AbstractHeavy-metal ions are common pollutants in wastewater and are thus attracting considerable attention. Herein, an eco-friendly biodegradable adsorbent, iminodisuccinic acid (IDS) modified attapulgite (ATP) is prepared by graft-polymerization to reduce Cu(II) in water, referred as IDS-ATP. The equilibrium adsorption capacity of IDS-ATP for Cu(II) is increased by 329.5% and 272% compared with raw ATP and non-degradable chelator ethylenediaminetetraacetic acid-modified ATP (EDTA-ATP), respectively. Moreover, the adsorption capacities for Cu(II) in combined system increased by 186% compared with in single system. The structure and surface properties of IDS-ATP are characterized, demonstrating that the IDS moieties are anchored on the surface of ATP without structural damage. In the aqueous Cu(II) (64 mg/L), the best adsorption pH is 5.0, the best dosage is 800 mg/L, and the adsorption equilibrium time is 4 h. The adsorption of IDS-ATP is chemical adsorption and regenerated adsorbent still exhibits high adsorption capacity. The adsorption mechanism includes the coordination of amino groups with Cu(II), the chelation of −COOH on heavy metals (HMs), and the ion exchange. Taking Cu(II) as an example to study the process of IDS-ATP in water, it is beneficial to apply this degradable material to reduce the other HMs.
Graphical Abstract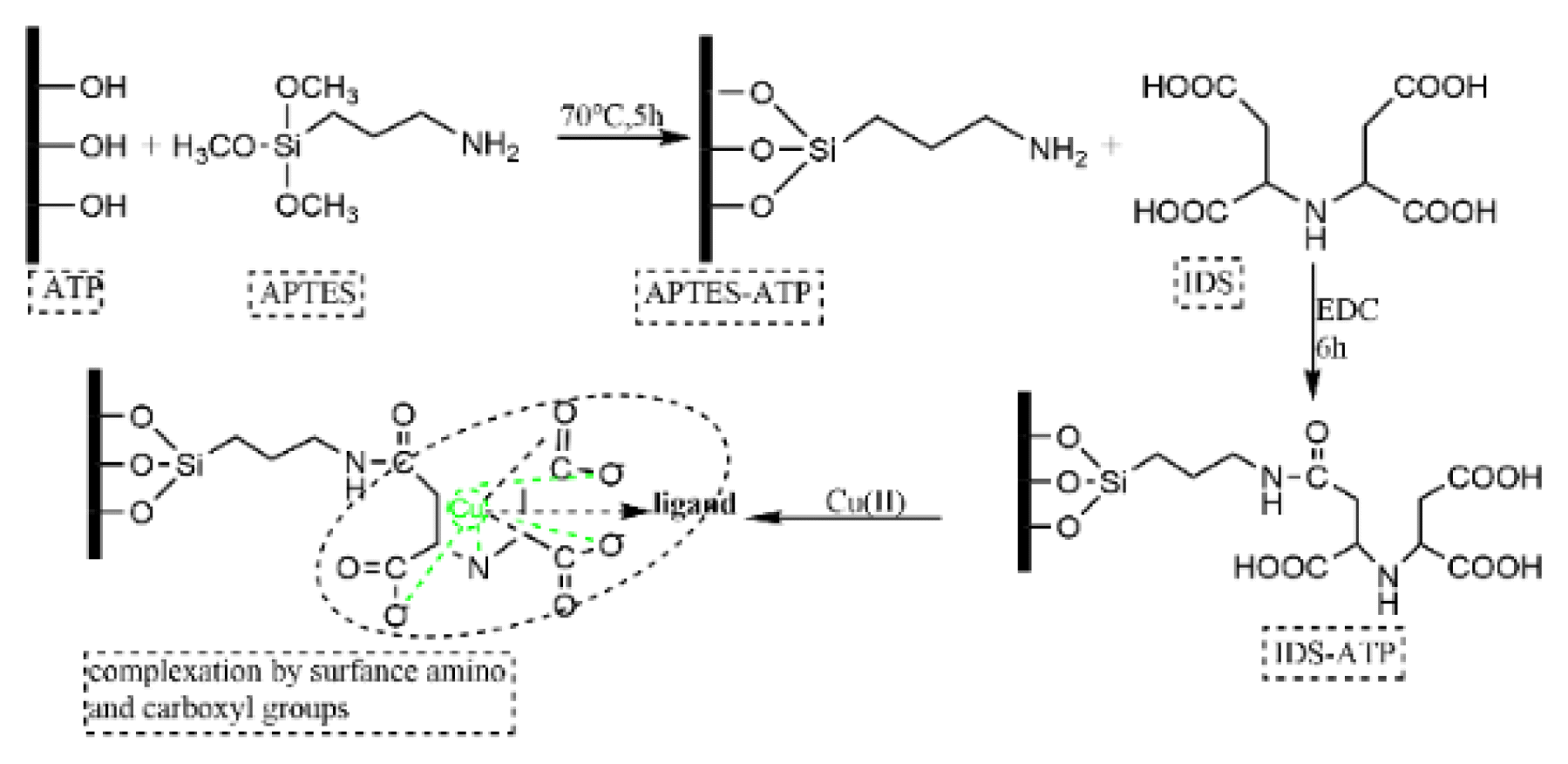
1. IntroductionHeavy metals (HMs) pose a more serious risk to animals, plants, and humans with the increasing concentration [1]. As a result, the pollution of HMs has become an important topic in recent years. Among the HMs, copper ion (Cu(II)) is an essential trace element required by living organisms at low levels [2]. However, it becomes an environmental and public health pollutant at certain concentrations in the environment [3]. Using copper-containing wastewater in agricultural activities can transfer Cu(II) from contaminated water and soil to crops, ultimately endangering human health [4–6]. The limit of Cu(II) is 2 mg/L in drinking water, according to the regulations of the World Health Organization (WHO), while it is 1 mg/L by the USA Environmental Protection Agency (EPA) [7]. Various treatment processes are required to reduce the adverse effects of Cu(II) on the environment and human health.
