and sulfur co-doped graphene into the coating enhances the oxygen overvoltage. This can be attributed to the preferred growth, a good crystallization of Ti/SnO2-Sb-NSG electrode and the larger surface areas of the NSG structure that provided more active reaction sites and less electroactivity for OER. Ti/SnO2-Sb-NSG anodes present the lower current density than that of the Ti/SnO2-Sb-NG and Ti/SnO2-Sb. Recently, Mohammad et al. [71] reported the degradation of reactive red dye 195 on a novel electrode material developed (Ti/TiHx/SnO2-Sb2O5-NiO-CNT). They have showed that the as-prepared material has an oxygen overvoltage of 1.91 V vs Ag/AgCl compared to 2.18 V vs SCE in this work. The difference observed from these works could be due to the different oxidizing species electrogenerated (ozone for Mohammad et al and hydroxyl radicals in the present study) therefore, the difference between the experimental conditions. When the materials present low current density and high oxygen overvoltage, they can restrain the oxygen evolution reaction and reduce the energy consumption and favor the electrocatalytic activity in DAO. Thus, Ti/SnO2-Sb-NSG present the best electrocatalytic performance.
In electrochemical processes, the electrode stability is one of the important factors related with the electrode performance. Accelerated service lifetime tests were employed to evaluate the stabilities of Ti/SnO2-Sb, Ti/SnO2-Sb-NG, and Ti/SnO2-Sb-NSG electrodes. Fig. 4b shows the results. The accelerated lifetime curve of Ti/SnO2-Sb-NSG is stable in low potential and exhibits a fast increase in the high potential, which suggests the electrode deactivation. The accelerated lifetime of Ti/SnO2-Sb, Ti/SnO2-Sb-NG and Ti/SnO2-Sb-NSG were 9.0 h, 23.2 h and 31.6 h respectively. Thus, Ti/SnO2-Sb-NSG electrode have a higher stability than Ti/SnO2-Sb and Ti/SnO2-Sb-NG. The stability of Ti/SnO2-Sb-NSG electrode is enhanced considerably; this can be due to the insertion of nitrogen and sulfur into graphene, which acts as a support for antimony doped tin oxide and intensifies the structure of the electrode [72]. In comparison of our findings to the results of other authors, the stability of antimony doped tin oxide-based electrodes have been significantly improved. Duan et al. [31] reported the preparation of Ti/Sb-SnO2-NGNS electrode and found that the electrode had an accelerated lifetime of 26.7 h, while in the present study we have found 31.6 h. Furthermore, studied the modification of SnO2-Sb electrode with carbon nanotube and chromium (Ti/SnO2–Sb2O4-CNT-Cr3C2) for the direct oxidation of phenol. The authors found that the as-prepared material had an accelerated lifetime of 7 h [73]. This low value could be due to the high concentration of sulfuric acid (1 M) used in their work compared to 0.5 M (H2SO4) in this work.
3.2. Optimization of COD Removal Efficiency by Direct Anodic Oxidation of Methyl Orange and Neutral Red on Ti/SnO2-Sb-NSGThe response corresponding to the combined effects of three variables was studied in their specified ranges as shown in Table S1. The experimental results obtained in the trials performed with the Box-Behnken design is presented in Table 1.
For MO degradation, the rate of COD removal varied between 57.58% and 96.78%. While for NR degradation, the rate of COD removal was between 53.48% and 80.71%. The second-order polynomial model Eq. (6) that established the correlation between the COD removal of MO (Y1) or NR (Y2) and the independent factors (in terms of real values) is shown in Eq. (8) and Eq. (9) for MO and NR, respectively.
Where Y1 and Y2 are the COD removal efficiency of MO and NR, respectively, X1, X2 and X3 are current density, electrolysis time, and initial dye concentration, respectively. From Eq. (8), all the variables have a positive effect on the response except the initial dye concentration. While from Eq. (9) all the variables have a positive effect on the COD removal efficiency.
The analysis of variance (ANOVA) was used to evaluate the statistical significance of the constructed models. Using the Fisher statistical test (F-test), the ANOVA consists of determining which factor significantly affect the response. Regression coefficient (R2) was used in this part in order to show the fitness of the model. The analysis of variance for surface response with MO and NR dyes are shown in Table S2 and Table S3 respectively. From both tables, it can be seen that the proposed models were highly significant due to a very low p-value (p 0.0001) and high F-value. From the quadratic models obtained Eq. (8) and Eq. (9), the experimental results lead to the optimum degradation conditions.
Pareto analysis was also used in this work in order to facilitate the interpretation of the results. It was applied according to the following equation.
Where, b represents the related regression coefficient of the parameter. The pareto graphics are shown in Fig. S1. They illustrate the percentage effect of each independent factor and their interaction on the response. Fig. S1a. shows that electrolysis time, current density, the quadratic effect of current density, and the quadratic effect between electrolysis time and initial dye concentration have a positive effect on the COD removal of MO. However, the other factors have a negative effect on the response.
It can be observed from Fig. S1b that electrolysis time, current density, initial dye concentration and the quadratic effect between current density and electrolysis time have a positive effect on the COD removal of NR. Therefore, it can be important to note that the electrolysis time and current density are the most influent factors during the electro-degradation of MO and NR. Among the quadratic effects for the degradation of MO and NR, electrolysis time (X22) and initial dye concentration (X32), respectively were the most significant.
In order to determine the interaction between variables on the dye degradations, the tree-dimensional (3D) response surface plots were used and presented in the following figures. Fig. 5A1 and Fig. 5B1 show the effect of current density and electrolysis time on MO and NR degradation respectively. It appears that increasing the current density and electrolysis time, increases the COD removal rate. This because the high current density promotes high generation of •OH, which degrade the pollutants by oxidation. The increase of the COD removal rates when the time increases could be due to the fact that, most of the intermediate compounds formed during electrolysis are degraded with high efficiency. During DAO, current density has shown to be one of the most important variable that can control the reaction rate of electrochemical degradation [2,74].
Fig. 5A2 and Fig. 5B2 show the effect of interaction between current density and initial dye concentration on MO and NR COD removal efficiency respectively. The response surface plot shows that the COD removal rate increases considerably when the current density increases and the initial dye concentration decreases. In general, organic pollutants are highly degraded at low initial dye concentration. Thus, in order to attain the efficient and complete degradation of synthetic dye, the textile effluents must be diluted before proceeding for catalytic treatment [75]. Therefore, low concentration would contribute to the total electro-degradation of organic pollutants. Similar results were also reported by others researchers [76]. However, the initial dye concentration shows a slight effect on the COD removal efficiency. Then we can note that the degradation of the organic compounds is mainly due by the •OH formed from the reaction (Eq. 4). In addition, as anode materials with high O2 overvoltage are used in DAO in sulfate media, other weaker oxidants as S2O82−, O3 and H2O2 can be competitively formed according to the following equations Eq. (11–13) [77]. These oxidants also contribute to the degradation of pollutants during the process.
