AbstractThe UV/H2O2 system is an effective advanced oxidation process (AOP) widely used for micropollutant abatement in drinking water and wastewater treatment plants. Recently, multiphysics simulations based on computational fluid dynamics (CFD) and chemical kinetics have shown promise by accurately depicting water treatment processes. This study demonstrates the feasibility of incorporating experimental results from a pilot-scale UV/H2O2 system into a multiphysics model. Various factors affecting contaminant degradation and oxidant production such as H2O2 dose, H2O2 injection methods, and reactor size were investigated. The obtained data became the basic building blocks when developing the numerical model. The model predictions exhibited a good correlation with the experimental results. 3-D visualizations using the model allow further in-depth analysis of the distribution of oxidants and target pollutant in the UV photoreactor, providing insights into the possibilities of reactor optimization.
Graphical Abstract
1. IntroductionOver the past few decades, numerous drinking water and wastewater treatment processes have been introduced and commercially implemented to combat the increasing threat of water pollutants, including pathogens and both organic and inorganic compounds. Advanced oxidation processes (AOPs) have recently gained attention as a possible solution that can effectively and economically treat such pollutants in the water system, usually without creating even more harmful (compared to parent) disinfection by-products (DBPs) [1]. AOPs use the powerful and nonselective OH radical (•OH) as the primary oxidant, which is a product of chemical reactions in AOP systems such as peroxone (O3/H2O2), UV/hydrogen peroxide (UV/H2O2), and heterogeneous photocatalysts [2,3].
This study focuses on the UV/H2O2 AOP system, in which H2O2 undergoes photolytic decomposition through UV irradiation to form •OH [4]. During the UV/H2O2 process, H2O2 photolysis is initiated with UV light absorption with a primary quantum yield of 0.5 [5] as reported in Eq. (1):
The UV/H2O2 process provides a number of advantages over other treatment methods for drinking water or wastewater treatment. Not only does direct UV photolysis serves as an additional tool for the degradation of contaminants or inactivation of pathogens, but the UV/H2O2 process is a cost-effective source of •OH utilizing a stable and available reactant (i.e., H2O2) that does not involve complicated operating procedures or gas-liquid mass transfer considerations [6–8].
Numerous studies have already focused on evaluating the effectiveness of UV/H2O2 process on the (bio)degradation of pollutants such as industrial discharge or pharmaceuticals in laboratory and pilot plant configurations, with varying degrees of success [9–12]. Researchers have previously explored the traditional kinetics behind the UV/H2O2 system by using key (photo)chemical reaction mechanisms or proposing degradation pathways. In some cases, selected reactions were compiled to develop kinetic models that could predict concentrations of oxidants or compounds of interest, which could then be compared with actual experimental results [13–18].
Recently, multiphysics computer software has proven to be invaluable in incorporating kinetics into 3-D reactors as to mimic their real-life counterparts. The mathematical equations governing the fluid dynamics inside the reactor as well as chemical and photolytic reactions occurring during the UV/H2O2 process are solved simultaneously using a multiphysics software. Elyasi and Taghipour [19] presented a general methodology for developing an integrated computational model of the UV/H2O2 system by evaluating hydrodynamic, radiation, and species concentration models separately, reducing the uncertainty of an integrated model. In addition, Alpert et al. [20] used PHOENICS CFD software to compare the effect of different turbulent (e.g., k-ɛ, RNG k-ɛ, k-ω) and UV fluence rate (e.g., RAD-LSI, MSSS) sub-models on model predictions while assuming pseudo-steady-state conditions for radical components. Barrera et al. [21] simulated UV-C and VUV lamp systems with or without H2O2 added as an oxidant. They focused on total organic carbon (TOC) removal using the local volumetric rate of energy absorption (LVREA) concept of UV fluence.
Furthermore, through a multiphysics program, it is possible to simulate various reactor/lamp configurations, target micropollutant (MP) degradation, turbulence models, etc., in a UV/H2O2 system. This makes it an insightful and cost-effective tool for the design optimization of reactors. Johnson and Mehrvar [22] studied single and multiple lamp arrangements for reactors in series to predict optimal initial H2O2 dose, concluding that a multiple lamp arrangement showed better MP removal efficiency and lower operating costs. Santoro et al. [23] and Ducoste and Alpert [24] compared single lamp or multi-lamp systems, respectively, using parallel and cross flow systems. In the latter research, different modeling approaches were taken, each with its advantages. The study suggested using an appropriate model depending on the purpose of the simulation (e.g., reactor design optimization, study on MP removal, etc.) and which reactor-related information is available. Wols et al. [25] analyzed a wide range of annular, cross-flow, and streamlined lamp reactors with different geometries for each reactor type using COMSOL multiphysics software, suggesting the flexibility of numerical simulations when modeled accurately. In an interesting approach, Mohajerani et al. [26] used COMSOL to optimize the radius of the UV/H2O2 reactor, where larger radii led to reduced UV LVREA distribution farther away from the UV lamp but with longer residence time overall, and smaller radii led to higher radiation energy within the reactor but led to shorter residence times.
This study aims to realistically model a pilot-scale UV/H2O2 process with the help of multiphysics software. Initial experiments were conducted using a pre-designed pilot-scale UV/H2O2 plant set up at a wastewater treatment plant with different configurations of initial H2O2 dose, injection method, or reactor diameter. These results provided important information for developing a numerical model that could accurately depict the various physical mechanisms and chemical reactions occurring in the system. The simulation data were compared with actual experimental results and visualized in 3-D for further analysis.