Several attempts have been made to reduce Cu(II) from aqueous media, including electrocoagulation [8], reverse osmosis [9], filtration [10], membranes [11], electrodialysis [12], biochemical treatment [13], adsorption/biosorption [14,15], etc. Among these treatments, the adsorption process is widely accepted for its cost-effectiveness and simplicity. In recent years, many efforts have been spent in developing the low-cost and high-effective adsorbents, such as natural materials, biosorbents, and solid wastes from agriculture and industry. Among them, natural clay minerals are familiar to mankind from the earliest days of civilization, and they are acknowledged to be exceptionally promising candidates as low-cost, sustainable, and effective adsorbents for the removal of HMs due to their abundance in most continents of the world, high adsorption properties, non-toxicity and exchangeable ions [16]. Attapulgite (ATP) (also known as palygorskite) was a typical kind of one-dimensional nano-sized aluminum-magnesium silicate mineral, usually in the form of a nanorod-like structure [17]. Under microscopic conditions, ATP takes the unique layered chain shape of bunches, flakes, etc. [18]. It has a certain elasticity, rich pores, large internal and external specific surface areas, and good adsorption performance. Zhang et al. modified ATP with zero-valent iron and its maximum adsorption capacity for Cr(III) could reach 95mg/g [19]. Yang et al. used ATP to remove various ionic dyes from water [14,20]. Potgieter et al. used ATP to remove multiple HMs in the solution, and the results showed that ATP has the best removal effect on Pb(II), followed by Cr(VI), Ni(II), and Cu(II) under the same conditions [21]. However, the existence of carbonate cements in the gaps and channels of ATP crystals leads to irregular pore structure, smooth surface morphology, and tight crystal stacking, which ultimately lead to low adsorption capacity [22].