The influence of electrolysis time and initial dye concentration on MO and NR degradation efficiencies were shown in Fig. 5A3 and Fig. 5B3. For MO degradation, the highest rate of degradation was achieved at a low concentration of the pollutant. Thus, it is obvious that increasing electrolysis time from 2 to 6 h at the MO concentration of 25 mg/L has led to the rise the MO dye from 66.58 % to about 96.78 %. Also, Fig. 5A3 shows that at initial dye concentration of 29.02 mg/L and electrolysis time of 6 h, MO degradation efficiency is about 98.71%. For NR degradation, the optimum value of the COD removal efficiency was about 82.72%. From Fig. 5B3 we can notice that the COD removal efficiency increases with NR concentration until up to 82.05 mg/L when the time also increases. Thus, at electrolysis time of 6 h and NR concentration of 82.05 mg/L, NR degradation efficiency is about 82.72%. Furthermore, the rate of degradation is higher at the beginning of electrolysis and diminishes with electrolysis time. However, we can note that the overall aromatic compounds are converted to aliphatic derivatives at the end of the bleaching period. The decrease in the degradation rate represented by the weakening of the slope can be explained by the transformation of the aromatic compounds into aliphatic derivatives by ring opening reactions. In addition, it is well known that carboxylic acids are formed during electro-degradation of organic compounds which react slowly with hydroxyl radicals. This result was also reported by Hammani et al. [78]. Nevertheless, it is important to mention that electrolysis time is the main important parameter on the MO and NR degradation in the experimental region investigated.
For MO COD removal efficiency, the optimum was 98.71% at 75.50 mA/cm2 of current density, 6.00 h of electrolysis time and 29.02 mg/L of MO initial dye concentration. While 82.73% of NR COD removal efficiency could be obtained at a current density of 52.08 mA/cm2, electrolysis time of 6.00 h and NR initial dye concentration of 82.05 mg/L. The results are shown in Table S4. Tarkwa et al. [79] have studied the degradation of orange G azo dye by the photo-Fenton process and have found that the dye was completely removed after 4 min and the total organic carbon (TOC) was removed until 93.41% after 180 minutes. The difference between the results of this previous work and our can be due to the fact that in the work carried out by Tarkwa and his collaborators, they have employed the coupling of photocatalysis and Fenton processes which has improved considerably the removal of the pollutant. Furthermore, Priya et al. [80] have conducted a study on the degradation of acid orange dye 7 using photocatalysis and photoelectrochemical processes. They have found that the complete removal of the organic dye was achieved at 73 min. However, the degradation studies of the textile synthetic effluent containing acid orange dye 7 as a pollutant was not conducted from the authors.
It was necessary to carry out some experiments with the optimum values obtained for testing the accuracy of their range. Thus, we have repeated thrice the experiments for each organic pollutant with their optimal conditions. The average COD removal efficiency of MO and NR were 96.59 and 81.10 % respectively. Thus, these results confirm that the obtained optimum for these processes could lead to high percentages of each dye degradation.
FTIR spectroscopy was used to investigate the intermediate formation during the electrooxidation of MO solutions before and after the process. Fig. 6a shows the FTIR spectra for samples before and after 6 h of treatment. After degradation, the characteristic absorption peaks of phenyl at 1,607, 1,520 and 1,446 cm−1 and the band at 1,423 cm−1 linked to the C-N bond of tertiary aromatic compounds disappeared [81]. Thus, it is important to mention that the C-N bond and phenyl ring were cleaved through the electro-degradation. Before degradation, we can observe a peak at 1,383 cm−1 which is ascribed to the azo bond absorption, this peak disappeared after 6h of electrolysis. The peak of the sulfonyl group at 1,041 cm−1 also vanished after the electro-degradation. However, the sulfonyl group disappearance was also confirmed by the generation of sulfate ion as mentioned above. Before degradation, we can observe a peak at 821 cm−1 which is ascribed to the C-H bonds of di-substituted benzene. This peak will be shifted to 835 cm−1 and 876 cm−1 after the DAO process. We can also remark that the peaks at 695 and 619 cm−1 changed to 683 and 621 cm−1. Therefore, new compounds have been formed during the treatment. For example, the newly formed band at 1,643 cm−1 after degradation was due to the absorption of the carbonyl group. Furthermore, the low absorption peak at 799 cm−1 could be ascribed to the (CH2)n wagging vibration. Based on these results, it can be seen that MO dye was attacked at several sites, and other compounds with carbonyl group were formed.
The degradation of MO is mainly ascribed to the DAO where •OH are produced during water electrolysis and readily react with the pollutants adsorbed on the anode [82]. The reactions between MO and •OH could occur twice because the electrode material is a non-active anode. The general pathway of MO degradation is as follows: firstly, •OH generated at the Ti/SnO2-Sb-NSG electrode; secondly, the disconnection of the conjugated structure of the azo bond by the oxidation with •OH which leads to the formation of some acid compounds like sulfanilic acid. The last chemicals were already identified by Du et al. [81]. Possible mechanism of electrochemical degradation of MO in presence of chlorine species where studied by LC-MS and GC-MS analysis [81]. However, no work has been found on the direct anodic oxidation of MO without chloride ions. Based on both the literature and the above results, the degradation pathway of methyl orange was proposed as shown in Fig. 7.
After the optimization of the operational conditions by BBD, we carried out the electro-degradation of the MO, NR and the mixture of two synthetic dye solutions (MO and NR). The optimal conditions for each dye degradation were selected and for the mixture, the optimal conditions of MO degradation were selected. Fig. 6b shows the COD removal efficiency of three synthetic solutions. From this figure, it can be observed that these conditions led to a maximum degradation efficiency (COD removal) of MO, NR, and MO + NR of 97.55%, 80.25%, and 86.66%, respectively. The degradation rate is higher at the beginning of the electrolysis and diminishes with electrolysis time. However, MO was almost completely degraded while NR was just degraded at only 80.25%. Thus, this fact could be justified by the structure of two pollutants, MO as an azo dye was rapidly attacked by the •OH during the electrolysis. Nevertheless, due to the complexity of the molecular structure of NR, it was not rapidly cleaved and destroyed.