2. Materials and Methods2.1. Site Description and Reactor SetupA sewage treatment plant located in Seoul, Republic of Korea, was selected due to the presence of treated wastewater discharge with acceptable UV transmittance values. Both conventional sludge and anaerobic-anoxic-aerobic (i.e., A2O) methods as well as Modified Ludzack-Ettinger (MLE) activated sludge process are used at this wastewater treatment plant, and treated wastewater effluent was used for pilot-scale UV/H2O2 experiments.
For initial experiments, two identical cylindrical reactors 1 m long and 90 mm in diameter were connected in series to set up the pilot plant (Fig. 1a and 1b). Each reactor consisted of a single, 8 kW medium pressure UV lamp (MPL) 55 cm long with a diameter of 24 mm (from ETA+) installed axially, surrounded by a quartz sleeve (from QSIL) resulting in a 40 mm diameter lamp/sleeve configuration. MPLs were used due to their high power density, reducing the number of lamps required and the complexity of the system. In order to determine the effect of diameter on the UV/H2O2 process, additional experiments were conducted using a single cylindrical reactor with specifications similar to the one described above, but with differing reactor diameters of 80 or 100 mm.
The input flow of wastewater effluent was kept at a constant of 7 L/min (equivalent to approx. 10 tons/day). The initial dissolved organic carbon (DOC) concentration of wastewater effluent was 4.0 mg/L, with a UV transmittance of 95% (at 254 nm, using 1 cm cell). H2O2 was added via side-stream at the beginning of each reactor. H2O2 concentration was adjusted by varying the pump flow rate (between 20 and 35 mL/min, depending on experiment condition) of the oxidant tank containing pre-prepared H2O2 stock solution (between 2,000 and 3,500 mg/L, depending on experiment condition). In order to determine the extent of •OH production, probe compound pCBA was selected due to its UV-resistant nature [27] and was spiked in low concentrations (10 μM) inside the raw water tank at the beginning of the UV/H2O2 process. For initial experiments, a low concentration (also 10 μM) of UV-resistant caffeine [28] was added to the raw water tank to compare actual abatement results with predicted MP abatement using •OH exposure (further discussed below).
2.2. Analytical MethodsThe concentration of H2O2 was measured using titanium sulfate (Ti(SO4)2) method provided by Eisenberg [29]. Colorimetric determinations were performed on-site using a DR1900 portable spectrophotometer (from Hach) at 405 nm to eliminate possible errors arising from delayed measurements.
Off-site, DOC concentration was measured using M5310 C TOC analyzer (from Sievers), and UV transmittance was assessed using LAMBDA 465 UV/Vis spectrophotometer (from PerkinElmer). pCBA and caffeine concentrations were measured by rapid separation liquid chromatography (RSLC) using UltiMate 3000 HPLC system separated by Acclaim 120 C18 column (from Thermo Fisher Scientific). From the concentration change of pCBA, the time-concentration value of •OH exposure during the UV/H2O2 process can be calculated using the following Eq. (2):
where k•OH,pCBA is the second-order rate constant between •OH and pCBA at 5×109 M−1 s−1 [30]. This approach can be used to predict caffeine decomposition using the second-order rate constant between •OH and caffeine at 5.9×109 M−1 s−1 [31] and compare with actual abatement values from real-world UV/H2O2 experiments. This can be achieved by slightly adjusting Eq. (2) into Eq. (3):
2.3. Multiphysics Simulation MethodsA multiphysics model was developed based on preliminary experimental results to simulate the behavior of the pilot plant UV/H2O2 system. In this model, the simulation domain is two UV photoreactors connected in series. The source water in question contains target compounds (i.e., pCBA and caffeine) and photoreactive chemical species (i.e., H2O2).
The shape of each photoreactor was a hollow cylinder with two cylinders connected tangentially at each end of the cylinder, acting as either an inlet or outlet. The two photoreactors are connected by a tube with the same diameter as the inlet/outlet. When necessary, a second injection occurred at the beginning of the connecting tube (referred to as two-step injection in this study). At the center portion (55% of its total length in the middle) of the inner wall of the hollow cylinder, a boundary wall condition was established to represent UV fluence. It was simulated by the emission of ultraviolent light at 254 nm with a radiation intensity of 1500 W/(cm2×sr). The 3-D simulation domain was prepared and discretized by tetrahedral mapped meshes.
Four physics modules in COMSOL Multiphysics software (version 6.0, COMSOL, Inc., Burlington, MA, USA) were incorporated for the numerical simulation: the turbulent flow module (spf) for the flow channel, the radiation in participating media module (rpm) for the UV lamp, the transport of diluted species module (tds) for the mass transport of chemical species, and the chemistry module (chem) for chemical reactions occurring during the UV/H2O2 process.
The simulation process was performed in two steps. In the initial step, the stationary solver calculated the equations of the turbulent flow module and the radiation in participating media module. Note that the velocity field, pressure field, and incident radiation were assumed to remain constant. With the inlet flow rate at 7 L/min, a stationary flow field (velocity field u and pressure p) was calculated solving Reynolds-averaged Navier-Stokes (RANS) equations for momentum conservation (Eq. (4)) and the continuity equation (Eq. (5)) for mass conservation with the equation for turbulent viscosity (μT ) of k-ɛ model (Eq. (6)):
where ρ is the density of the solution, μ is the dynamic viscosity, F is the volume force vector, Cμ is the model constant, k is the turbulent kinetic energy, and ɛ is the turbulent dissipation rate.