Huang et al. finds that surface modifications can improve the adsorption capacity and selectivity of ATP toward HMs, and hydroxyl, amino groups and carboxyl groups are the widely used functional groups [23]. The chelating agents, containing ligands with two or more coordinating atoms, are used to interact with the metal atoms or ions to form a complex with a cyclic structure [24]. Commonly used chelating agents include diethylenetriaminepentaacetic acid (DTPA), ethylenediaminetetraacetic acid (EDTA), citric acid (CA), etc. [25–29]. EDTA has attracted a surprising amount of attention as a cleaning agent because of its high metal extraction efficiency, solubility, and the high thermodynamic stability of the metal complexes formed [30–33]. However, it shows the obvious disadvantage of limited biodegradability and considerable persistence in the soil, leading to potential adverse effects on soil function [34–38]. Whereas iminodisuccinic acid (IDS) has attracted extensive attention due to its environmental friendliness and excellent degradability. The study show that IDS can be degraded by approximately 80% a week in the environment, and it is converted into aspartic acid (Asp) mainly by lyase [39–41]. Experimental results of Cokesa et al. show that complexes generated by IDS with some metal ions are also biodegradable, such as manganese and copper ions, the complexes formed with IDS can degrade 55% and 40% after 28 days, respectively [42]. Wu et al. used the IDS to extract HMs from industrial sludge, the removal rate of Cu and Ni are 91.3% and 90.7%, respectively [43]. IDS was used to remove Cu(II), Co(II), Ni(II) from industrial effluents, and the removal rate of them are 98%, 82%, 67%, and 55%, respectively [44]. Therefore, IDS is recommended as an easily biodegradable alternative to DTPA, EDTA, and CA.
Based on this consideration, the effect of IDS on morphology, structure, crystallinity, acid-base property of ATP was systematically investigated in this study. Grafting IDS moieties on the surface of ATP to obtain a straw-based adsorbent with abundant carboxyl and amino groups. The influence of modification on adsorption kinetic, capacity and mechanism were evaluated. This research contributed to understand the influence mechanism and its environmental consequences.
2. Materials and Methods2.1. MaterialsThe ATP used in the study was locally obtained in Xuyi County, Jiangsu Province, China. IDS was purchased from Shandong Yuanlian Chemical Co., Ltd. 3-Aminopropyltriethoxysilane (APTES), and EDTA was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. All the chemicals were of analytical grade. The ultrapure water was used in all solutions. 1 mol/L HCl or NaOH was used to adjust pH.
2.2. Synthesis of IDS-ATP and EDTA-ATPATP and APTES are put into ethanol. The solution is stirred at 70°C for 5 h and then left to stand. The prepared powder was filtered and washed 3 times with deionized water. After drying at 80°C for 12 h, the powder was referred as APTES-ATP. The specific methods were similar to those of wang et al. with slight modification [24].
APTES-ATP was added to IDS and soaked for 12 h. After washing with deionized water, 300 mL of phosphate buffer was added, and the mixture was ultrasonically dispersed for 5 minutes. Then 1-(3-Dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (EDC) solution was added, sonicated for 25 min, and brought to room temperature for 6 h. After filtration and washing with 0.1 mol/L NaH2PO4, the supernatant was washed with deionized water and dried at 50°C for 12 h to obtain IDS-ATP. The preparation process of IDS-ATP was shown in Fig. S2, and the relevant results on comparative experiments for modification condition are shown in Fig. S3. EDTA-ATP was also synthesized under the same condition replacing IDS with EDTA.