4. ConclusionsThis study was carried out in order to optimize the degradation of MO and NR dyes in the electrochemical oxidation system using Ti/SnO2-Sb-NSG anode. An efficient Ti/SnO2-Sb-NSG anode was successfully prepared by microwave irradiation and sol-gel methods. Raman and XRD analyses showed that the nitrogen and sulfur co-doped graphene was synthetized and have a high crystalline structure. In addition, the lifetime of the elaborated material was significantly improved and possesses higher oxygen evolution potential. BBD method with 18 experiments (including 6 repetitions of central points) was used in this study in order to obtain the optimized conditions for the degradation of synthetic dye effluent of MO and NR. MO dye was degraded at 98.71%, the obtained optimums are current density 75.00 mA/cm2, electrolysis time 6.00 h and initial dye concentration 29.02 mg/L. For NR degradation, the optimum of the degradation was 82.73% taking current density at 52.08 mA/cm2, electrolysis time at 6.00 h and NR initial concentration at 82.05mg/L. The obtained results also show that the electrolysis time and current density are the most influent factors during the direct anodic oxidation process. However, the mechanism of the MO degradation through DAO via hydroxyl radical as a mediator was proposed and detailed. These conditions were applied on a mixture of both dyes and an acceptable response achieved. Finally, considering the enhanced service lifetime and electrocatalytic properties, the Ti/SnO2-Sb-NSG electrode can be expected to limit significantly the cost of the organic pollutant wastewater treatment and reveals a good practical application prospect.
AcknowledgementsThe authors wish to thank the Department of Chemistry of the Faculty of Science at the University of Ngaoundere for they provided all support to our disposition in order to work in this project. We also want to thank Pr Deepak Kumar Pattanayak at CSIR-CECRI in India for the help concerning the electrode material’s characterization.
References1. Semra C., Geyikc F., Elevli S.. Adsorption of neutral red dye from an aqueous solution onto natural sepiolite using full factorial design. Clays Clay Miner. 2011;59:617–625.
https://doi.org/10.1346/CCMN.2011.0590607
2. Reza M., Dargahi A., Shabanloo A.. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode : Optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab J. Chem. 2020;13:6847–6864.
https://doi.org/10.1016/j.arabjc.2020.06.038
3. Ganiyu S.O., Van H.E., Cretin M., Esposito G., Oturan M.A.. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep Purif. Technol. 2015;156:891–914.
https://doi.org/10.1016/j.seppur.2015.09.059
4. Djonga W.G., Noubissié E., Noumi G.B.. Discoloration test of a slaughterhouse effluent by adsorption on two adsorbents produced from sawdust of Khaya senegalensis and Pinus sp. Results Eng. 2019;4:1–8.
https://doi.org/10.1016/j.rineng.2019.100191
5. Tsafam A., Domga R., Dikdim J., et al. Adsorption of Cr (VI) onto clay modified by sodium chloride and clay modified by aluminum hydroxide of Karewa (North Cameroon). Int. Res J. Adv. Eng. Sci. 2019;4:247–255.
6. Shertate R., Thorat P.. Biotransformation of textile dyes: A bioremedial aspect of marine environment. Am. J. Environ. Sci. 2014;10:489–499.
https://doi.org/10.3844/ajessp.2014.489.499
7. Babu R., Parande A., Kumar S., Bhanu U.. Treatment of dye effluent by electrochemical and biological processes. Open J Saf. Sci. Technol. 2011;1:12–18.
https://doi.org/10.4236/ojsst.2011.11002
8. Massoudinejad M., Ghaderpoori M., Azari R.. The Removal of COD and color from textile industry by chlorine hypochlorite. Int. J. Adv. Sci. Technol. 2015;76:1–11.
https://doi.org/10.14257/ijast.2015.76.05
9. Peralta-Hernández J., Godínez L.. Electrochemical hydrogen peroxide production in acidic medium using a tubular photo-reactor: Application in advanced oxidation processes. J. Mex Chem. Soc. 2014;58:348–355.
https://doi.org/10.29356/jmcs.v58i3.144
10. Ndjomgoue-Yossa A., Nanseu N.J., Kengne L., Ngameni E.. Effect of electrode material and supporting electrolyte on the treatment of water containing Escherichia coli by electrocoagulation. Int. J. Environ. Sci. Technol. 2015;12:2103–2110.
https://doi.org/10.1007/s13762-014-0609-9
11. Kacem B., Clematis D., Elaoud S., Barbucci A., Panizza M.. A flexible electrochemical cell setup for pollutant oxidation in a wide electrical conductivity range and its integration with ultrasound. J. Water Process Eng. 2022;46:102564.
https://doi.org/10.1016/j.jwpe.2022.102564
12. Teguia R., Noumi G., Ngobtchok B., Domga . Tannery wastewater treatment by electro-Fenton and electro-persulfate processes using graphite from used batteries as free-cost electrode materials. Case Stu. Chem. Environ. Eng. 2022;5:100190.
https://doi.org/10.1016/j.cscee.2022.100190
13. Kong J., Shi S., Kong L., Zhu X., Ni J.. Preparation and characterization of PbO2 electrodes doped with different rare earth oxides. Electrochim. Acta. 2007;53:2048–2054.
https://doi.org/10.1016/j.electacta.2007.09.003
14. Jawad N., Najim S.. Removal of methylene blue by direct electrochemical oxidation method using a graphite anode. IOP Conf Ser. Mater. Sci. Eng. 2018;454:1–9.
https://doi.org/10.1088/1757-899X/454/1/012023
15. Panizza M., Cerisola G.. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009;109:6541–6569.
https://doi.org/10.1021/cr9001319
16. Comninellis C., Vercesi G.. Characterization of DSA-type electrodes: choice of a coating. J. Appl. Electrochem. 1991;21:335–345.
https://doi.org/10.1016/0040-6031(91)80257-J
17. Subba R.A., Venkatarangaiah V.. Metal oxide-coated anodes in wastewater treatment. Environ. Sci. Pollut. Res. 2014;21:3197–3217.
https://doi.org/10.1007/s11356-013-2313-6
18. Feng L., Hullebusch V.E., Rodrigo M., Esposito G., Oturan M.. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013;228:944–964.
https://doi.org/10.1016/j.cej.2013.05.061
19. Bonyadinejad G., Sarafraz M., Khosravi M., et al. Electrochemical degradation of the Acid Orange 10 dye on a Ti/PbO2 anode assessed by response surface methodology. Korean J. Chem Eng. 2016;33:189–196.
