AbstractThe research aims for the simultaneous use of chlorine and UV irradiation (UV/Chlorine) to decolorize and detoxify Navy Blue 250 (NB250) and raw dye wastewater. The UV/Chlorine was the most effective in removing NB250 and color intensity (American Dye Manufacturers Institute, ADMI unit). The UV/Chlorine removed NB250 approximately 1.47–1.50 times faster than chlorination alone. The degradation constants in UV/Chlorine were 0.3317 mM-1min-1 and 0.3384 mM-1min-1 for NB250 and its ADMI color intensity, respectively. The optimum pH for decolorization was between 6 and 7. The UV/Chlorine with a chlorine dose of 1.0–2.0 mM was recommended for decolorization to meet the industrial discharge standard (300 ADMI). Reactive chlorine species (RCS) were more significant oxidants for NB250 removal than HO•. The presence of NB250 inhibited the germination of morning glory (Ipomoea aquatica) seeds, and exposure to raw dye wastewater (color = 6,658 ± 227 ADMI) completely inhibited seed germination. However, the samples treated by the UV/Chlorine were less toxic to seed germination and root growth. Therefore, the UV/Chlorine has a high potential in the decolorization and detoxification of dye wastewater. This is the first study to use the UV/Chlorine to treat industrial dye wastewater.
Graphical Abstract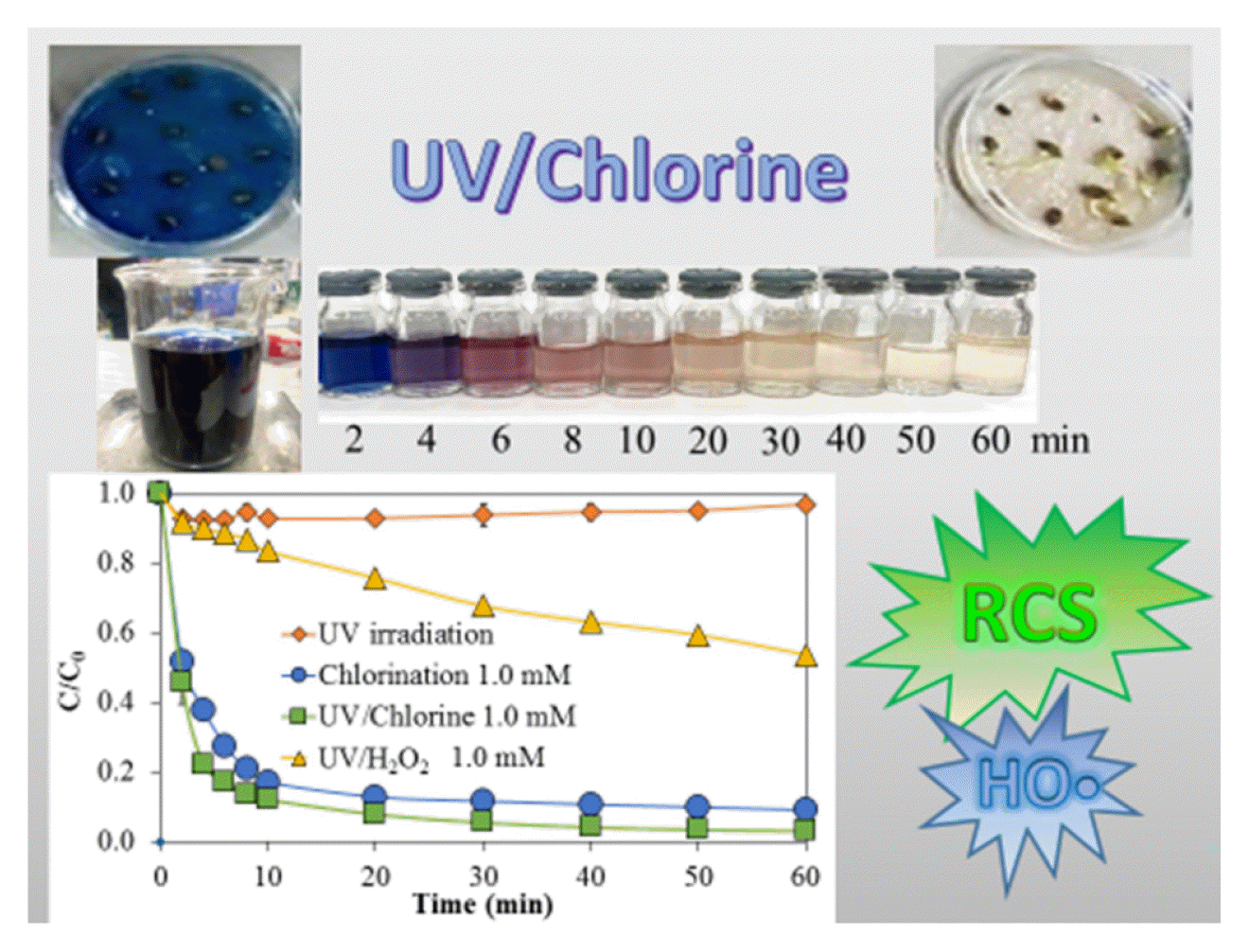
1. IntroductionSynthetic dyes are extensively used in textile and dyeing factories. Among the industrial groups potentially polluting color to the environment, the textile and dyeing industries are the most frequently reported [1]. Due to insufficient dyeing processes and limitations in the dye-absorption capacity of fabrics [2], 30–40% of dyes generally remains and is released into wastewater [3, 4]. Most synthetic dyes consist of an azoic bond (N = N) [3, 5, 6], and the presence of azoic structures leads to a lowered degradation efficiency of conventional wastewater treatment processes [7, 3, 8]. The dye residuals in effluent can cause aesthetic problems, even at low concentrations (10–50 mg L−1) [9], and dye residuals can endanger aquatic, as well as human ecosystems [10, 11, 1].
Navy Blue 250 (NB250), a reactive dye, has been commonly used in the cellulose-dyeing process, and its structure includes two chromophores of diazo bonds connected with aromatic structures (Fig. S1). This diazoic dye is not easily biodegraded [12, 13, 14], and the use of biological treatment processes requires specific conditions, such as sufficient retention times, specific and various microbial species, and adequate nutrients [8, 15, 1], leading to practice limitations. Therefore, chemical oxidation is an interesting option for dye removal to avoid potential adverse effects on ecosystems, as mentioned above. Because treated water from wastewater treatment plants can be discharged into receiving water resources, where it is occasionally used for irrigation, phytotoxicity tests should be carried out to provide biosafety information for water reclamation.
Recently, the simultaneous use of chlorine and UV irradiation (UV/Chlorine) has been introduced as a novel advanced oxidation process [16]. UV photolysis of free chlorine (HOCl and OCl−) produces several free radicals, including hydroxyl radical (HO•), chlorine radical (Cl•), and its derivative radicals, including ClO•, ClOH•−, Cl2•−, etc. (called reactive chlorine species [RCS]) [17, 18], as well as promotes the degradation of recalcitrant compounds. HO• is considered the strongest oxidant typically used in wastewater treatment, as its oxidation potential is 2.7 V. Although the oxidation potential of RCS (1.6–2.4 V) is lower [18], its presence also enhances the removal of benzoic acid by 62–65% [16]. The molar absorption coefficients of HOCl and OCl− at 254 nm are 59 ± 1 and 66 ± 1 L.(mol·cm)−1, respectively [19], whereas that of H2O2 is only 18.4 L.(mol·cm)−1 [20]. Thus, the UV/Chlorine process is expected to realize greater treatability and more economy than conventional advanced oxidation processes (AOPs) (e.g., UV/H2O2) [21]. In addition, the UV/Chlorine process can suppress various disinfection byproduct formations (trichloromethane, chloral hydrate, dichloropropanone, trichloropropanone, trichloronitromethane, dichloroacetonitrile, and trichloroacetonitrile), leading to lower toxicity than chlorination alone [22]. Several research teams found that the oxidation products from the UV/Chlorine process were less toxic than their parent compounds [23, 24, 25].