2.3. CharacterizationsThe crystals of the synthesized samples are characterized by X-ray diffraction (XRD, RIGAKU D/MAX 2550/PC, Japan) at a scanning speed of 2°/min from the range of 10–80°(2θ). Fourier transform infrared (FTIR) spectra are acquired using KBr pellets in the range of wavenumbers 400~4000 cm−1 with a Bruker MPA FTIR spectrometer (Bremen, Germany). The data obtained in the experiment are analyzed and processed by Omnic 9.2. SEM images are acquired using a Hitachi S-4800 scanning electron microscope (Tokyo, Japan). The X-ray photoelectron spectroscopy analysis is obtained using PHI 5000 VP II (UIVAC-PHI, Japan). The Brunauer-Emmentt-Teller (BET) surface areas were determined by N2 adsorption/desorption technique (ASAP 2010 M+C Micromeritics Inc., USA). The chemical stability of the material is analysised by the element analyzer (EA2400 II, PerkinElmer Enterprise Management Co., Ltd.). The concentration of Cu(II) is analysised by Atomic Absorption Spectroscopy(TAS-990, Beijing Puxi General Instrument Co., Ltd).
2.4. Batch Adsorption StudiesThe materials were soaked in buffer solutions (pH = 2, 4, 7, 10) to test their stability. The effect of the functionalization on the material was tested by adding 80 mg different adsorbents into 50 mL of 64 mg/L Cu(II) solution, whose dosage is 1600 mg/L. Adsorption isotherms for Cu(II) adsorption on the adsorbent were carried out with 800 mg/L dosage at concentrations ranging from 100 to 300 mg/L. In the adsorption kinetics experiments, the dosage of adsorbent in 64 mg/L Cu(II) solution was 800 mg/L. The effect of adsorbent dosage on Cu(II) was investigated with dosage ranging from 400 mg/L to 2000 mg/L. The effect of pH was studied in the range of 2–8.
3.75mg/L HCl solution and 85mg/L NaNO3 solutions were used as desorbent. The solid-liquid was separated by high speed tabletop centrifuge (TD-4M, Shandong Boke Scientific Instrument Co., Ltd) at 8000 rpm for 10 min. The result of the desorption experiment was measured by Cu(II) concentration in the supernatant. A Cu(II)-Cr(VI) solution (10–300 mg/L) with 800 mg/L dosage to find out the effect of combined pollution.
The adsorption behavior of Cu(II) was studied experimentally at 25°C. The pH was 5 in batch experiments, and the adsorbent was mixed with Cu(II) solution in adsorption tubes and shaken at 120 rpm for 6 hours on a thermostatic shaker. After reaching adsorption equilibrium, the concentration of Cu(II) in the supernatant was detected by atomic absorption spectrophotometer, and the amount of desorption was calculated.
3. Results and Discussions3.1. Structural CharacterizationThe morphology of ATP, APTES-ATP, IDS-ATP, and EDTA-ATP are employed by SEM (Fig. 1). The morphology of the original ATP presents a tightly reunited bar crystal structure ((6~7) μm length × (0.3~0.6) μm diameter), which is difficult to disperse. After functionalization, the rod-like structure of ATP is complete, the rod-like crystals ((1.9~4.4) μm length × (0.2~0.63) μm diameter for IDS-ATP; (2.1~5.2) μm length × (0.34~0.83) μm diameter for EDTA-ATP) are relatively dispersed, and the surface is surrounded by substances, indicating the adherence of IDS organic matter on the surface. It leads to a higher degree of dispersion than the original ATP.
The XRD spectra of ATP, APTES-ATP, IDS-ATP, and EDTA-ATP in Fig. 1 (e) shows similarities to each other. The reflections at 2θ = 8.86°, 20.8°, and 19.72° closely match those of the standard ATP (PDF No. 31–0783), corresponding to the characteristic concave bump diffraction at (110), (121), and (040) respectively. The reflection at 2θ = 26.7° corresponds to quartz (SiO2) in ATP, APTES-ATP, IDS-ATP, and EDTA-ATP [45]. Nevertheless, characteristic reflections of ATP are observed in all of functionalized ATP composites, indicating that the functionalization process did not destroy the characteristic ATP structure. In conclusion, the functionalization process takes place on the surface. The BET surface area of IDS-ATP and EDTA-ATP are 55.439 m2/g and 74.676 m2/g, which is significantly lower than that of ATP (154.054 m2/g). This is possibly because some channels in ATP were stocked during the process of functionalization [46].