Simultaneously, the incident radiation (G) was calculated using the P1 approximation method as presented by Eq. (7):
where DP1 is the P1 diffusion coefficient, κ is the absorption coefficient, and Ib is the blackbody radiative intensity.
In the subsequent step, the time dependent solver calculated the equations of the transport of diluted species module and the chemistry module. The mass balance equation for the chemical species i is given as Eq. (8):
where Di is the diffusion coefficient of i, ci is the concentration of i, and u is the flow velocity vector obtained from the CFD calculation of the first step. Ri is the source term from the photolysis reaction of the UV/H2O2 process calculated using the chemistry module.
The chemical and photochemical reactions of the UV/H2O2 process included in the numerical simulation are summarized in Table 1, which includes a detailed array of intermediates and radical components participating in various reactions. Note that the rate of reaction #1 was governed by Eq. (9), which interconnected radiation in participating media module and chemistry module:
where Φ4 is the quantum yield, G is the incident radiation obtained from the DOM, U254 is the molar photon energy at 254 nm (= 471528 J/Ein), ɛH2O2 is the molar absorption coefficient (19.6 M−1 cm−1), CH2O2 and is the concentration of H2O2 in the specific part of simulation domain [32].
The numerical calculation was based on the finite element method (FEM) using a 64-core processor workstation (AMD Ryzen Threadripper PRO 5995WX CPU 2.70GHz, 512 GB memory). A direct solver MUMPS was utilized in the flow, radiation, mass transfer, and mass transport problem, and the backward differentiation formula (BDF) was used to solve time-dependent problems in the second step. Further details about the numerical simulations can be found in Fig. S1 and Tables S1 and S2 of the of the supplementary data.
3. Results and Discussion3.1. Pilot plant UV/H2O2 Experiments: H2O2 InjectionExperiments were conducted using different initial concentration and injection configurations for H2O2 to extract empirical values important for modeling. Initially, two default, 90 mm diameter reactors in series were used to determine the extent of •OH formation under various experimental conditions. Two versions of H2O2 injection approaches were used: a single injection method in which the entire initial H2O2 dose was inserted at the beginning of the UV/H2O2 process, or a two-step injection method in which the initial dose was divided in half to be injected equally at the beginning of each reactor. Two-step injection experiments were conducted to determine whether splitting the injections leads to higher oxidant yield efficiency, revisiting an idea from a previous study using a different AOP system [33]. The initial H2O2 dose was 5, 10, or 15 mg/L. Thus, for two-step injection experiments, H2O2 was added in two sets of 2.5, 5, or 7.5 mg/L, correspondingly. For these experiments, initial, intermediate (i.e., between two reactors), and output concentrations of H2O2, pCBA, and caffeine were measured (Fig. S2 of the supplementary data).
When comparing experiments with the same total H2O2 dose but differing in the number of injections, H2O2 consumption at the end of the UV/H2O2 process was relatively similar for total doses of 5, 10, or 15 mg/L regardless of injection type (Fig. 2a). However, abatement of •OH radical probe pCBA was higher for single H2O2 injection experiments than its two-step injection counterparts (Fig. 2b). Single injection methods benefit from receiving a large amount of H2O2 right at the beginning of the first reactor, generally with higher concentrations of H2O2 in UV reactors for photolysis during the entire process. Although the total injected concentration of H2O2 was the same, excluding very high H2O2 concentration operating conditions which may lead to scavenging of •OH, single injection experiments had a higher yield of •OH production and subsequent pCBA degradation. Using Eq. (2), this naturally led to higher •OH exposure values for single injection experiments (Fig. 2c).
Supplementary results regarding caffeine abatement performed in the same experiments confirmed above trends (Fig. 2d). Additionally, Eq. (3) could now be used to predict caffeine abatement and compare it with degradation results from UV/H2O2 experiments. As •OH oxidation is the primary source of abatement for both pCBA and caffeine, caffeine abatement can be confidently predicted using the experimental results of pCBA degradation during the same UV/H2O2 process. Overall, the predictions showed high accuracy when compared with experimental results (Fig. S3 of the supplementary data).
3.2. Pilot plant UV/H2O2 Experiments: Reactor DiameterAdditional UV/H2O2 experiments were conducted by converting the current pilot plant setup from two reactors in series to a single reactor and by varying the reactor diameter. Two additional reactors, one smaller and one larger reactor in diameter (80 and 100 mm, respectively), were manufactured to determine the effect of diameter on the current UV/H2O2 process. As these experiments used only a single reactor, only H2O2 and pCBA concentrations before and after flowing through the reactor were measured. All experiments were conducted using a single injection method with an initial H2O2 dose of 15 mg/L.
The results showed that the outlet H2O2 concentration decreased as diameter increased, alluding to the possible increase in H2O2 photolysis reactions occurring in reactors with a larger diameter (Fig. 3a). pCBA abatement results revealed that the smaller reactor showed less micropollutant abatement compared to the original reactor, while the wider reactor displayed better degradation results (Fig. 3b). From these results, the •OH exposure value of the 100 mm diameter reactor was calculated to be the largest among the three reactors at 5.86×10−10 M s, around 25% larger than the •OH exposure measured from the smallest, 80 mm diameter reactor at 4.67×10−10 M s (Fig. 3c).