The formation of free radicals depends on various factors: chlorine dose and UV intensity can be directly proportional to the amount of free radicals formed, while chlorine speciation induces different formation pathways of free radicals [19, 16, 21]. The dissociation of HOCl by UV photolysis stoichiometrically forms HO• and Cl•, and the reaction between OCl− and UV protons preferentially generates O• and Cl•. Because a change in pH forms different active chlorine species, the effect of pH cannot be negligible. A previous study found that higher chlorine doses enhanced the removal of Reactive Red-2 dye, and decolorization was highest at pH 7 [6]. On the other hand, HOCl, a key substance in HO• formation, is predominant in acidic conditions, leading to a higher oxidation capability [26, 27]. This contradiction may be caused by water matrixes, pollutant characteristics, and operating conditions. Although several research teams reported dye degradation by the UV/Chlorine process [12, 28], most conducted the experiment with specific dyes in synthetic waters. One study attempted to conduct the UV/Chlorine process in environmental samples by adding reactive green 12 in seawater and municipal wastewater effluent [29]. This circumstance may not perfectly simulate actual industrial dye wastewater, as various constituents that co-occur with dye compounds can affect the efficiency of dye removals. From a practical viewpoint, it is therefore crucial to examine the treatment performance using actual wastewaters. In addition, the role of free radicals in NB250 degradation has not been revealed. An understanding of operating factors can provide useful information for the control of dye compounds.
The research aims to assess the removal of NB250 dye by the UV/Chlorine process in comparison with chlorination or UV irradiation alone. To the best of our knowledge, this is the first study to investigate the use of the UV/Chlorine process to treat NB250 dye and raw dye wastewater. The effect of the operating parameters (chlorine dose, contact time, and pH) on dye degradation was also investigated, and degradation kinetics were revealed. Scavengers, including benzoic acid (BA) and nitrobenzene (NB), were used to determine the role of free radicals in NB250 removal. Because dyes can react differently with active agents (e.g., chlorine, free radicals, H2O2), the removal of NB250 via the UV/Chlorine process was compared with that via the UV/H2O2 process, which is a conventional AOP [30]. Finally, the phytotoxicity was examined to provide complete information on biosafety for water reclamation in irrigation. NB250 was used as a model compound in this study, as it is UV-resistant and has a high potential to remain in treated wastewater. The research findings are beneficial for the development of conventional chemical oxidation (e.g., chlorination) to an advanced oxidation process (UV/Chlorine) to enhance the removal of recalcitrant dye.
2. Materials and Methods2.1. Chemical and SampleC.I. Navy blue 250 (NB250) was obtained from a textile industry. A synthetic dye water was prepared with an initial NB250 concentration of 100 mg L−1, responsible for a color intensity in the range of 6,800–7,400 according to the American Dye Manufacturers Institute (ADMI) color scale. This dye color exhibits a color intensity equivalent to the raw wastewater obtained from a textile dyeing industry located in Bangkok, Thailand. The quality of raw wastewater was pH 8.4, color 6,400–7,100 ADMI, chemical oxygen demand (COD) 184 mg L−1, biochemical oxygen demand (BOD) 32 mg L−1 and dissolved organic carbon (DOC) 132 mg L−1. The wastewater exhibited a low BOD to COD ratio (0.17), reflecting a large amount of non-biodegradable organic matter. Sodium hypochlorite (NaOCl 4 – 6%) was purchased from Roongsub Chemical Ltd. A phosphate buffer solution (40 mM) prepared from a mixture of sodium phosphate dibasic heptahydrate (Na2HPO4.7H2O) and sodium phosphate monobasic monohydrate (NaH2PO4.H2O) was used to control pH during the reaction.
2.2. ExperimentUV/Chlorine oxidation was conducted in a 250-mL beaker illuminated with three UV-C lamps (16W, Shubyue). The UV-C lamps in a quartz sleeve (i.d., 20 mm; L, 355 mm) were installed 15 cm above the water surface. The UV-C lamps were warmed for 20 min before starting the experiment. The corresponding average UV intensity was 3.54 mW.cm−2, determined by potassium iodide-iodate actinometer [31]. The chlorine was diluted in the sample solution to achieve the targeted dose in a range of 0.1–2.0 mM. The mixture was placed under the UV lamps and continuously stirred with a magnetic agitator at an ambient temperature. The change in the temperature of the solutions was minor during the reaction (25 ± 3 °C). The pH value of the dye solution was controlled at 6, 7, 8 and 9 by the phosphate buffer solution. The difference between the initial and final pH values was less than 0.2. The samples were treated for 0–60 min contact time. 1 mM Na2S2O3 was used to quench the residual chlorine. 5 mM Nitrobenzene (NB), purchased from PanReac AppliChem (Germany), was used as a scavenger for HO•, while 5 mM benzoic acid (BA), obtained from KEMAUS (Australia), was added to trap HO• and RCS [16]. To compare the efficiency with the UV/Chlorine process, the UV/H2O2 process was run with 1 mM of H2O2, pH 7 ± 0.2, and a UV intensity of 3.54 mW.cm−2. All experiments were conducted in triplicate.
2.3. Analytical MethodThe NB250 concentration was determined by a spectrophotometer (4001/4, GENESYS 20) with a light absorbance at the wavelength of 595 nm. The color scale, according to ADMI units, was measured by a spectrophotometer (DR 600, HACH) within the range of 0–300 ADMI. The concentration of the chlorine was measured by the N,N-diethyl-p-phenylenediamine (DPD) method [32].
The phytotoxicity test was modified from a previous study [33]. The sample was evaluated on germination of morning glory (Ipomoea aquatica) seeds. Briefly, 20 mL of samples (e.g., treated and untreated samples) was fed in a Petri dish lined with cultivation paper. The germination of target seeds in ultrapure water was considered the control sample. Each treatment was added with 10 seeds. Germination was monitored for 7 d. All conditions were repeated in triplicate. The relative seed germination index (RSGI) and germination rate (GR) were calculated following Eq. (1) and Eq. (2), respectively, and the root inhibition (RI) was determined by Eq. (3).
where SG is a number of germinated seeds in the sample, and TS is total seeds in the sample.
where SGs is the number of germinated seeds in the sample, and SGc is the total germinated seeds in the control sample.
where A is the root length in the control sample, and B is root length in the sample.
3. Results and Discussion3.1. Comparative Dye Removals between UV/Chlorine, UV/H2O2, Chlorination, and UV IrradiationThe different methods for removing NB250 and its color intensity (ADMI) are shown in Fig 1a and 1b, respectively. The NB250 concentration and its color were slightly changed, although UV irradiation was carried out for 60 min, indicating this dye compound was highly resistant to UV photolysis. This result is similar to a previous finding that a reactive dye compound (i.e., C.I. Reactive Orange 16) was not degraded during UV irradiation (28 W, two UV-C lamps) [34]. Chlorination at 1 mM rapidly removed NB250 dye by 95.4%, indicating a high reactivity of NB250 to chlorine. The ADMI color intensity was decreased by 90.9%. An intermediate or oxidized product could be a chromatic compound, resulting in the total target pollutants presented in the ADMI color analysis being higher than those in the parent compound alone (i.e., NB250). Thus, a lower removal of dye intensity was observed, compared to the removal of NB250. A previous study found that free chlorine (e.g., HOCl and OCl−) partially removed and transformed Disperse Red-1 dye into intermediates, such as 1-chloro-4-nitrobenzene and 2-((4-chlorophenyl) (ethyl)amino)-ethanol [35]. Although NB250 reacted highly to the chlorine, the color of the treated water with a 1-mM chlorine dose was still unacceptable, as its color intensity (577 ADMI) was higher than the industrial standard for effluent in Thailand (300 ADMI) [36]. Dye removal by the UV/Chlorine process was the most effective, as it quickly decreased the NB250 concentration and ADMI color intensity in 10 min. The NB250 dye and ADMI color intensity were removed at rates of 99.6% and 96.9%, respectively. A slight enhancement in decolorization via the UV/Chlorine process, compared with chlorination, possibly resulted from the UV-screening effect of dye, causing a significant reduction in UV light when high dye concentrations are presented [37]. However, the ADMI color intensity was reduced to below the color standard after 40 min contact time (275 ADMI), and it could further decrease to 200 ADMI at 60 min contact time. Direct photolysis of HOCl/OCl− produced HO• and RCS, which enhanced the oxidation of organic compounds into smaller molecules [38]. The degradation product from UV/Chlorine was more biodegradable, compared to its parent compound (NB250), as indicated by the slight increase in BOD concentrations (4.8 mg L−1 for the pristine sample and 9.6 mg L−1 for the treated sample). The oxidation capacities of HO• and Cl• are much higher than that of free chlorine, and the standard oxidation potentials of HO• and Cl• are +2.7 and +2.4 V, respectively, compared to free chlorine (+1.395 V) [39]. The UV/H2O2 process could remove only 40.6% and 46.5% of the NB250 dye and the ADMI color intensity, respectively. This low effectiveness possibly resulted from an insufficient H2O2 dose. The dosage between H2O2 and COD at 0.5 was generally suggested as an optimum ratio [40], while the H2O2 to COD ratio used in our study was 0.25. In addition, the molar absorption coefficient of free chlorine (i.e., HOCl/OCl−) was 3.2–3.6 times higher than that of H2O2 [20, 19]; thus, the performance of the UV/Chlorine process was 2.09–2.45 times better than the UV/H2O2 process.