Fig. 1 (f) shows the FT-IR spectra of ATP, APTES-ATP, IDS-ATP, and EDTA-ATP with the considerable variation found in the 400–4000 cm−1 region. The band at 466 cm−1 and 788 cm−1 are bending vibration absorption peak of Si-O-Si, and vibrational absorption peak of Si-O, respectively. The band at 1025 cm−1 is an asymmetric stretching vibrational absorption peak of Si-O bridge noninternal-Si. −OH, a stretching band at 3396 cm−1, was observed for the structural hydroxyl group due to the structural water attached to the Mg and Al octahedra of ATP [24].
Compared with the original ATP, a stretching vibration peak of −NH at 685 cm−1 appears in the spectra of APTES-ATP, indicating that APTES successfully modified the surface of ATP. Some distinctive bands are obtained at 685, 1213, 1645, and 2941 cm−1 in IDS-ATP compared to the original ATP. The new peaks at 685 cm−1 and 1645 cm−1 in IDS-ATP can be traced to the stretching vibration of −NH. The new peaks at 1213 cm−1and 2941 cm−1 can be ascribed to the stretching vibration of C-N and −OH respectively. In addition, the peak becomes higher at 1645cm−1, possibly due to the −NH of IDS grafted to the surface of ATP, or the amide bond generated during functionalization [24]. The new peaks of EDTA-ATP can be explained by the symmetrical stretching peak of −COOH (1406 cm−1) and −NH (1644cm−1). Besides, the peak at 1644 cm−1 becomes higher, which may be due to the formation of amide bonds. The new peaks at 2522 and 2941 cm−1 are the results of the presence of the hydroxyl (−OH) group [47]. These observed changes prove that IDS and EDTA are grafted onto the ATP surface successfully.
3.2. The Chemical Stability of Functionalized ATPAfter treating with different Ph buffer solution, some elemental composition of ATP is shown in Table 1. As seen from the table, the mass contents of C, N, and H on the surfaces of IDS-ATP and EDTA-ATP are slightly different. After treatment, the C, N, and H content of IDS-ATP are reduced by 4.41%~13.24%, 2.04%~8.16%, 2.87%~6.32%, respectively; the C, N, and H content of the treated EDTA-ATP are reduced by 0.94%~3.77%, 7.46%~20.90%, and 10.06%~12.29%. The content of various elements on the surface of the material did not change much, indicating that ATP functionalized by IDS and EDTA has good stability under acidic, neutral, and alkaline conditions. But overall, the stability of IDS-ATP and EDTA-ATP in strong acid and strong alkaline solutions is slightly weaker. This may be caused by the formation of −NH3+COO− during functionalization, which is formed by the reaction between the −COOH on the surface of the chelate agent (IDS and EDTA) and −NH2 on the surface of ATP [11]. Under strongly acidic conditions, H+ recombines with COO- to form −COOH; under strongly alkaline conditions, OH− recombines with NH3+ to form −NH, so that part of IDS or EDTA is detached from the material.
3.3. Cu(II) Removal Behaviors3.3.1. Cu(II) removed by ATP, APTES -ATP, IDS -ATP and EDTA-ATP
Fig. 2 (a) demonstrates the impact of functionalization on the adsorption of Cu(II). The equilibrium adsorption of Cu(II) on ATP is 2.20 mg/g, which indicates that ATP has a poor adsorption capacity for Cu(II). The equilibrium adsorption of APTES-ATP, IDS-ATP, and EDTA-ATP increase to 2.32 mg/g, 9.45 mg/g, and 2.56 mg/g, respectively. Compared with ATP, the adsorption of Cu(II) increased by 329.55% for IDS-ATP and by 16.36% for EDTA-ATP. It is found that the adsorption capacity is improved by grafting nitrogen-containing groups and carboxyl groups on the surface of the ATP during functionalization.