For the smaller 80 mm diameter reactor, it is presumed that the attenuation effect of UV radiance is reduced to a degree and higher UV fluence may reach H2O2 in the target water, which at first glance, possibly suggests higher micropollutant degradation efficiency. However, since the volume occupied by the lamp and surrounding quartz sleeve remains constant inside the reactors, a reduction in diameter of just 10 mm results in a loss of working volume of more than 26% when compared to the original reactor. The small working volume of the reactor leads to much shorter hydraulic retention time (HRT) within the reactor, resulting in an overall lower formation of •OH. On the other hand, a wider reactor with a diameter of 100 mm may contain radial segments in the outer edge of the reactor that suffer from low UV fluence resulting from UV attenuation. However, a larger diameter provides additional working volume for the reactor, granting a longer HRT for further photolysis of H2O2 during the process, likely resulting in higher H2O2 consumption and subsequent •OH production.
3.3. Modeling of UV/H2O2 Pilot PlantWith the experimental results of different injection methods, initial H2O2 concentrations, and reactor diameters, COMSOL was used to first design a 3-D model of the two UV photoreactors in series setup (Fig. 1c). Simulation of the fluid dynamics in the present system suggested that the flow of water proceeds in a vortex-like fashion along the cylindrical axis as the wastewater effluent enters the reactor perpendicularly to the reactor at a high flow rate (Fig. 1d).
Simulated values from the numerical model were compared with initial experimental results varying the injection methods and initial H2O2 concentrations (Fig. 2). Overall, the simulation results showed a good correlation with their real-life counterparts for outlet H2O2 concentration, micropollutant abatement, and •OH exposure. The •OH exposure value for the two-step injection of 15 mg/L H2O2 dose can be considered an exceptional case (Fig. 2c). However, due to the logarithm in the numerator of Eq. (2), •OH exposure can be significantly exaggerated near 100% abatement, resulting in what may initially appear to be a large discrepancy between experimental results and simulated values. Simulation values and experimental results of variable reactor diameters were also compared, highlighting the high accuracy of simulations when developed directly with field data (Fig. 3).
By plotting 3-D models and graphs of compound concentration and •OH exposure change over the reactor length, it was possible to clearly visualize the overall reactions and changes occurring within the reactor as the diameter varies (Fig. 4). While H2O2 concentrations are similarly high at the initial third for all three reactors due to the lack of UV fluence and consequent lack of photolysis, the larger diameter reactor shows a darker blue hue (i.e., more H2O2 consumption) at the end of the reactor, agreeing with experimental results. The concentration profile of H2O2 clearly shows a higher consumption of H2O2 for reactors with larger diameters as water is processed through the reactor (Fig. 4a). The abatement of pCBA shows a visual pattern similar to the aforementioned H2O2 concentration change, with more degradation occurring in the center of 100 mm diameter reactor. The pCBA concentration profile graph confirms the higher abatement of micropollutant as the reactor diameter increases (Fig 4b). Previously, •OH-related values could only be discerned through indirect approaches such as using a probe compound to calculate •OH exposure. However, 3-D simulations can provide considerable details in any part of the reactor that would otherwise be impossible to obtain, such as the concentration profile of •OH. As expected, •OH concentration is the highest near the lamp/sleeve region of the reactor for all three diameter configurations, gradually decreasing with increasing distance from the center of the cylindrical axis. As the diameter expands to 100 mm, the outer edge sections of the reactor near the wall begin to show a significant drop in •OH concentration, indicating an optimum diameter for the current reactor setup. •OH exposure plotted over reactor length also shows the advantage of a larger reactor diameter in this study (Fig. 4c). Overall, the UV/H2O2 pilot plant with its various setups and experiment conditions could be accurately modeled in 3-D using multiphysics software, providing accurate simulated values that match well with real-world measurements (Fig. 5).
4. ConclusionsIn this study, various UV/H2O2 experiments were conducted in a pilot plant using wastewater effluent by varying the initial dosage of H2O2, number of H2O2 injections, and diameter length. The experimental results were critical in developing a multiphysics model that can accurately depict a real-life UV/H2O2 process. The simulation results showed a good correlation with the experimental results for significant values of the UV/H2O2 process, such as outlet H2O2 concentration, micropollutant abatement, and •OH exposure. A reliable multiphysics simulation can be very beneficial to retain, as numerical simulations can be conducted beforehand or even in place of experiments. Rather than fabricating and experimenting with each diameter configuration, diameter optimization can be achieved by modeling additional reactors with different diameters to determine the optimal parameter for maximum •OH production and confirmed with a smaller number of tests. Suppose when a specific target micropollutant known for its toxicity with a pre-established rate constant with •OH is selected, the abatement of said target can be predicted with a high level of confidence using a multiphysics simulation. Thus, there would be no need to spike it in the source water, which can be a cumbersome or an unrealistic course of action due to cost or environmental concerns.