https://doi.org/10.1007/s11814-015-0115-x
20. Taehee K., Vinayak G., Hae-Noo-Ree J., et al. Facile synthesis of SnO2 aerogel/reduced graphene oxide nanocomposites via in situ annealing for the photocatalytic degradation of methyl orange. Nanomaterials. 2019;9:358.
https://doi.org/10.3390/nano9030358
21. Xia L., Cairu S., Jiangang Y., Kaigui Z.. Preparation and investigation of nickel–antimony co-doped tin oxide anodes for electro-catalytic oxidation of organic pollutions. Int. J. Electrochem Sci. 2019;14:205–218.
https://doi.org/10.20964/2019.01.23
22. Farbod S., Edward P.L.. Electrochemical oxidation of an organic dye adsorbed on tin oxide and antimony doped tin oxide graphene composites. Catalysts. 2020;10:263–271.
https://doi.org/10.3390/catal10020263
23. Chen A., Zhu X., Xi J., Qin H., Ji Z.. Ultra-high oxidation potential of Ti/Cu-SnO2 anodes fabricated by spray pyrolysis for wastewater treatment. J. Alloys Compd. 2016;683:501–505.
https://doi.org/10.1016/j.jallcom.2016.05.075
24. Maharana D., Xu Z., Niu J., Rao N.. Electrochemical oxidation of 2,4,5-trichlorophenoxyacetic acid by metal-oxide-coatedTi electrodes. Chemosphere. 2015;136:145–152.
https://doi.org/10.1016/j.chemosphere.2015.04.100
25. Brychy R., Rguiti M., Rhazzane N., et al. Electrochemical Degradation of Crystal Violet Using Ti/Pt/SnO2 Electrode. Appl Sci. 2021;11:1–18.
https://doi.org/10.3390/app11188401
26. Sofia D.. Preparation, characterization and environmental applications of SnO2-Sb2Ox films. J. Appl. Electrochem. 2016;39:55–67.
27. Yin M., Xu J., Li Q., et al. Highly active and stable Pt electrocatalysts promoted by antimony-doped SnO2 supports for oxygen reduction reactions. Appl. Catal. B: Environ. 2014;23:331–339.
https://doi.org/10.1016/j.apcatb.2013.07.007
28. Costa C., Botta R., Espindola E., Olivi P.. Electrochemical treatment of tannery wastewater using DSA electrodes. J. Hazard. Mater. 2007;153:616–627.
https://doi.org/10.1016/j.jhazmat.2007.09.005
29. Maizelis A., Bairachniy B.. Electrochemical Formation of Multilayer SnO2-Sbx Oy Coating in Complex Electrolyte. Nanoscale Res. Lett. 2017;12:1–6.
https://doi.org/10.1186/s11671-017-1902-6
30. Feng H., Cui X., Chen W.. Pulse electro-codeposition of Ti/SnO2-Sb2O4-CNT electrode for phenol oxidation. Electrochem Solid State Lett. 2010;13:20–23.
31. Duan T., Wen Q., Chen Y., Zhou Y., Duan . Enhancing Electrocatalytic Performance of Sb-doped SnO2 Electrode by Compositing Nitrogen-doped Graphene Nanosheets. J. Hazard Mater. 2014;55:1–11.
https://doi.org/10.1016/j.jhazmat.2014.08.018
32. Zhao C., Wu Y., Liang H., Chen X., Tang J., Wang X.. N-doped graphene and TiO2 supported manganese and cerium oxides on low-temperature selective catalytic reduction of NOx with NH3
. Int. J. Electrochem. Sci. 2018;13:197–206.
33. Nair K., Kumaravel V., Pillai C.. Carbonaceous cathode materials for electro-Fenton technology : Mechanism, kinetics, recent advances, opportunities and challenges. Chemosphere. 2021;269:e129325.
https://doi.org/10.1016/j.chemosphere.2020.129325
34. Domga , Karnan M., Oladoyinbo F., et al. A simple, economical one-pot microwave assisted synthesis of nitrogen and sulfur co-doped graphene for high energy supercapacitors. Electrochim. Acta. 2020;341:e135999.
https://doi.org/10.1016/j.electacta.2020.135999
35. Ren L., He H.. Effect of the addition of graphite oxide on the morphology of the alumina aerogels. AIP Adv. 2020;8:55–63.
https://doi.org/10.1063/1.5016901
36. Rochman R., Wahyuningsih S., Ramelan A., Hanif Q.. Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. Mater. Sci Eng. 2019;509:e012119.
https://doi.org/10.1088/1757-899X/509/1/012119
37. Domga , Bertrand G., Joseph S., Bosco J.. Synthesis of nitrogen and phosphorus co-doped graphene as efficient electrocatalyst for oxygen reduction reaction under strong alkaline media in advanced chlor-alkali cell. Carbon Trends. 2021;4:e100043.
https://doi.org/10.1016/j.cartre.2021.100043
38. Zhao Y., Zhang C., Liu T., Xue J., et al. Low temperature green synthesis of sulfur-nitrogen co-doped graphene as efficient metal-free catalysts for oxygen reduction reaction. Int. J Electrochem. Sci. 2017;12:3537–3548.
https://doi.org/10.20964/2017.04.67
39. Jiang F., Zhang J., Li N., et al. Nitrogen-doped graphene prepared by thermal annealing of fluorinated graphene oxide as supercapacitor electrode. J. Chem. Technol. Biotechnol. 2019;94:3530–3537.
https://doi.org/10.1002/jctb.6147
40. Cai B., Shao C., Qu L., Meng Y., Jin L.. Preparation of sulfur-doped graphene fibers and their application in fl exible fibriform micro-supercapacitors. Front. Mater. Sci. 2019;13:145–153.