3.3.2. The kinetic of Cu(II) removalThe influence of contact time on the adsorption of Cu(II) is shown in Fig 2 (a). The adsorption rate of IDS-ATP to Cu(II) is fast and gradually slows down, reaching adsorption equilibrium at 240 min. That of EDTA-ATP is 120 min. The adsorption kinetic parameters of functionalized ATP for Cu(II) are shown in Table S1 and S2 [48,49]. Quasi-first-order and quasi-second-order dynamic fitting of Cu(II) adsorption change were shown in Fig. S3. Compared with the quasi-second-order kinetics, the theoretical maximum adsorption capacity derived by the quasi-first-order kinetic equation differently from the experimental value, and the correlation coefficient R2 of the quasi-first-order kinetic equation is much smaller than that for the quasi-second-order kinetic equation. Thereby the adsorption process of IDS-ATP and EDTA-ATP on Cu(II) can be fitted by a quasi-second-order kinetic equation model. Therefore, adsorption is an irreversible reaction, and the Cu(II) fixed on the weak energy sites for a long time [50].
3.4. Study of the Adsorption Parameters3.4.1. The effects of adsorbent dosageFor the adsorption of Cu(II), when the adsorbent dosage is 800mg/L, IDS-ATP and EDTA-ATP have the best adsorption effect on Cu(II), and the adsorption amounts are 11.75 mg/g and 2.88 mg/g, respectively (Fig. 2 (b)). When the adsorbent dosages of IDS-ATP and EDTA-ATP are increased to 2000mg/L, the amounts of Cu(II) adsorbed are 10.75 mg/g and 2.25 mg/g, respectively. It can be observed that the removal rate of Cu(II) increased with increasing dose of adsorbent, and the adsorption capacity decreased gradually with increasing adsorbent dosage. When the dose of adsorbent is more than 800mg/L, a decrease in the adsorption capacity of Cu(II) was seen; therefore, 800mg/L was chosen as the best dosage.
3.4.2. The effects of pHpH is a key factor in the formation of stable complexes between metal ions and chelating agents. As shown in Fig. 2 (c), the adsorption capacity of IDS-ATP for Cu(II) is significantly better than that of EDTA-ATP. In addition, the adsorption of Cu(II) on IDS-ATP and EDTA-ATP increased as the pH increased. When the pH value of the solution is 2.0 and 3.0, the adsorption capacity is low. The adsorptions of IDS-ATP and EDTA-ATP for Cu(II) are only 4.4 mg/g and 2.9 mg/g, respectively. This may be related to the competition between the adsorption of H+ and Cu(II), and the surface-bound groups are −COOH in solution under a low pH value [51]. Furthermore, the electrostatic repulsion between Cu(II) and surface-bound groups is enhanced for the protonation of amino groups at low pH, which also reduces Cu(II) adsorption on the adsorbents [24]. The adsorption of Cu(II) increases sharply at pH>5.0. When the pH increases to 8.0, the adsorptions of IDS-ATP and EDTA-ATP on Cu(II) increase to 35.23 mg/g and 33.75 mg/g, respectively. This is due to the formation of Cu(II) hydroxide precipitates, which lower the solution concentration of Cu(II). Therefore, a pH of 5.0 is chosen as the optimum pH for the adsorption of Cu(II).
3.5. Adsorption Isotherms of Cu(II) RemovalThe adsorption isotherm curve of functionalized ATP for Cu(II) is presented in Fig. 2 (d). The result shows that with the increasing of Cu(II) concentration over a specific range, the adsorption capacity of functional ATP will increase until reaching the maximum adsorption capacity. Moreover, the adsorption of IDS-ATP was better than that of EDTA-ATP. With the increasing of initial concentration of Cu(II), the adsorption capacity of IDS-ATP and EDTA-ATP on Cu(II) increased from 6.24 mg/g to 17.90 mg/g and from 2.5 mg/g to 3.5 mg/g, respectively. Cu(II) is rapidly adsorbed on the functionalized ATP surface, and a few adsorption sites are occupied under a low initial concentration of Cu(II). Hence, the functionalized ATP shows a minor difference with ATP on the adsorption of Cu(II). More and more adsorption sites are occupied with the increasing of Cu(II) initial concentration, and the adsorption will tend to equilibrate under sufficiently high concentration.