Additional visual representations provided a better starting point for optimization of the reactor or process. For example, as most of pCBA has already degraded by the midpoint of the 100 mm diameter reactor, the lamp length does not need to remain as long as it currently is. Streamline plots can provide valuable information on so-called “dead zones” within the reactor to improve reactor design from a hydraulic perspective. Additionally, UV fluence plots can determine the viability of the current UV/H2O2 setup when interacting with waters of different UV transmittance characteristics. With endless possibilities for adaptation, this study confirms the feasibility and benefits of developing a numerical simulation model from real-life experiments for further optimization.
AcknowledgmentsThis work was supported by the Korea Environment Industry & Technology Institute (KEITI) through the Developing Innovative Drinking Water and Wastewater Technologies Project, funded by the Korea Ministry of Environment (MOE) (2019002710003).
NotesAuthor Contributions D.C. (Ph.D. student) conducted all pilot plant experiments and wrote the manuscript. G.L (Master’s student) conducted all simulations and compiled references. H.J. (Ph.D. student) provided academic support for simulations and revised the manuscript. J.Y. (Professor) and C.L. (Professor) revised the manuscript. References1. Gligorovski S, Strekowski R, Barbati S, Vione D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015;115:13051–13092.
https://doi.org/10.1021/cr500310b
2. Hoigné J. Chemistry of Aqueous Ozone and Transformation of Pollutants by Ozonation and Advanced Oxidation Processes. Hrubec J, editorQuality and Treatment of Drinking Water II, Part of The Handbook of Environmental Chemistry (Part C: Water Pollution). Berlin: Springer; 1998. p. 83–141.
3. Helz GR. Aquatic and Surface Photochemistry. Boca Raton: CRC Press; 1994. p. 451–468.
4. Wang GS, Hsieh ST, Hong CS. Destruction of humic acid in water by UV light-catalyzed oxidation with hydrogen peroxide. Water Res. 2000;34:3882–3887.
https://doi.org/10.1016/S0043-1354(00)00120-2
5. Liao CH, Gurol MD. Chemical oxidation by photolytic decomposition of hydrogen peroxide. Environ. Sci. Technol. 1995;29:3007–3014.
https://doi.org/10.1021/es00012a018
6. Legrini O, Oliveros E, Braun AM. Photochemical processes for water treatment. Chem. Rev. 1993;93:671–698.
https://doi.org/10.1021/cr00018a003
7. Jo CH, Dietrich AM, Tanko JM. Simultaneous degradation of disinfection byproducts and earthy-musty odorants by the UV/H2O2 advanced oxidation process. Water Res. 2011;45:2507–2516.
https://doi.org/10.1016/j.watres.2011.02.006
8. Zhang Z, Chuang YH, Szczuka A, et al. Pilot-scale evaluation of oxidant speciation, 1,4-dioxane degradation and disinfection byproduct formation during UV/hydrogen peroxide, UV/free chlorine and UV/chloramines advanced oxidation treatment for potable reuse. Water Res. 2019;164:114939.
https://doi.org/10.1016/j.watres.2019.114939
9. Adams CD, Kuzhikannil JJ. Effects of UV/H2O2 preoxidation on the aerobic biodegradability of quaternary amine surfactants. Water Res. 2000;34:668–672.
https://doi.org/10.1016/S0043-1354(99)00186-4
10. Andreozzi R, Canterino M, Marotta R, Paxeus N. Antibiotic removal from wastewaters: The ozonation of amoxicillin. J. Hazard. Mater. 2005;122:243–250.
https:/doi.org/10.1016/j.jhazmat.2005.03.004
11. Shahrestani MM, Rahimi A. Evaluation of electrical energy consumption in UV/H2O2 advanced oxidation process for simultaneous removal of NO and SO2
. Environ. Eng. Res. 2019;24:389–396.
https://doi.org/10.4491/eer.2018.276
12. Laftani Y, Boussaoud A, Chatib B, Hachkar M, Makhfouk ME, Khayar M. Photocatalytic degradation of a diazo-dye in artificial seawater matrix: Optimization of UV/H2O2 process on the Ponceau S decolorization by using central composite design. Environ. Eng. Res. 2021;27:210002.
https://doi.org/10.4491/eer.2021.002
13. Glaze WH, Lay Y, Kang JW. Advanced Oxidation Processes. A kinetic model for the oxidation of 1,2-dibromo-3-chloropropane in water by the combination of hydrogen peroxide and UV radiation. Ind. Eng. Chem. Res. 1995;34:2314–2323.
https://doi.org/10.1021/ie00046a013
14. Stefan MI, Hoy AR, Bolton JR. Kinetics and mechanism of the degradation and mineralization of acetone in dilute aqueous solution sensitized by the UV photolysis of hydrogen peroxide. Environ. Sci. Technol. 1996;30:2382–2390.
https://doi.org/10.1021/es950866i
15. Crittenden JC, Hu S, Hand DW, Green SA. A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Res. 1999;33:2315–2328.
https://doi.org/10.1016/S0043-1354(98)00448-5
16. Stefan MI, Mack J, Bolton JR. Degradation pathways during the treatment of methyl tert-butyl ether by the UV/H2O2 Process. Environ. Sci. Techno. 2000;34:650–658.
https://doi.org/10.1021/es9905748
17. Edalatmanesh M, Dhib R, Mehrvar M. Kinetic modeling of aqueous phenol degradation by UV/H2O2 process. Int. J. Chem. Kinet. 2008;40:34–43.