Because several factors affect decolorization, the kinetic degradation of the NB250 concentration and ADMI color intensity can be simplified and explained by the pseudo first-order reaction. The pseudo first-order kinetic degradation (k′) is determined following Eqs. (4)–(5), and the k′ values are summarized in Table 1. The k′ values of NB250 in the UV/Chlorine process and with chlorination alone were 0.3574 and 0.2322 min−1, respectively. Similarly, the k′ values for decreasing the ADMI color intensity via the UV/Chlorine process and via chlorination only were 0.3814 and 0.2603 min−1, respectively. Removal of the ADMI color intensity was faster than that of the NB250 compound, indicating that the chromatic compounds of intermediates or oxidized products were still reactive to chlorines and free radicals. In addition, the UV/Chlorine process eliminated NB250 and its color approximately 1.47–1.54 times faster than chlorination alone, resulting in a smaller UV/Chlorine reactor footprint. Thus, the UV/Chlorine process achieved the synergistic removal of chromatic compounds generated from intermediates. Use of the UV/H2O2 process resulted in k′ values equivalent to 0.0097 and 0.0207 min−1 for NB250 and its color removals, respectively. A previous study on the UV/H2O2 process (4.1 mM H2O2) for the removal of Reactive Red-141 (25 mg L−1) achieved a k′ value of 0.0187 min−1 (R2 = 0.94) [41]. Comparing the k′ values between the UV/Chlorine and UV/H2O2 processes indicated that the former was the most effective in the removal of NB250 and its color intensity.
Chlorine residuals for chlorination at 60 min contact time remained at 42%, compared with the initial chlorine dose (Fig. S2) UV irradiation accelerated the decomposition of chlorine, compared with chlorination alone. In total, 61% of chlorine in the UV/Chlorine process was rapidly consumed in the first 10 min contact time, and 95% of chlorine was decomposed after 60 min. These results confirmed that chlorine was transformed during UV irradiation to form free radicals.
The electrical energy per order of pollutant removal (EEO), a powerful scale-up parameter, is a measure of the electrical energy consumption required to degrade a pollutant by one order of magnitude in a fixed volume of contaminated water [42]. The EEO (kWh.m−3 per order) can be calculated following Eq. (6).
where P is the power (kW) of the UV system, t is the irradiation time (h), V is the sample volume (L), and Ci and Cf (mg L−1) are the initial and final dye concentrations, respectively.
The EEO values for NB250 and ADMI removal via the UV/Chlorine process were 25.6 and 24 kWh.m−3 per order, respectively. Higher EEO values correspond to lower removal efficiencies. Currently, the cost of industrial electricity is approximately 4.6 BTH per kWh. Thus, the estimated costs of electrical energy consumption for NB250 and ADMI removal by one order of magnitude are 118 and 110 BTH.m−3, respectively. The EEO values for NB250 and ADMI removal in the UV/Chlorine process were 37 and 19 times lower than those in the UV/H2O2 process (948 and 444 kWh.m−3 per order for NB250 and ADMI removal, respectively). The chlorine cost (lab grade) was approximately 2.05 BTH/mM. Therefore, the unit costs for NB250 and ADMI removal by the UV/Chlorine process at a 1-mM chlorine dose were 120 and 112 BTH.m−3, respectively.
3.2. Change in Absorbance SpectrumThe pristine sample of dye wastewater exhibited the highest absorbance at the wavelength of 595 nm, followed by the wavelength of 312 nm (Fig. 2). The spectrum of samples treated by UV irradiation for 60 min was similar to the pristine sample and consistent with the insignificant removal of NB250. When the samples were treated by the UV/Chlorine process and chlorination alone for 60 min, the peak absorbances at 595 nm completely disappeared. In addition, the second largest peak (at 312 nm) was decreased. The UV/Chlorine process and chlorination alone presumably removed the chromatic structure of NB250. It is worth noting that the visible color of the chlorinated samples changed from dark blue to purple and red-orange, while the color of the samples treated with the UV/Chlorine process was purple followed by light orange and pale yellow, respectively (Fig. S3). A previous study concluded that UV/Chlorine oxidation could decompose the azo bonds in a reactive dye compound (RR2), as indicated by a decrease in the peak wavelength of 538 nm of 94.2% compared to the pristine sample [6].
3.3. Effects of Chlorine Dose and pH on NB250 DegradationNB250 removal via the UV/Chlorine process at different chlorine doses (0.1–2.0 mM) is shown in Fig. 3a. An increase in the chlorine dose removed more of the NB250 and ADMI color in the UV/Chlorine process. On the other hand, the chlorine dose used was reciprocal with the contact time required to achieve the color level benchmark (i.e., 300 ADMI). The higher amount of free radicals (e.g., HO• and RCS) generated from the photolysis of free chlorine (HOCl/OCl−) could shorten the required contact time. Although color removal by the UV/Chlorine process was greatly improved with a higher chlorine dose of 0.1–0.8 mM, the color levels could not meet the effluent standard (Fig. S4a). The limitation in decolorization could be explained by an insufficient chlorine dose, as the chlorine residual was hardly detected after 20 min contact time. Increasing the chlorine dose above 1.0 mM caused a slight improvement in decolorization (Fig. 3b), implying that a chlorine dose of 1.0 mM could be considered an optimum dose. The acceptable color intensity of effluents was achieved via the UV/Chlorine process with a chlorine dose of 1.0–1.2 mM and 40 min contact time. Intensifying the chlorine dose to 1.5 and 2.0 mM could shorten the contact time to 30 min or less. The lowest color intensity was found in the UV/Chlorine process with a 2.0-mM chlorine dose and 60-min contact time, responsible for 40 ADMI. To achieve an acceptable color level, a chlorine dose in the range of 1.0–2.0 mM was recommended for the UV/Chlorine process. It is worth noting that the chlorine residual was in the range of 4–41 mgCl2.L−1 for the chlorine range of 1.0–2.0 mM (Fig. S5a). Higher chlorine doses also enhanced the decolorization efficiency of chlorination alone (Fig. S4b). If chlorination alone is preferred, a chlorine dose above 2.0 mM is recommended to achieve the allowable color level. Although chlorination at a chlorine dose of 2 mM offered similar decolorization via the UV/chlorine process at a 1-mM chlorine dose, the chlorine residual with chlorination alone at 2.0 mM after 60 min contact time was extremely high, at 95 mgCl2.L−1, exhibiting 33% chlorine consumption (Fig. S5b). Therefore, dechlorination should be implemented following chlorination to avoid any adverse effects of exposing excessive chlorine residuals to ecosystems, and the formation of disinfection byproducts should be controlled.