The Langmuir and Freundlich adsorption isotherm models (Table. S3) are fitted to Fig. 2 (d), and the relevant parameters obtained are given in Table. S4. The Langmuir and Freundlich adsorption isotherm models are shown in Table. S5. The correlation coefficient R2 (0.9802) of the Langmuir equation for IDS-ATP absorbed Cu(II) is significantly higher than that of the Freundlich equation (0.9102). The results reflect that the adsorption of Cu(II) on IDS-ATP tends to be a homogeneous, monolayer adsorption process. However, the fitting correlation coefficient R2 (0.9523) of the Freundlich equation for EDTA-ATP adsorption on Cu(II) is significantly higher than that of the Langmuir equation (0.7655). Therefore, the Freundlich equation is better than the Langmuir equation. This indicates that Cu(II) adsorption onto EDTA-ATP is a major heterogeneous adsorption process. This may be due to the inhomogeneous functional group formation of the functionalization. The fitting results show that the equilibrium constant RL of the Langmuir equation is 0< RL <1, so the adsorption of Cu(II) is preferential [52].
3.6. Adsorption Effect of Combined Pollution SystemThe removal rate of heavy metal ions in a combined system is compared with a single system, the adsorption capacity of the prepared adsorbent is shown in Fig. 3. The Cr(VI) was added in the form of Cr2O72−. Adsorption of functionalized ATP for Cu(II) and Cr(VI) with different concentrations under single and combined HMs pollution systems. Fig. 3 (a) shows that the adsorption of Cu(II) by IDS-ATP and EDTA-ATP significantly increases under the combined pollution system. After adsorbing one of the HMs (Cu(II) or Cr(VI)) in the combined HMs pollution systems, the carboxyl group of the surface of functionalized ATP adsorbs another electrical heavy metal depending on its electrical properties of the adsorbed heavy metal [53]. Therefore, the adsorption of Cu(II) increased by 186.31% (51.25 mg/g) under the combined pollution system. Fig. 3 (b) shows that the adsorption of Cr(VI) increased by 376.47% (21.25 mg/g) under the combined pollution system. However, EDTA-ATP has poor treatment under the combined pollution system.
In summary, IDS-ATP has better pollution treatment under the combined pollution system, and the adsorption of combined pollution by IDS-ATP is better than that of EDTA-ATP.
3.7. Adsorption StabilityTo verify the adsorption stability of functional ATP, 85 mg/L NaNO3 solution was used as the desorption solution to desorb the adsorbed ATP. The results of the desorption of Cu(II) can be used to distinguish physical adsorption and chemical adsorption. Fig. 3 shows that the physical adsorption of IDS-ATP and EDTA-ATP for Cu(II) are 0.02 mg/g (0.21%) and 0.10 mg/g (3.89%), respectively. Therefore the adsorption of Cu(II) by functionalized ATP is mainly based on stable chemical adsorption.
3.8. Proposed Reaction MechanismTo understand the removal mechanism of IDS-ATP for Cu(II), the XPS spectra of IDS-ATP is partially changed after reacting with Cu(II) (Fig. 5 (b)). The appearance of the characteristic peak of Cu 2p 1/2 (953.8 eV), satellite peak (942.55 eV), and Cu 2p 3/2 (933.6 eV) are observed, indicating that Cu(II) has successfully adhered to the surface of the functionalized material. The content of cations (Ca, Si, Al, etc.) are reduced after reaction with Cu(II) for the ion exchange of Cu(II) with cations.