41. Ouyang Z., Lei Y., Chen Y., et al. Preparation and Specific Capacitance Properties of Sulfur, Nitrogen Co-Doped Graphene Quantum Dots. Nanoscale Res. Lett. 2019;14:1–9.
https://doi.org/10.1186/s11671-019-3045-4
42. Han J., Li Q., Peng C., et al. Increasing S dopant and specific surface area of N/S-codoped porous carbon by in-situ polymerization of pedot into biomass precursor for high performance supercapacitor. Appl. Surf. Sci. 2020;502:e144191.
https://doi.org/10.1016/j.apsusc.2019.144191
43. Balaji S., Elavarasan A., Sathish M.. High performance supercapacitor using N-doped graphene prepared via supercritical fluid processing with an oxime nitrogen source. Electrochim Acta. 2016;200:37–45.
https://doi.org/10.1016/j.electacta.2016.03.150
44. Wang X., Qin Y., Zhu L., Tang H.. Nitrogen-doped reduced graphene oxide as a bifunctional material for removing bisphenols: Synergistic effect between adsorption and catalysis. Environ Sci. Technol. 2015;21:147–158.
https://doi.org/10.1021/acs.est.5b01059
45. Jing Z., Qi S., Tao X., Yu Z.W., Qiao Y.. Materials Active-screen plasma multi-functionalization of graphene oxide for supercapacitor application. J. Mater. Sci. 2020;12:46–58.
https://doi.org/10.1007/s10853-020-05410-y
46. Tasis D.. Recent progress on the synthesis of graphene-based nanostructures as counter electrodes in DSSCs based on iodine/iodide electrolytes. Catalysts. 2017;7:85–99.
https://doi.org/10.3390/catal7080234
47. Bundaleska B., Henriques J., Abrashev M., et al. Large-scale synthesis of free-standing N-doped graphene using microwave plasma. Sci. Rep. 2018;8:1–11.
https://doi.org/10.1038/s41598-018-30870-3
48. Lu G., Zhou J., Zhang L., Gao W.. Experimental investigation on the influence of microwave exposure on the cutting performance of TBM disc cutter cutting of hard rocks. Results Eng. 2021;12:100285.
https://doi.org/10.1016/j.rineng.2021.100285
49. Wang K., Bing C.J., Han M.J., Li W.L.. Preparation of nitrogen-doped graphene with solid microwave method. Chinese J. Inor. Chem. 2020;29:1–7.
https://doi.org/10.3969/j.issn.1001-4861.2013.00.328
50. Yang H., Liang J., Zhang L., Liang Z.. Electrochemical oxidation degradation of methyl orange wastewater by Nb/PbO2 electrode. Int. J. Electrochem. Sci. 2016;11:1121–1134.
51. De León , Recio F., Walsh C.. Decolorization of methyl orange dye at IrO2-SnO2-Sb2O5 coated titanium anodes. Sep. Sci Technol. 2013;25:123–129.
https://doi.org/10.1002/ceat.201200231
52. Sotgiu G., Montanaro D., Orsini M., Petrucci E.. Manganese-containing mixed oxide electrodes as anode materials for degradation of model organic pollutants. Chem. Eng. Trans. 2017;57:1639–1644.
https://doi.org/10.3303/CET1757274
53. Azami M., Bahram M., Nouri S., Naseri A.. Central composite design for the optimization of removal of the azo dye, methyl orange, from waste water using Fenton reaction. J. Serbian Chem. Soc. 2012;77:235–246.
https://doi.org/10.2298/JSC110315165A
54. Domga R., Tcheka C., Arnaud G., et al. batch equilibrium adsorption of methyl orange from aqueous solution using animal activated carbon from gudali bones. Int. J. of Innov. and Sci Res. 2016;5:798–805.
55. Teguia R., Noumi G., Akoulah J., Domga . Indirect electrochemical degradation of methyl orange dye on graphite bifunctional electrode. Int. J. Appl. Res. 2020;6:1–13.
https://doi.org/10.6084/m9.figshare.1216120
56. Teguia R., Noumi G., Domga . Dip coating deposition of manganese oxide nanoparticles on graphite by sol gel technique for the indirect electrochemical oxidation of methyl orange dye : Parameter’s optimization using box-behnken design. Case Stud. Chem. Environ. Eng. 2021;3:e100068.
https://doi.org/10.1016/j.cscee.2020.100068
57. Shanmugam S., Muthiah P., Harinarayan J., Chiya A., Swaminathan G.. Box Behnken design-based optimization of solar induced photo catalytic decolourization of textile dye effluent. Cent. Eur. J. Eng. 2013;3(1)135–144.
http://dx.doi.org/10.2478/s13531-012-0039-8
58. Djenouhat M., Bendebane F., Bahloul L., Samar M., Ismail F.. Optimization of methylene blue removal by stable emulsified liquid membrane using Plackett–Burman and Box–Behnken designs of experiments. R. Soc. Open Sci. 2018;5:171220.
http://dx.doi.org/10.1098/rsos.171220
59. Kaouthar O., Mohammed L.. Optimization of textile azo dye degradation by electrochemical oxidation using Box-Behnken Design. Mediterr. J. Chem. 2019;8(5)410–419.
http://dx.doi.org/10.13171/mjc851907103ko
60. Benrabah B., Bouaza A., Hamzaoui S., Dehbi A.. Sol-gel preparation and characterization of antimony doped tin oxide (ATO) powders and thin films. EPJ Appl. Phys. 2009;48:55–64.
https://doi.org/10.1051/epjap/2009171
61. Yao Y., Cui L., Li Y., et al. Electrocatalytic degradation of methyl Orange on PbO2-TiO2 nanocomposite electrodes. Int. J. Environ Res. 2015;9:1357–1364.
https://doi.org/10.22059/ijer.2015.1028
62. Nidheesh P., Zhou M., Oturan M.. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere. 2018;197:207–227.
https://doi.org/10.1016/j.chemosphere.2017.12.195
63. Rodier J., Legube B., Nicole M.. L’analyse physico-chimique et microbiologique des eaux. 9ème edParis-Dunod: 2009. p. 3335–3398.
64. Teguia R., Noumi G., Domga . Electrochemical degradation of synthetic textile wastewater by C/MnO2 electrode assessed by surface response methodology. Avicenna J. Environ. Health Eng. 2021;8(2)1–10.
https://doi.org/10.34172/ajehe.2021.15
65. Tahir H., Shah A., Iqbal S., Kifayatullah H.. The statistical optimization of indirect electrochemical oxidation process for the treatment of dye from simulated textile discharge. Int. J. Environ Sci. Nat. Resour. 2017;2:1–17.
https://doi.org/10.19080/ijesnr.2017.02.555583
66. Silwana B., Van D.H., Iwuoha E., Somerset V.. Synthesis, characterisation and electrochemical evaluation of reduced graphene oxide modified antimony nanoparticles. Thin Solid Films. 2015;592:124–134.
https://doi.org/10.1016/j.tsf.2015.09.010
67. Thaneswari T., Lourduraj A.. Large Scale Synthesis and Characterization of Few Layer Graphene Nanosheets for Supercapacitor Applications. Int. Res. J. Eng. Technol. 2017;12:327–329.