https://doi.org/10.1002/kin.20286
18. Ghafoori S, Mehrvar M, Chan PK. Free-radical-induced degradation of aqueous polyethylene oxide by UV/H2O2: Experimental design, reaction mechanisms, and kinetic modeling. Ind. Eng. Chem. Res. 2012;51:14980–14993.
https://doi.org/10.1021/ie3005995
19. Elyasi S, Taghipour F. Simulation of UV photoreactor for degradation of chemical contaminants: Model development and evaluation. Environ. Sci. Techno. 2010;44:2056–2063.
https://doi.org/10.1021/es902391t
20. Alpert SM, Knappe DRU, Ducoste JJ. Modeling the UV/hydrogen peroxide advanced oxidation process using computational fluid dynamics. Water Res. 2010;44:1797–1808.
https://doi.org/10.1016/j.watres.2009.12.003
21. Barrera M, Mehrvar M, Gilbride KA, et al. Photolytic treatment of organic constituents and bacterial pathogens in secondary effluent of synthetic slaughterhouse wastewater. Chem. Eng. Res. Des. 2012;90:1335–1350.
https://doi.org/10.1016/j.cherd.2011.11.018
22. Johnson MB, Mehrvar M. Aqueous metronidazole degradation by UV/H2O2 process in single-and multi-lamp tubular photoreactors: Kinetics and reactor design. Ind. Eng. Chem. Res. 2008;47:6525–6537.
https://doi.org/10.1021/ie071637v
23. Santoro D, Raisee M, Moghaddami M, et al. Modeling hydroxyl radical distribution and trialkyl phosphates oxidation in UV-H2O2 photoreactors using computational fluid dynamics. Environ. Sci. Techno. 2010;44:6233–6241.
https://doi.org/10.1021/es1000962
24. Ducoste JJ, Alpert SM. Computational fluid dynamics modeling alternatives for UV-initiated advanced oxidation process. Water Qual. Res. J. Canada. 2015;50:4–20.
https://doi.org/10.2166/wqrjc.2014.035
25. Wols BA, Hofman JAMH, Beerendonk EF, Uijttewall WSJ, van Dijk JC. A systematic approach for the design of UV reactors using computational fluid dynamics. AlChe J. 2011;57:193–207.
https://doi.org/10.1002/aic.12255
26. Mohajerani M, Mehrvar M, Ein-Mozaffari F. Photoreactor design and CFD modelling of a UV/H2O2 process for distillery wastewater treatment. Can. J. Chem. Eng. 2012;90:719–729.
https://doi.org/10.1002/cjce.20569
27. Rosenfeldt EJ, Linden KG. The ROH,UV concept to characterize and the model UV/H2O2 process in natural waters. Environ. Sci. Techno. 2007;41:2548–2553.
https://doi.org/10.1021/es062353p
28. Sun P, Lee WN, Zhang R, Huang CH. Degradation of DEET and caffeine under UV/Chlorine and simulated sunlight/chlorine conditions. Environ. Sci. Techno. 2016;50:13265–13273.
https://doi.org/10.1021/acs.est.6b02287
29. Eisenberg GM. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943;15:327–228.
https://doi.org/10.1021/i560117a011
30. Neta P, Dorfman LM. Pulse Radiolysis Studies. XIII. Rate Constants for the Reaction of Hydroxyl Radicals with Aromatic Compounds in Aqueous Solutions. Hart EJ, editorRadiation Chemistry Volume I. Aqueous Media, Biology, Dosimetry. Washington, D.C: American Chemical Society; 1968. p. 222–230.
31. Broséus R, Vincent S, Aboulfadl K, et al. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res. 2009;43:4707–4717.
https://doi.org/10.1016/j.watres.2009.07.031
32. Semitsoglou-Tsiapou S, Templeton MR, Graham NJD, et al. Low pressure UV/H2O2 treatment for the degradation of the pesticides metaldehyde, clopyralid and mecoprop – Kinetics and reaction product formation. Water Res. 2016;91:285–294.
https://doi.org/10.1016/j.watres.2016.01.017
33. Lee C, Yoon J, von Gunten U. Oxidative degradation of N-nitro-sodimethylamine by conventional ozonation and the advanced oxidation process. Water Res. 2007;41:581–590.