The k′ values in the UV/Chlorine process were increased with higher chlorine doses (Table 1). The k′ value in Eq. (5) includes an excessive reactive agent (i.e., chlorine doses). To understand the effect of chlorine dose, the correlation between chlorine concentrations and k′ values can fit the second-order degradation rate following Eqs. (7)–(8). The second-order degradation rate constant (k″) can be obtained from a slope. The k″ values for the removal of NB250 and its color intensity via the UV/Chlorine process were 0.3317 mM−1min−1 and 0.3384 mM−1min−1, respectively (Fig. S6a and 6b). The k″ values for chlorination alone for removals of NB250 and color intensity were 0.2238 mM−1min−1 and 0.2244 mM−1min−1, respectively. The different rates between the two treatment processes were similar to the aforementioned results showing the UV/chlorine process enhanced the degradation rate of NB250 and ADMI color by 1.48–1.51 times compared to chlorination alone.
The effect of pH on the removal of NB250 by the UV/Chlorine process is shown in Fig. 4a. At 60 min contact time, the NB250 removal was similar during pH 6–9. However, the highest k′ values were observed at pH 6 and 7 (Table 1). The dissociation of HOCl/OCl− (pKa= 7.5) is sensitive to the solution pH. At a pH lower than 7.5, HOCl dominates in the speciation of free chlorine, while OCl− is the predominant species at a higher pH [19, 12]. In addition, the quantum yields of HOCl and OCl− photolysis at 254 nm at an ambient temperature were 1.40 and 0.97, respectively [16]. Consequently, the formations of HO• and Cl• were enhanced with lower pH values, leading to greater decolorization efficiency. A similar explanation was also given for the observation of the lowest removal efficiency and k′ values of ADMI color intensity at pH 9. The predominance of OCl− at an alkaline pH led to a greater potential for scavenging free radicals, causing a lower degradation rate [43]. The k′ values for NB250 removal in the UV/Chlorine process at pH 6, 7, 8 and 9 were 0.3273, 0.3574, 0.2717, and 0.1778 min−1, respectively (Fig. S7a), while the k′ values for reducing ADMI color in the UV/Chlorine process at pH 6, 7, 8 and 9 were 0.3846, 0.3814, 0.2951, and 0.2172 min−1, respectively (Fig. S7b). Equivalent results were found for chlorination alone. The highest removal rate was observed with chlorination alone at pH 6 and 7, while a higher pH led to a slower decolorization rate (Fig. S8a and 8b). The k′ values for NB250 removal via chlorination alone at pH 6, 7, 8 and 9 were 0.2247, 0.2322, 0.1836, and 0.1089 min−1, respectively, while the k′ values for reducing ADMI color intensity via chlorination alone at pH 6, 7, 8 and 9 were 0.2845, 0.2603, 0.2177, and 0.1683 min−1, respectively. Predominant chlorine species in an acidic condition; HOCl, which is a stronger oxidant than OCl−, could be responsible for quicker decolorization. Therefore, the color removals by the UV/Chlorine process and chlorination alone were most effective and likely similar in effectiveness between pH 6 and 7, which can be regarded as the optimum pH (Fig. 4b).
3.4. Effects of Free Radicals on NB250 Removal and DecolorizationThe NB addition caused a decrease in the removal of NB250, compared with the absence of scavengers (Fig. 5). At 60 min contact time, the presence of NB in the UV/Chlorine process suppressed the removal of NB250 to 95.6%, whereas 99.6% of NB250 was removed in the absence of scavengers. The minor difference indicated that the presence of HO• is slightly involved in decolorization. The result of NB250 removal by the UV/Chlorine process in the presence of NB was similar to that by chlorination alone (95.4%). When both radicals (e.g., HO• and RCS) were trapped for the sample spiked with BA, the amount of NB250 removed at 60 min was significantly reduced to 66.4%. Hence, both HO• and RCS formations affected the decrease in NB250. Comparing efficiencies between the sample spiked with NB and the sample spiked with BA revealed that RCS played a significant role in the removal of NB250 and ADMI color, rather than HO• (Fig. S9). This finding was dissimilar to that of a previous research team, who found that use of the UV/Chlorine process to remove food color additive (Brilliant Blue FCF) was mainly responsible for HO• formation [12]. The explanation is attributed to the characteristics of different pollutants and the lifetimes of radicals [6]. The presence of dual chromatic bonds (N = N) in the NB250 structure may result in more toleration, rather than a triarylmethane structure in Brilliant Blue FCF. Cl• is a selective oxidant to electrophilic functional groups, such as acetic acid, benzoic acid, phenol, greater than hydroxyl radicals [6]. In addition, RCS are highly reactive to olefins and benzene derivatives [44], while HO• is a non-selective electrophilic reagent [18]. Hence, RCS can more precisely react with the target compound in the presence of interferences (i.e., degradation and intermediate products). The selective characteristics could lead to a longer half-life, compared with HO•. Because the NB250 structure is composed of disulfonic acids and phenolic groups, the presence of RCS could promote NB250 decolorization.
3.5. Effects of Dye WastewaterChlorination alone at 1 mM for 60 min contact time decreased the ADMI color intensity by 90.5%, resulting in the final color intensity of 663 ADMI (Fig. 6). Similar color removal efficiency was also found in the UV/Chlorine process at 1 mM. Although the UV/Chlorine process at 1 mM for 60 min could reduce the color intensity to the acceptable level in synthetic waters, color removal in the dye wastewater was less effective due to interferences inducing higher chlorine consumption (Fig. S10). This higher chlorine consumption probably resulted from effluent organic matter, as the DOC concentration was 132 mg L−1, compared with 97 mgC.L−1 in the synthetic wastewater. The reduction in the color intensity of the wastewater was 91.1%, resulting in a final color intensity of 587 ADMI. Increasing the chlorine dose to 2 mM succeeded in decolorizing the sample to meet the discharge standard. The color intensity of the samples treated by the UV/Chlorine process with 2 mM of chlorine for 30 min contact time was below the discharge standard, presenting at 223 ADMI. Prolongation to 60 min improved the decolorization, and the final color intensity was 93 ADMI. Therefore, the use of 2 mM chlorine in the UV/Chlorine process was recommended to treat dye wastewater with an initial color intensity of 6,658 ± 227 ADMI. Dye wastewater with a greater color intensity may require an operational adjustment, such as a higher chlorine dose or UV intensity, longer contact time, lower pH, and additional pre-treatment.
3.6. PhytotoxicityThe germination rate (GR) of seeds in the control sample was 83%, indicating a high reliability of the seeds used. The germination in the control sample can be used to normalize the germination in the solution, represented as relative seed germination index (RSGI). The RSGI in the synthetic wastewater was 76%. Because there should be no inhibitor of root growth in the control sample, root inhibition (RI) could not be determined. The presence of NB250 can have an adverse effect on the plant germination. Previous studies found that the inhibition of seeding germination was increased depending on dye or effluent concentrations [45, 46]. The presence of dye (i.e., Reactive Blue 160) that its category similar to that used in our study caused a decrease in the germination of various plants (i.e., corn, green gram, ground nut, and black gram) [47]. Although chlorination was effective in removing NB250, the RSGI did not increase. This possibly implies the toxic potential of chlorinated products. It is worth nothing that the chlorine residual was quenched before the germination test. Treatment with the UV/Chlorine process resulted in higher germination than in the non-treatment sample, indicating that the degradation product of NB250 was less toxic in nature than the pristine compound. Therefore, the UV/Chlorine process was recommended for decolorizing dye wastewater. However, not all treatments clearly increased the root growth in the synthetic wastewater (p > 0.05). The phytotoxicity of dyes was more clearly observed when raw wastewater was used. Seed germination was completely inhibited in the raw wastewater, resulting in undetectable RSGI and RI. Thus, discharging the untreated dye wastewater to the environment can decrease the fertility of fields if the contaminated water resource is used for agriculture. Water after treatment by UV irradiation, chlorination alone, and the UV/Chlorine process promoted seed germination (p < 0.05). The different results from synthetic waters were likely from a high contamination of unknown substances (i.e., effluent organic matters) as the COD (and DOC) concentration in raw wastewater was higher. The COD concentration in raw wastewater was 184 mg L−1, while that in synthetic wastewater was 51 mg L−1. Although the UV light did not significantly reduce the color intensity, it can disinfect flora pathogens [48, 49]. The results suggest that the exposure of seeds to a low concentration of dye was less toxic to seed germination and root growth. Several research studies reported comparable results that seed germination in treated dye wastewater was enhanced, compared with untreated wastewater [33, 47].