68. Wang Y., Ni Z., Yu T., et al. Raman studies of monolayer graphene: the substrate effect. Environ. Eng. Res. 2008;4:10637–10640.
https://doi.org/10.1016/eer2008.02.014
69. Pimenta M., Dresselhaus G., Dresselhaus M., Canc L.. Studying disorder in graphite-based systems by Raman spectroscopy. Chem. Phys. Lett. 2007;355:1276–1291.
https://doi.org/10.1039/b613962k
70. Costa Y., Colmenares Y., Pizani P., Leite E., Chiquito A.. Sb doping of VLS synthesized SnO2 nanowires probed by Raman and XPS spectroscopy. Chem. Phys. Lett. 2018;695:125–130.
https://doi.org/10.1016/j.cplett.2018.02.014
71. Mohammad K., Seyed M., Ali B.. Novel Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode for electrochemical ozone generation for degradation of toxic textile azo dyes. Environ. Eng. Res. 2022;27(3)200429.
https://doi.org/10.4491/eer.2020.429
72. Fangcheng C., Jingyi T., Shengping Z., Hongqing W., Caizhen Y., Yuan L.. Preparation and recent developments of Ti/SnO2-Sb electrodes. J. Chem. 2021;2107939.
https://doi.org/10.1155/2021/2107939
73. Hu F., Dong Q., Cui X., Chen W.. Improved SnO2–Sb2O4 based anode modified with Cr3C2 and CNT for phenol oxidation. Electrochim. Acta. 2011;56:1576–1580.
https://doi.org/10.1016/j.electacta.2011.13.022
74. Zhang G., Huang X., Ma J., Wu E., Zhou T.. Ti/RuO2-IrO2-SnO2 anode for electrochemical degradation of pollutants in pharmaceutical wastewater : optimization and degradation performances. Sustainability. 2021;13:1–12.
https://dx.doi.org/10.3390/su13010126