https://doi.org/10.1016/j.watres.2006.10.033
Fig. 1(a) Photoreactors containing UV lamp before installation and (b) two UV photoreactors installed in series. (c) 3-D model and (d) streamline plot of two UV photoreactors in series, with intermediate inlet for two-step injection experiments. 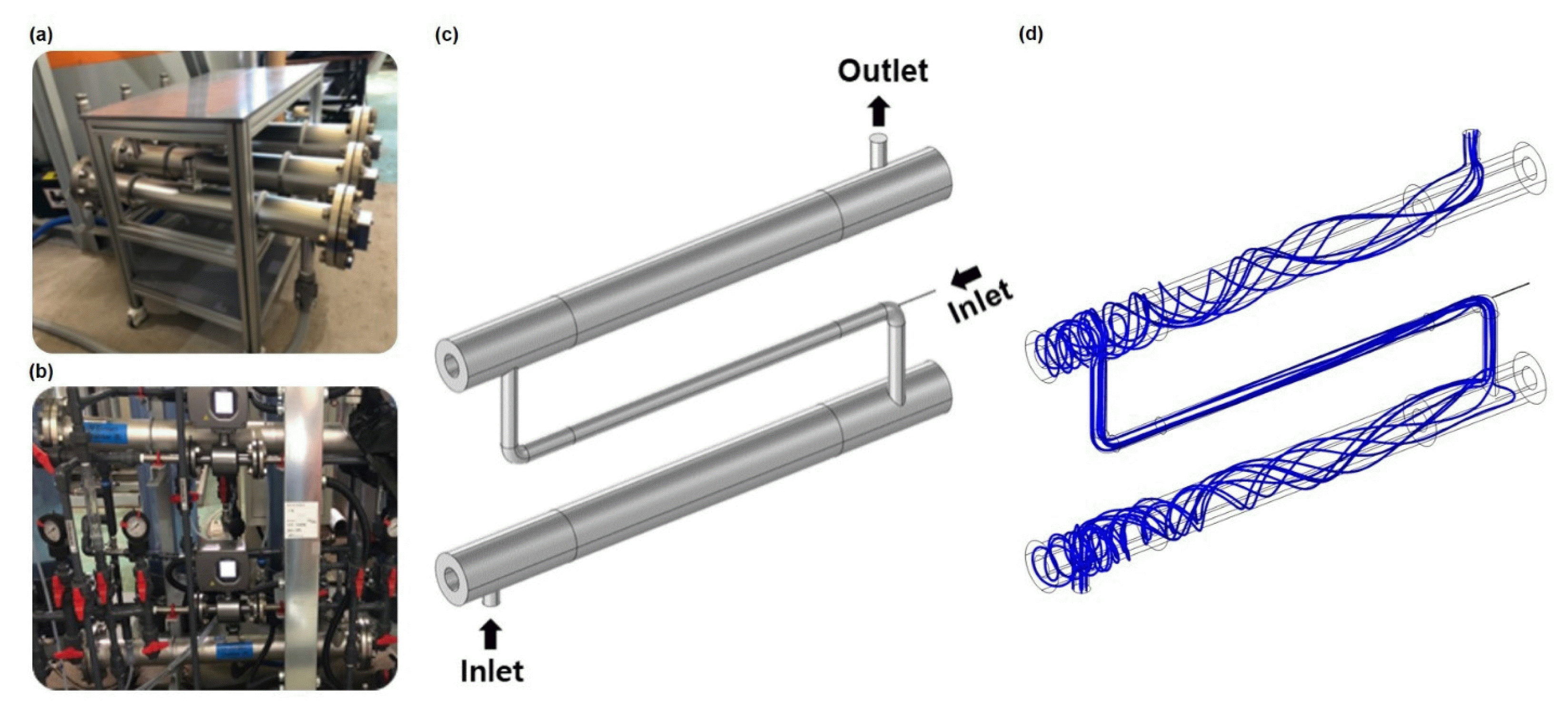
Fig. 2Experimental results vs. simulated values of (a) outlet H2O2 concentration, (b) pCBA abatement, (c) •OH exposure, and (d) caffeine abatement for single and two-step injection experiments ([pCBA]0 & [caffeine]0 = 10 μM). 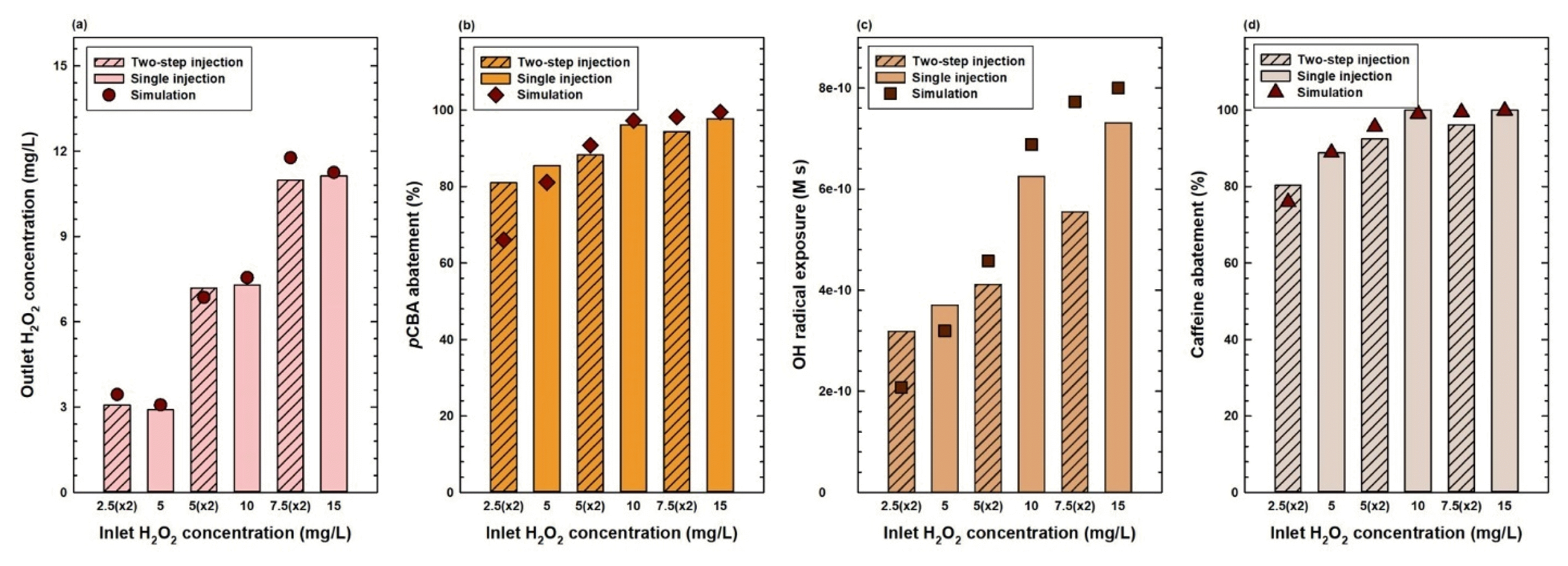
Fig. 3Experimental results vs. simulated values of (a) outlet H2O2 concentration, (b) pCBA abatement, and (c) •OH exposure for variable reactor diameter experiments ([H2O2]0 = 15 mg/L, [pCBA]0 = 10 μM). 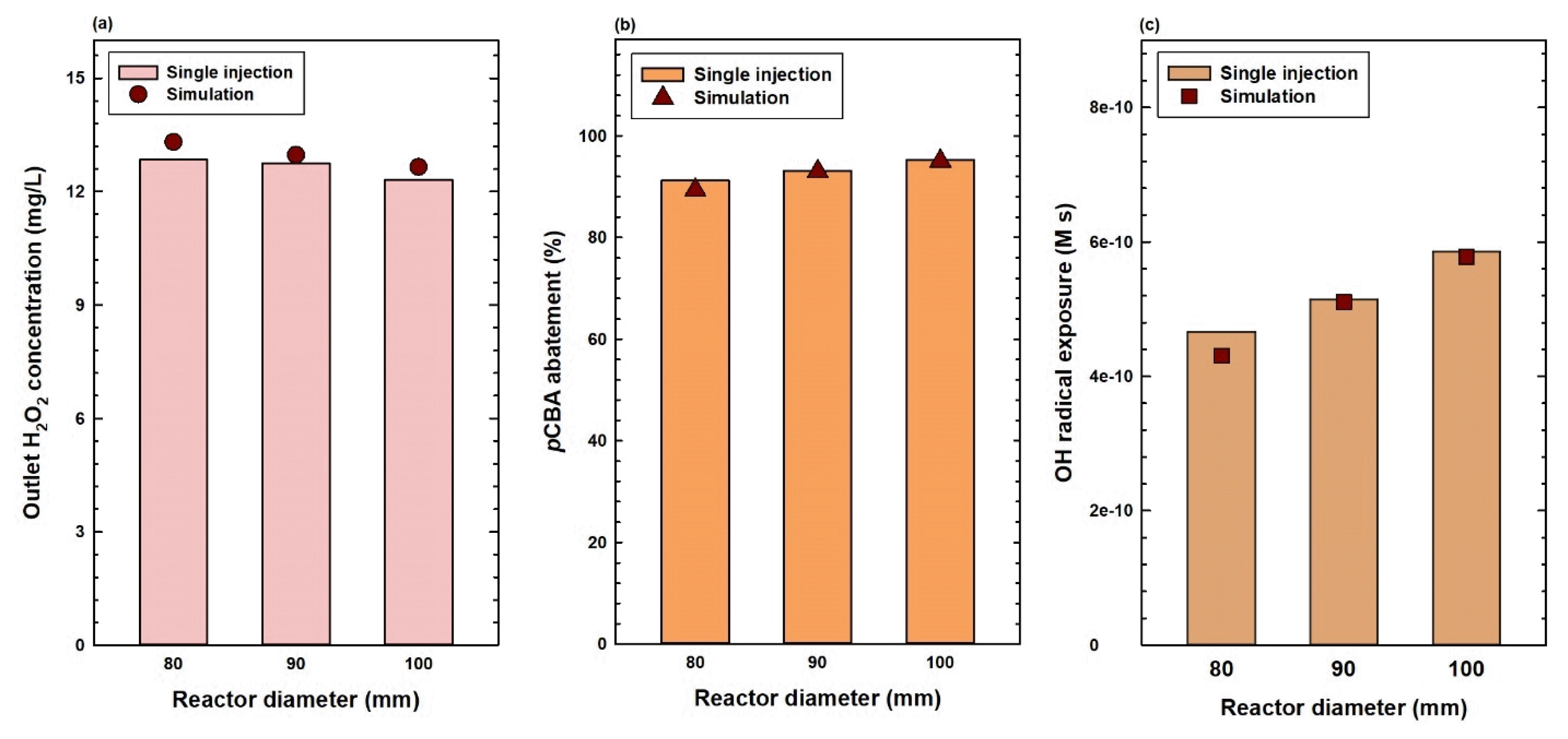
Fig. 43-D simulated concentration values of (a) H2O2, (b) pCBA, and (c) •OH for variable reactor diameter experiments ([H2O2]0 = 15 mg/L, [pCBA]0 = 10 μM). Colored dots for bottom row plots indicate simulation values from Fig. 3 for their respective diameter experiments. 
Fig. 5Measured vs. simulated (a) outlet H2O2 concentration and (b) micropollutant abatement for all UV/H2O2 experiments at the end of the first UV photoreactor (regardless of single or two-step injection, [pCBA]0 & [caffeine]0 = 10 μM). 
Table 1Chemical and photochemical reactions occurring during the UV/H2O2 process
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||