4. ConclusionsAmong various treatments in this study, the UV/Chlorine process was the most effecting in removing NB250 compound and its ADMI color, followed by chlorination alone. The UV/Chlorine process provided a synergistic effect on decolorization, resulting in the removal of NB250 at a rate approximately 1.47–1.50 times faster than the use of chlorination alone. The decolorization rates of chlorination alone and the UV/Chlorine process were increased with higher chlorine doses. In addition, the degradation constants (k″) for the removal of NB250 and ADMI color intensity by the UV/Chlorine process were 0.3317 mM−1min−1 and 0.3384 mM−1min−1, respectively. The optimum pH was between 6 and 7. The UV/Chlorine process at a chlorine dose of 1.0–2.0 mM was recommended for the decolorization of raw wastewater to meet the industrial standard of discharge. RCS played a more prominent role in the removal of NB250 and ADMI color intensity than HO•.
The presence of NB250 inhibited the germination of morning glory (Ipomoea aquatica) seeds. The removal of NB250 by chlorination alone did not increase seed germination. Thus, the UV/Chlorine process was recommended for the decolorization of NB250 solution, as the treated sample was less toxic to plant growth compared to pristine water. Seed germination was completely inhibited in the raw dye wastewater (color = 6,658 ± 227 ADMI), but exposing seeds to treated effluents, which had a lower color intensity, was less toxic to seed germination and root growth. To achieve both outcomes of meeting the industrial standard of discharge and biological safety, the UV/Chlorine process can be used as an effective treatment for dye wastewater.
AcknowledgementThis work was supported by the National Research Council of Thailand (NRCT) for funding the research with the Mid-career researcher grant number N41A640147.
NotesAvailability of data and materials All data generated or analyzed during this study are included in this published article and its supplementary information files. Authors’ contributions A.D. (Master student) conducted research methodology, data curation, visualization, and writing-original draft. J.B. (Assistant Professor) performed data curation, writing, and editing. S.P. (Associate Professor) processed conceptualization, data curation, visualization, supervision, and writing-original draft and editing. All authors read and approved the final manuscript. References1. Katheresan V, Kansedo J, Lau S. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018. 6:4676–4697.
https://doi.org/10.1016/j.jece.2018.06.060
2. Nguyen T, Fu CC, Juang RS. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013;219:109–117.
https://doi.org/10.1016/j.cej.2012.12.102
3. Mass R, Chaudhari S. Adsorption and biological decolourization of azo dye Reactive Red 2 in semicontinuous anaerobic reactors. Process Biochem. 2005;40:699–705.
https://doi.org/10.1016/j.procbio.2004.01.038
4. Nourmoradi H, Zabihollahi S, Pourzamani HR. Removal of a common textile dye, navy blue (NB), from aqueous solutions by combined process of coagulation-flocculation followed by adsorption. Desalin. Water Treat. 2016;57(11)5200–5211.
https://doi.org/10.1080/19443994.2014.1003102
5. Gomare S, Kalme S, Govindwar S. Biodegradation of Navy Blue-3G by Brevibacillus laterosporus MTCC 2298. Acta Chim Slov. 2009;56:789–796.
http://www.dlib.si/details/URN:NBN:SI:DOC-E08AHTRH
6. Wu Q, Li Y, Wang W, Wang T, Hu H. Removal of C.I. reactive red 2 by low pressure UV/chlorine advanced oxidation. J Environ. Sci. 2016;41:227–234.
https://doi.org/10.1016/j.jes.2015.06.013
7. Brown D, Hamburger B. The degradation of dyestuffs: part III-investigations of their ultimate degradability. Chemosphere. 1987;16(7)1539–1553.
https://doi.org/10.1016/0045-6535(87)90094-4
8. Saratale R, Saratale G, Kalyani D, Chang J, Govindwar S. Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR. Bioresour. Technol. 2009;100(9)2493–2500.
https://doi.org/10.1016/j.biortech.2008.12.013
9. Banat I, Nigam P, Singh D, Marchant R. Microbial decolorization of textile-dyecontaining effluents: A review. Bioresour. Technol. 1996;58(3)217–227.
https://doi.org/10.1016/S0960-8524(96)00113-7
10. Riera-Torres M, Gutierrez-Bouzan G, Crespi M. Combination of coagulation-flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination. 2010;252(1–3)53–59.
https://doi.org/10.1016/j.desal.2009.11.002
11. Panda K, Mathews A. Ozone oxidation kinetics of Reactive Blue 19 anthraquinone dye in a tubular in situ ozone generator and reactor: Modeling and sensitivity analyses. Chem. Eng J. 2014;255:553–567.
https://doi.org/10.1016/j.cej.2014.06.071
12. Nikravesh B, Shomalnasab A, Nayyer A, Aghababaei N, Zarebi R, Ghanbari F. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater. J. Environ. Chem. Eng. 2020;8(5)104244.
https://doi.org/10.1016/j.jece.2020.104244
13. Benkhaya S, M’rabet S, Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6:e03271.
https://doi.org/10.1016/j.heliyon.2020.e03271
14. Hashemi S, Kaykhaii M. Chapter 15-Azo dyes: Sources, occurrence, toxicity, sampling, analysis, and their removal methods. Emerging freshwater pollutants: Analysis, fate and regulations. Elsevier; 2022. 267–287.
https://doi.org/10.1016/B978-0-12-822850-0.00013-2
15. Sandhya S. Biodegradation of azo dyes under anaerobic condition: Role of azoreductase. Atacag Erkurt H, editorBiodegradation of azo dyes. The handbook of environmental chemistry. 9:Berlin: Springer; 2010.
https://doi.org/10.1007/698_2009_43
16. Fang J, Fu Y, Shang C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ Sci. Technol. 2014;48(3)1859–1868.
https://doi.org/10.1021/es4036094
17. Nowell LH, Hoigné J. Photolysis of aqueous chlorine at sunlight and ultraviolet wavelengths: I. Degradation rates. Water Res. 1992;26(5)593–598.
https://doi.org/10.1016/0043-1354(92)90232-S
18. Kishimoto N. State of the art of UV/Chlorine advanced oxidation processes: Their mechanism, byproducts formation, process variation, and application. J. Water Environ. Technol. 2019;17(5)302–335.
https://doi.org/10.2965/jwet.19-021
19. Feng Y, Smith D, Bolton J. Photolysis of aqueous free chlorine species (HOCl and OCl−) with 254 nm ultraviolet light. J Environ. Eng. Sci. 2007;6:277–284.
https://doi.org/10.1139/s06-052
20. Stefan MI, Hoy AR, Bolton JR. Kinetics and mechanism of the degradation and mineralization of acetone in dilute aqueous solution sensitized by the UV photolysis of hydrogen peroxide. Environ. Sci. Technol. 1996;30(7)2382–2390.
https://doi.org/10.1021/es950866i
21. Gao YQ, Zhang J, Li C, Tian FX, Gao NY. Comparative evaluation of metoprolol degradation by UV/chlorine and UV/H2O2 processes. Chemosphere. 2020;243:125325.
https://doi.org/10.1016/j.chemosphere.2019.125325
22. Zhu Y, Wu M, Gao N, Chu W, Li K, Chen S. Degradation of phenacetin by the UV/chlorine advanced oxidation process: Kinetics, pathways, and toxicity evaluation. Chem. Eng. J. 2018;335:520–529.
https://doi.org/10.1016/j.cej.2017.10.070
23. Yin K, Deng Y, Liu C, He Q, Wei Y, Chen S, Liu T, Luo S. Kinetics, pathways and toxicity evaluation of neonicotinoid insecticides degradation via UV/chlorine process. Chem. Eng J. 2018;346:298–306.