75. Javaid R., Qazi U.. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health. 2019;16:1–27.
https://doi.org/10.3390/ijerph16112066
76. Panizza M., Oturan M.. Degradation of Alizarin Red by electro-Fenton process using a graphite-felt cathode. Electrochim Acta. 2011;56:7084–7087.
https://doi.org/10.1016/j.electacta.2011.05.105
77. Brillas E., Garrido J., Rodríguez R., Arias A., Cabot P., Centellas F.. Wastewaters by electrochemical advanced oxidation processes using a BDD anode and electrogenerated H2O2 with Fe(II) and UVA light as catalysts Port. Electrochim. Acta. 2008;26:15–46.
https://doi.org/10.4152/pea.200801015
78. Hammami S., Oturan N., Bellakhal N.. Oxidative degradation of direct orange 61 by electro-Fenton process using a carbon felt electrode : Application of the experimental design methodology. J. Electroanal. Chem. 2007;610:75–84.
https://doi.org/10.1016/j.jelechem.2007.07.004
79. Tarkwa J., Nihal O., Elie A., Samuel L., Mehmet A.O.. PhotoFenton oxidation of Orange G azo dye: process optimization and mineralization mechanism. Environ. Chem. Lett. 2019;17:473–479.
https://doi.org/10.1007/s10311-018-0773-0
80. Priya A., Senthil R., Selvi A., et al. A study of photocatalytic and photoelectrochemical activity of as-synthesized WO3/g-C3N4 composite photocatalysts for AO7 degradation. Mater Sci. Energy Technol. 2020;3:43–50.
https://doi.org/10.1016/j.mset.2019.09.013
81. Du L., Wu J., Qin S., Hu C.. Degradation mechanism of Methyl Orange by electrochemical process on RuOx–PdO/Ti electrode. Water Sci. Technol. 2011;6:1539–1545.
https://doi.org/10.2166/wst.2011.414
82. Oturan M., Aaron J.. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014;44:2577–2641.
https://doi.org/10.1080/10643389.2013.829765
Fig. 1Oxidation Electrochemical device. 1. magnetic stirrer; 2. magnetic bar; 3. Electrolytic cell; 4. cathode (carbon felt); 5. Anode (Ti/SnO2-Sb-NSG); 6. D.C power supply. 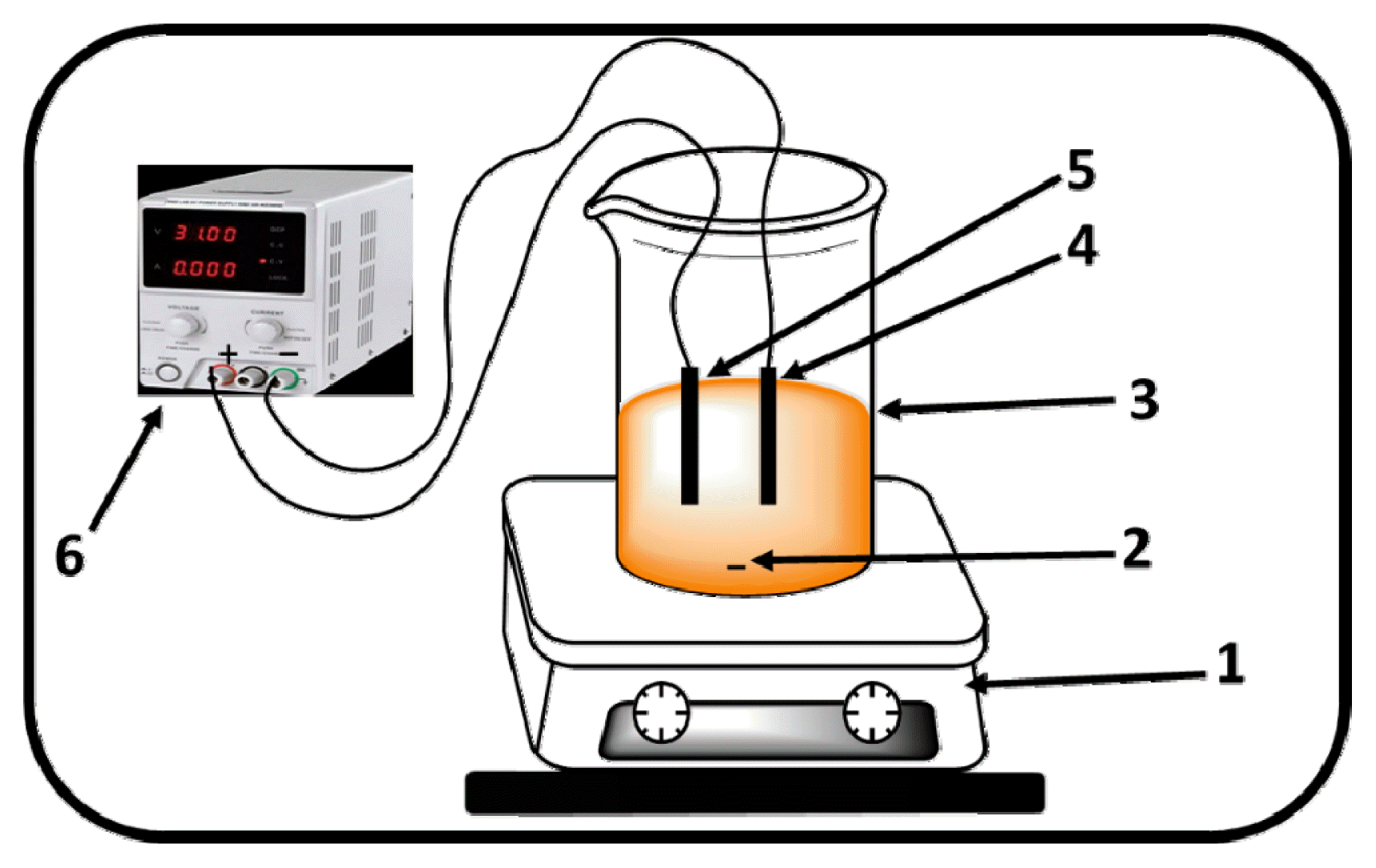
Fig. 2(a) FTIR spectra and (b) Raman spectra of graphite (G), graphene oxide (GO), nitrogen sulfur codoped graphene (NSG) and SnO2-Sb-NSG. 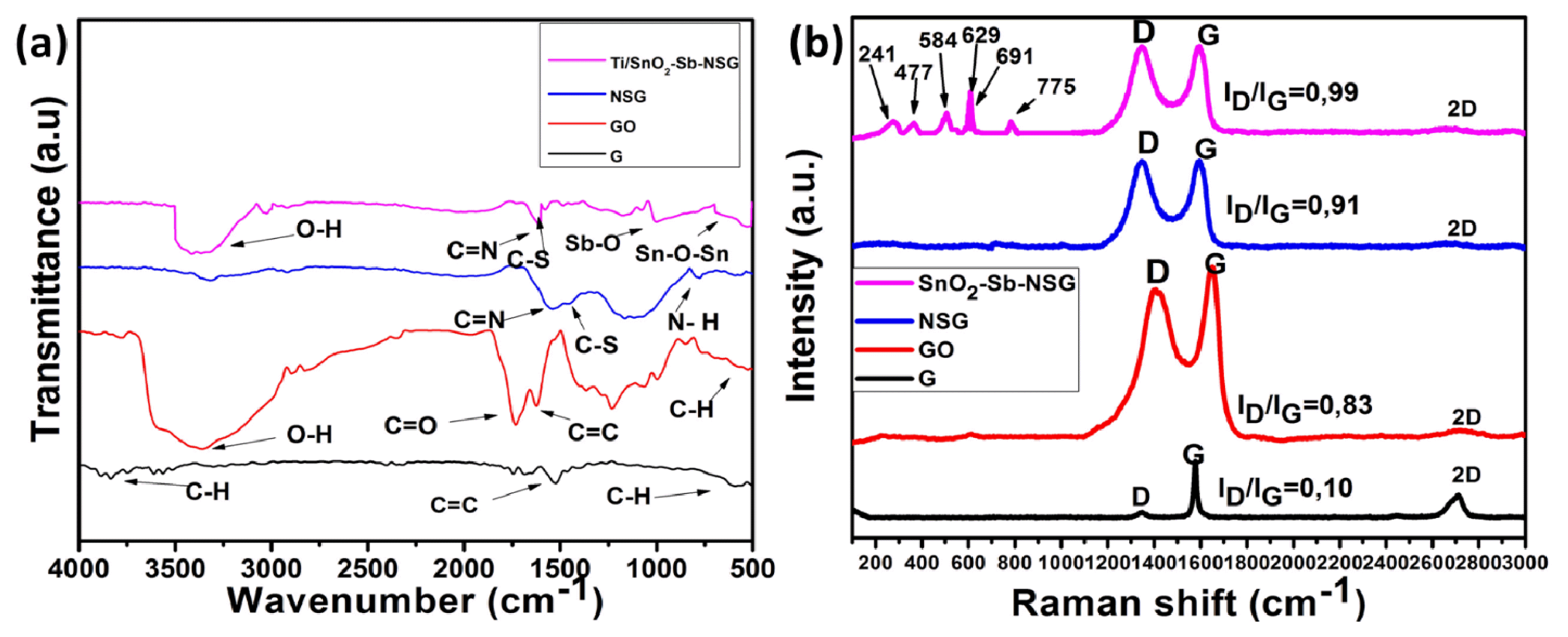
Fig. 3(a) Typical XRD patterns of graphite (G), graphene oxide (GO), nitrogen sulfur codoped graphene (NSG) and SnO2-Sb-NSG and (b) TGA/DSC analysis of SnO2-Sb-NSG powder. 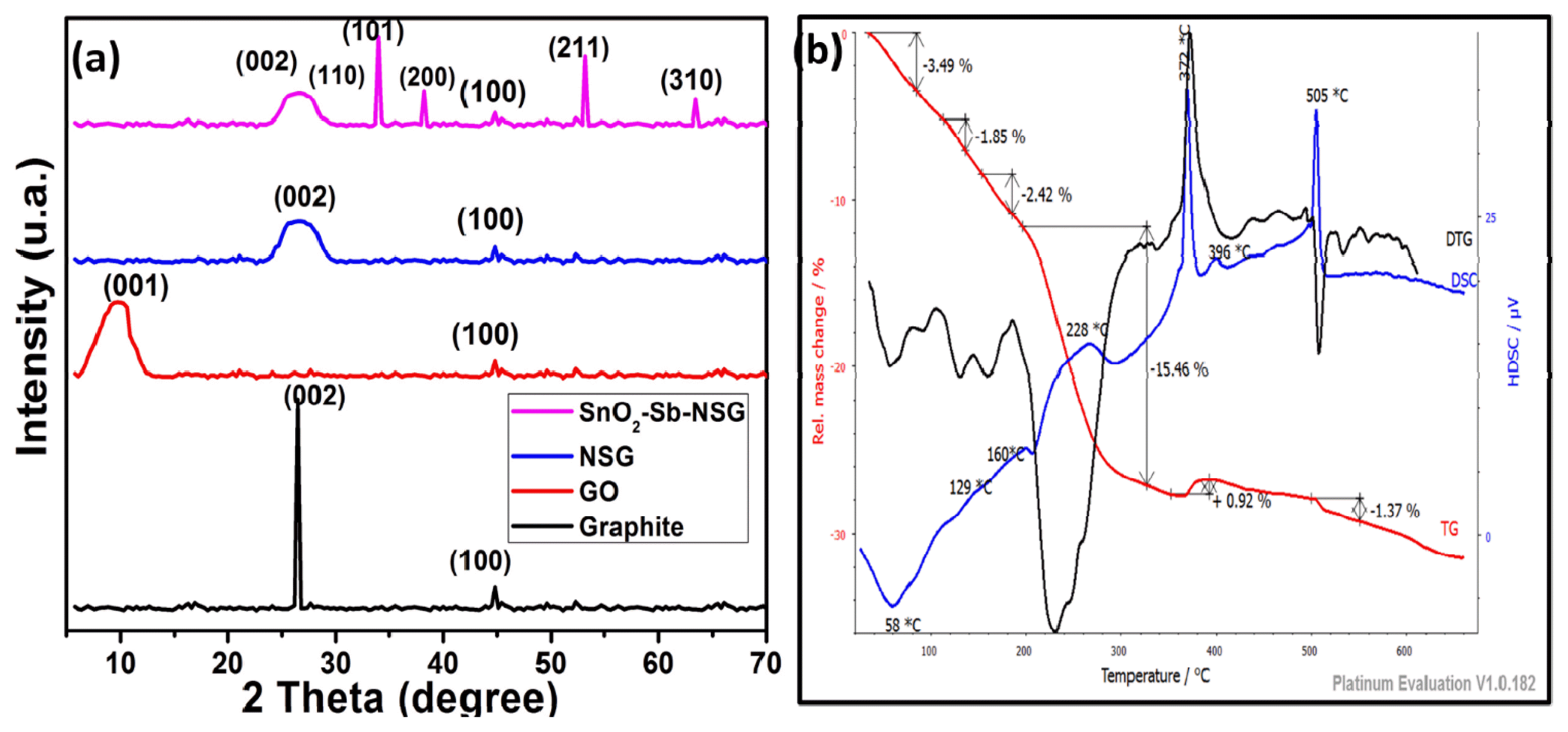
Fig. 4(a) Linear sweet voltammogram of electrode materials performed in 0.25M Na2SO4 solution with sweep speed of 10 mV/s and (b) Accelerated lifetime curves of electrode materials. 
Fig. 5The response surface of MO (A) and NR (B) removal efficiencies (%) as function of (A1 and B1) current density and electrolysis time, (A2 and B2) current density and initial dye concentration, and (A3 and B3) electrolysis time and initial dye concentration. 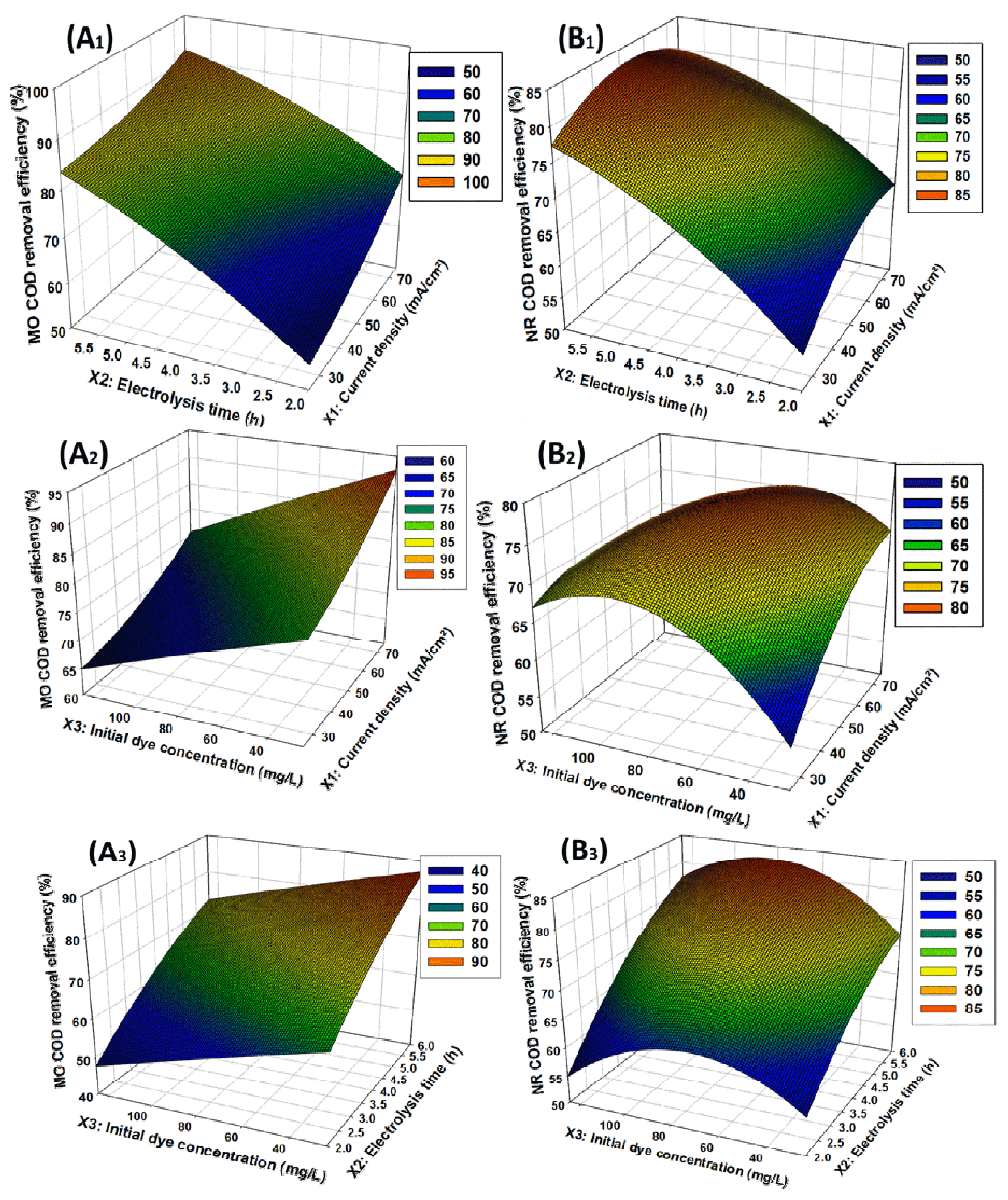
Fig. 6a) FTIR of MO before and after degradation and, b) COD removal efficiencies of MO, NR and MO + NR. 
Table 1Full Factorial Design Used for the DAO of MO and NR Dyes on Ti/SnO2-Sb-NSG Electrode. |
|
||||||||||||||||||||||||||||||||||||