https://doi.org/10.1016/j.cej.2018.03.168
24. Cai WW, Peng T, Zhang JN, Hi LX, Yang B, Yang YY, Chen J, Ying GG. Degradation of climbazole by UV/chlorine process: Kinetics, transformation pathway and toxicity evaluation. Chemosphere. 2019;219:243–249.
https://doi.org/10.1016/j.chemosphere.2018.12.023
25. Li Q, Lai C, Yu J, Luo J, Deng J, Li G, Chen W, Li B, Chen G. Degradation of diclofenac sodium by the UV/chlorine process: Reaction mechanism, influencing factors and toxicity evaluation. J. Photochem. Photobiol. A. 2022;425:113667.
https://doi.org/10.1016/j.jphotochem.2021.113667
26. Jin J, El-Din MG, Bolton JR. Assessment of the UV/Chlorine process as an advanced oxidation process. Water Res. 2011;45(4)1890–1896.
https://doi.org/10.1016/j.watres.2010.12.008
27. Yang H, Li Y, Chen Y, Ye G, Sun X. Comparison of ciprofloxacin degradation in reclaimed water by UV/chlorine and UV/persulfate advanced oxidation processes. Water Environ. Res. 2019;91(12)1576–1588.
https://doi.org/10.1002/wer.1144
28. Rafiei N, Fatehizadeh A, Amin M, Pourzamani H, Ebrahimi A, Taheri E, Aminabhavi T. Application of UV/chlorine processes for the DR83:1 degradation from wastewater: Effect of coexisting anions. J. Environ. Manage. 2021;297:113349.
https://doi.org/10.1016/j.jenvman.2021.113349
29. Belghit A, Merouani S, Hamdaoui O, Bouhelassa M, Al-Zahrani S. The multiple role of inorganic and organic additives in the degradation of reactive green 12 by UV/chlorine advanced oxidation process. Environ. Technol. 2022;43(6)835–847.
https://doi.org/10.1080/09593330.2020.1807609
30. Tchobanoglous G, Burton F, Tsuchihashi R, Stensel H, editorsMetcalf & Eddy Inc. Wastewater engineering, treatment and reuse. 4th edNew York: McGraw-Hill; 2003. p. 1196–1200.
31. Rahn RO, Bolton JR, Stefan MI. The Iodide/Iodate actinometer in UV disinfection: Determination of the fluence rate distribution in UV reactors. Photochem. Photobiol. 2006;82(2)611–615.
https://doi.org/10.1562/2005-06-10-RN-570
32. APHA. Standard method for the examination of water and wastewater. 19th edNew York: MaGraw-Hill; 1995.
33. Ebency C, Rajan S, Murugesan ARR, Elayarajah B. Biodegradation of textile azo dyes and its bioremediation potential using seed germination efficiency. Int. J. Curr. Microbiol Appl. Sci. 2013;2(10)496–505.
https://www.ijcmas.com/Archives-11.php
34. Mitrović J, Radović MD, Bojić D, Anđelković T, Purenović M, Bojić A. Decolorization of the textile azo dye reactive orange 16 by the UV/H2O2 process. J. Serbian Chem. Soc. 2012;77(4)465–481.
https://doi.org/10.2298/JSC110216187M
35. Vacchi FI, Albuquerque AF, Vendemiatti JA, Morales DA, Ormond AB, Freeman HS, Zocolo GJ, Zanoni MVB, Umbuzeiro G. Chlorine disinfection of dye wastewater: implications for a commercial azo dye mixture. Sci. Total Environ. 2013;442:302–309.
https://doi.org/10.1016/j.scitotenv.2012.10.019
36. Pollution Control Department. Water standard Internet. [cited 1 September 2021]. Available from: https://www.pcd.go.th/laws/
37. Behin J, Farhadian N. Response surface methodology and artificial neural network modeling of reactive red 33 decolorization by O3/UV in a bubble column reactor. Adv. Environ. Technol. 2016. 2133–44.
https://doi.org/10.22104/AET.2016.361
38. Watts MJ, Linden KG. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007;41(13)2871–2878.
https://doi.org/10.1016/j.watres.2007.03.032
39. Beitz T, Bechmann W, Mitzner R. Investigations of reactions of selected azaarenes with radicals in water. 2. Chlorine and bromine radicals. J. Phys. Chem. A. 1998;102(34)6766–6771.
https://doi.org/10.1021/jp980655a
40. Collivignarelli MC, Pedrazzani R, Sorlini S, Abba A, Bertanza G. H2O2 based oxidation processes for the treatment of real high strength aqueous wastes. Sustainability. 2017;9(2)244.
https://doi.org/10.3390/su9020244
41. Gultekin I, Ince NH. Degradation of reactive azo dyes by UV/H2O2: Impact of radical scavengers. J. Environ. Sci. Health-Toxic/Hazard. Subst. Environ. Eng. 2004;39(4)1069–1081.
https://doi.org/10.1081/ESE-120028414
42. Bolton JR, Bircher KG, Tumas W, Tolman CA. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric-and solar-driven systems. Pure Appl. Chem. 2001;73(4)627–637.
https://doi.org/10.1351/pac200173040627
43. Lee JE, Kim MK, Lee JY, Lee YM, Zoh KD. Degradation kinetics and pathway of 1H-benzotriazole during UV/chlorination process. Chem. Eng. J. 2019;359:1502–1508.
https://doi.org/10.1016/j.cej.2018.11.026
44. Guo K, Wu Z, Shang C, Yao B, Hou S, Yang X, Song W, Fang J. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water. Environ. Sci. Technol. 2017;51(18)10431–10439.
https://doi.org/10.1021/acs.est.7b02059
45. Mahawar P, Akhtar A. Impact of dye effluent on growth and chlorophyll content of Alfalfa (Medicago sativa L.). Ann. Plant Sci. 2016;5(9)1432–1435.
https://doi.org/10.21746/aps.2016.10.002
46. Rahman MA, Rayhan MYH, Chowdhury MAH, Mohiuddin KM, Chowdhury MAK. Phototoxic effect of synthetic dye effluents on seed germination and early growth of red amaranth. Fundam. Appl. Agric. 2018;3(2)480–490.
https://doi.org/10.5455/faa.299239
47. Barathi S, Karthik C, SN , Padikasan IA. Biodegradation of textile dye Reactive Blue 160 by Bacillus firmus (Bacillaceae: Bacillales) and non-target toxicity screening of their degraded products. Toxicol. Rep. 2020;7:16–22.
https://doi.org/10.1016/j.toxrep.2019.11.017
48. Urban M, Motteram J, Jing HC, Powers S, Townsend J, Devonshire J, Pearman I, Kanyuka K, Franklin J, Hammond-Kosack K. Inactivation of plant infecting fungal and viral pathogens to achieve biological containment in drainage water using UV treatment. J. Appl. Microbiol. 2011;110(3)675–687.
https://doi.org/10.1111/j.1365-2672.2010.04917.x
49. Younis B, Mahoney L, Schweigkofler W, Suslow K. Inactivation of plant pathogens in irrigation water runoff using a novel UV disinfection system. Eur. J. Plant Pathol. 2019;153:907–914.
https://doi.org/10.1007/s10658-018-01608-8
50. Liu F, Ying GG, Tao R, Zhao JL, Yang JF, Zhao LF. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ. Pollut. 2009;157:1636–1642.
https://doi.org/10.1016/j.envpol.2008.12.021
Fig. 1Removal of NB250 concentration (a) and ADMI (b) (NB250 = 100 mg L−1, pH 7± 0.2, temperature 25 ± 3 °C) 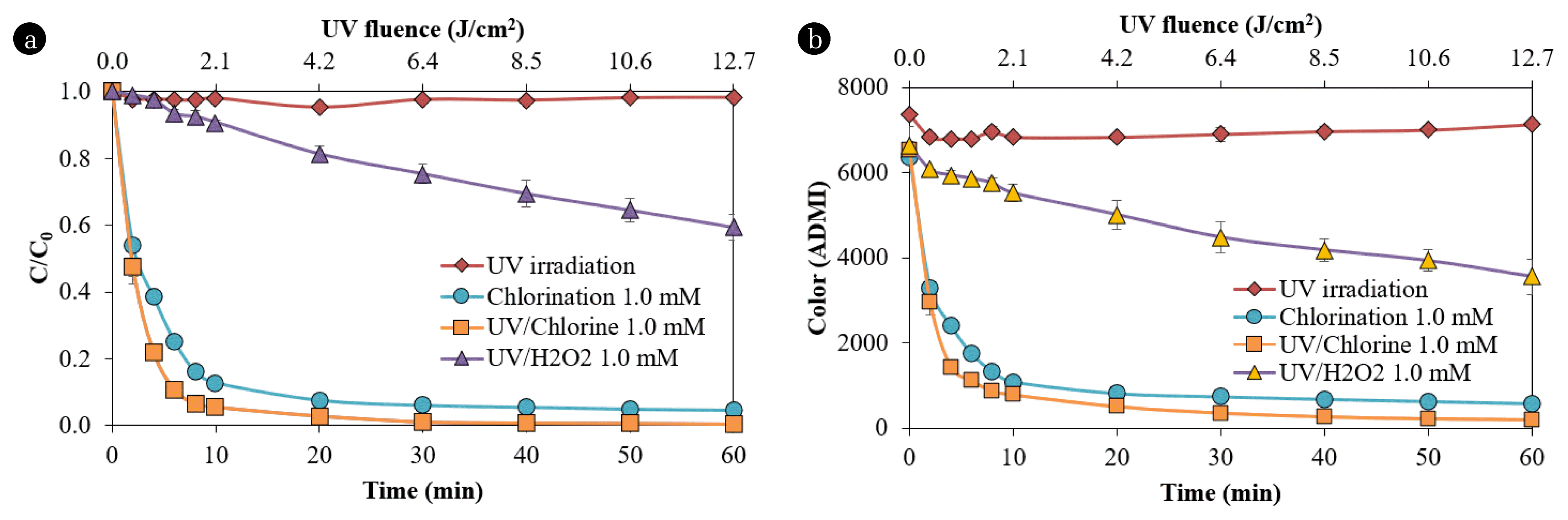
Fig. 3Removal of NB250 (a) and its efficiency (b) by the UV/Chlorine process under various chlorine doses (NB250 = 100 mg L−1, pH 7 ± 0.2, temperature 25 ± 3 °C) 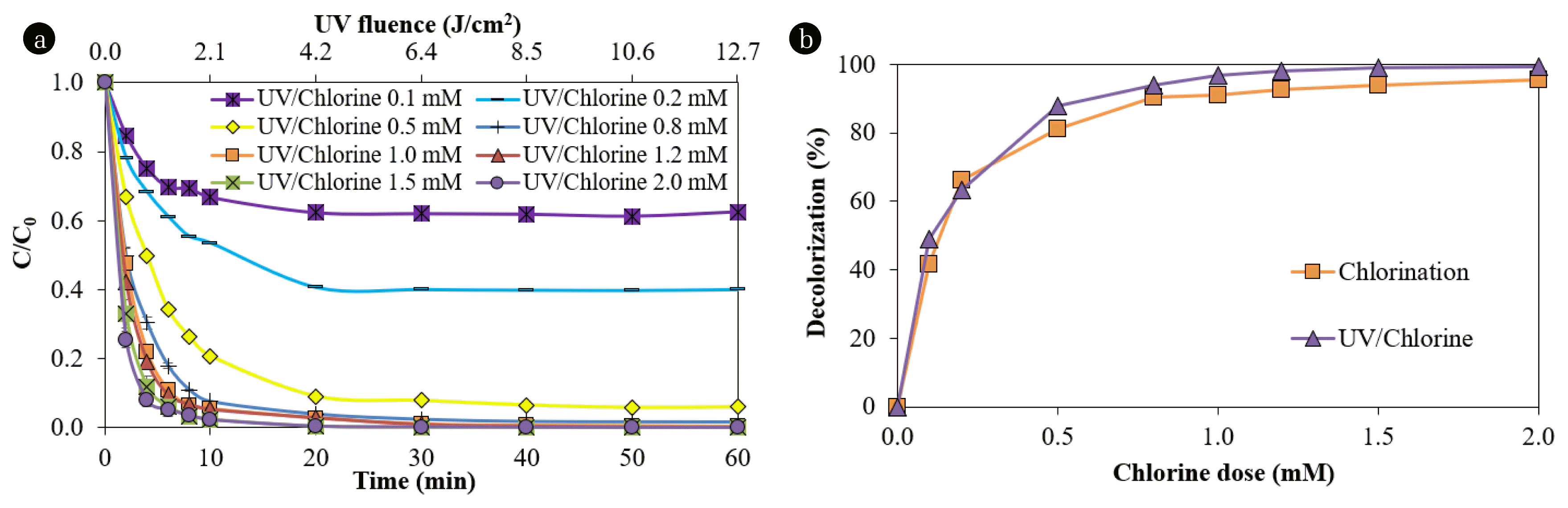
Fig. 4Effect of pH on the UV/Chlorine process for the removal of NB250 (a) and k′ values (b) of NB250 (solid line) and ADMI (dash line) (Chlorine = 1.0 mM, NB250 = 100 mg L−1, temperature 25 ± 3 °C) 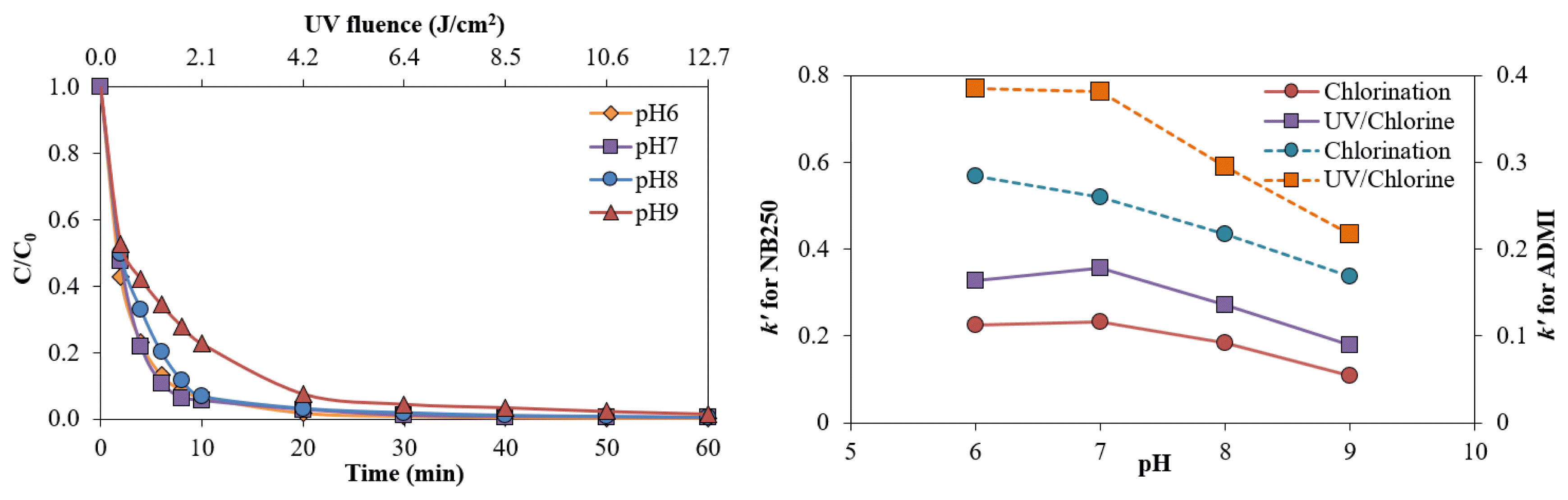
Fig. 5The removal of NB250 in the presence of scavengers (NB250 = 100 mg L−1, pH = 7 ± 0.2, Chlorine = 1.0 mM, BA and NB = 5 mM) 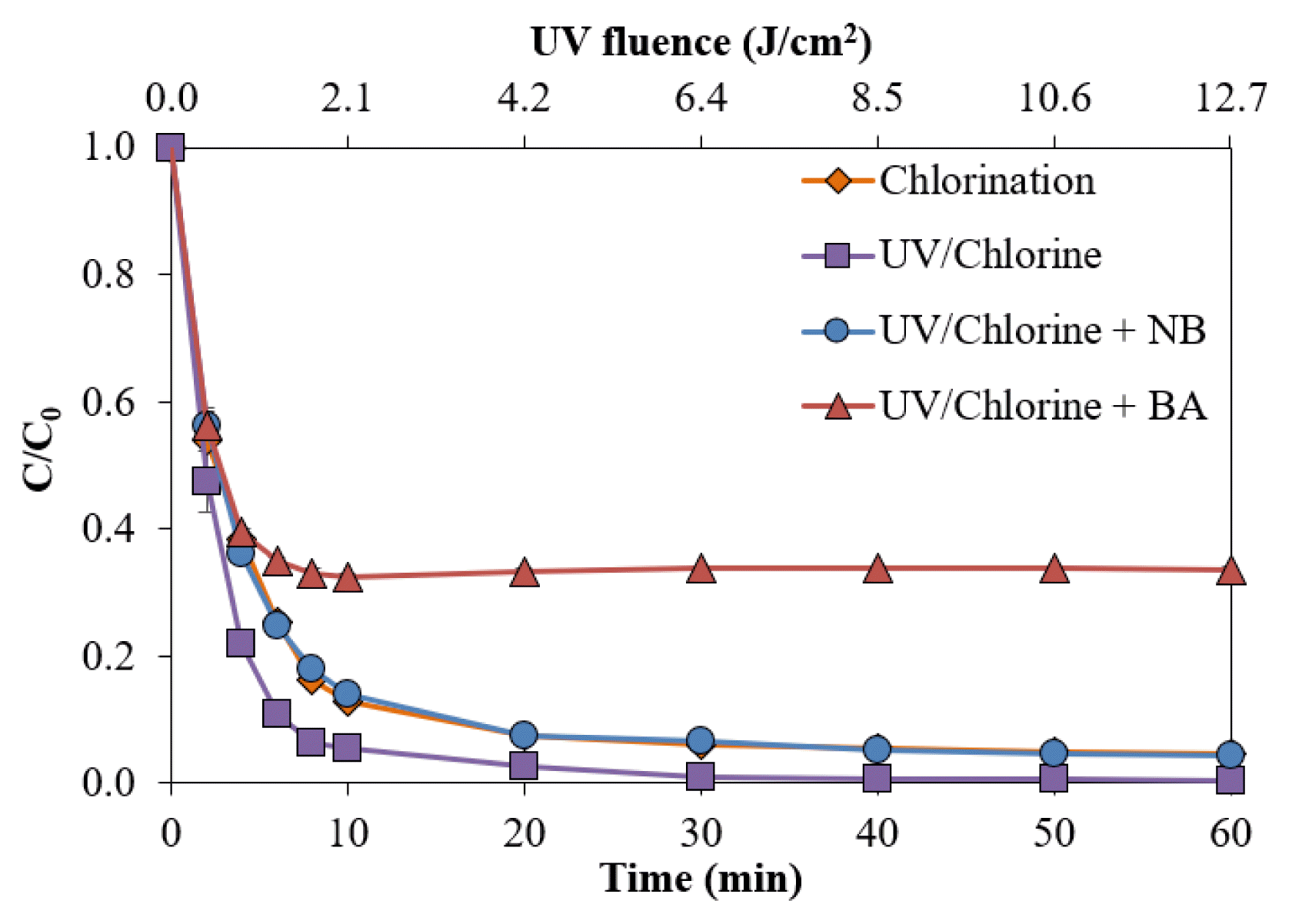
Fig. 6The removal of color intensity in raw dye wastewater (initial color intensity = 6,658 ± 227 ADMI, pH = 7 ± 0.2) 
Fig. 7Phytotoxicity on germination of morning glory (Ipomoea aquatic) seeds (pH = 7 ± 0.2, contact time = 60 min) 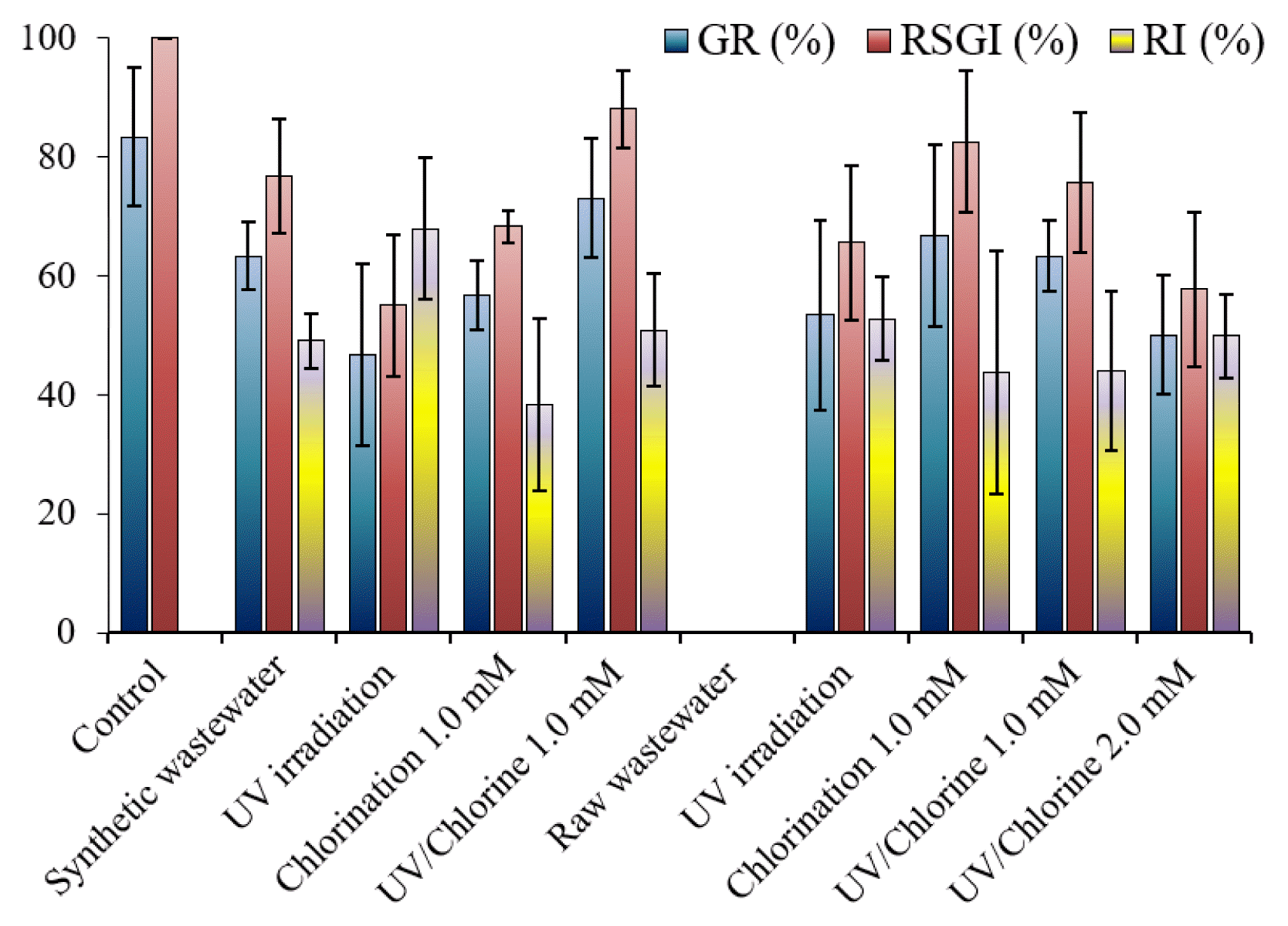
Table 1The pseudo first-order kinetic constant (k′) and second-order kinetic constant (k″) for the removal of NB250 and color intensity |
|
||||||||||||||||||||||||||||||||||||||||