FTIR spectra of different IDS-ATP were compared and analyzed (Fig. 5 (a)). Fig. 5 (a) shows that the absorption peaks near 2941 cm−1 and 1645 cm−1 are weakened after the reaction with Cu(II). Here are the stretching vibration peaks of −OH and −NH, which means the change of −OH and −NH. And the stretching vibration peak of −CN (1213cm−1) is shifted after the reaction with Cu(II) [27]. The carboxyl and nitrogen-containing groups on the IDS-ATP surface participate in the adsorption process and chemically interact with Cu(II).
The C 1s spectra before and after the reaction of ATP-IDS with Cu(II) are shown in Fig. 5 (c). It can be seen that the C 1s spectra of IDS-ATP were C-C, C-N, and C=O peaks at 284.96 eV, 285.05 eV, and 288.30 eV, respectively [22]. After the reaction with Cu(II), the binding energy corresponding to the characteristic peak was reduced because the chemical bonds were broken during removal. The peak area ratio of C-C, O-C=O, and C-N are changed, and the peak area of O-C=O decreased, indicating that the chemical composition around the C atom changes after the reaction. The reason is that the carboxyl groups on the surface of the material produced chelation of Cu(II) [24].
The N 1s spectra of IDS-ATP before and after reaction with Cu(II) are shown in Fig. 5 (d). The two mainly composed peaks of N 1s spectra of IDS-ATP are at 398.68 eV (C-N and −NH) and 400.50 eV (O=C-NH- and −NH3+). The binding energy corresponding to the characteristic peak increases after the reaction, indicating that a metal-N coordination bond is formed, and N atoms share electrons with Cu. This evidence shows that the −NH2 participate in the reaction [54].
After analysing the characterization and experimental results, the removal mechanism of Cu(II) on IDS-ATP can be inferred. 1) the ion exchange between Cu(II) and the cations of the surface of IDS-ATP(Ca(II), Al(III), Si(IV)); 2) −COOH on the surface of the material produces chelation of Cu(II); 3) The coordination atom N on the surface of the material is coordinated with Cu(II). The possible adsorption mechanism is illustrated in Fig. 6.
3.9. Reuse Performance
Fig. 7 shows that the adsorption capacity of IDS-ATP for Cu(II) decreases slightly after repeated use 5 times. HCl solution (3.75mg/L) is used as desorbent. The removal of IDS-ATP on Cu(II) decreases from 55.15% to 49.76% and remains unchanged in the last two cycles, indicating that IDS-ATP has high stability and a good application prospect in the adsorption of heavy metal ions.
4. ConclusionsIn this study, the functional ATP (IDS-ATP) was successfully synthesized through functionalization with chelating agent IDS and was developed for the removal of HMs from aqueous solutions. The stability of IDS-ATP under acidic and neutral conditions was stable, and the risk of secondary pollution was significantly reduced. The IDS-ATP was characterized by SEM, FTIR, XRD, and BET. IDS was successfully grafted to the surface of ATP, and the crystal structure of ATP was not changed. Due to the anchored amino and carboxyl groups on the material surface, IDS-ATP could rapidly reduce Cu(II) in an aqueous solution, and the Cu removal ability was better than that of EDTA-ATP. The dosage of IDS-ATP was 800 mg/L, the pH was 5, the initial concentration was 64 mg/L, and the adsorption equilibrium time was 4 h. The adsorption of Cu(II) was enhanced by introducing IDS, which was 11.75 mg/g, 4.31 times as much as that of ATP, and 3.72 times as much as that of EDTA-ATP. The adsorption was stable chemical adsorption and preferential adsorption. In addition, the adsorption capacity of IDS-ATP is better in a combined pollution system than in a single pollutant system. Grafted IDS groups can provide active sites for the adsorption, and coordination of −NR, chelation of −COOH, and ion exchange between HMs and cations of the surface were predominant reactions involved in sorptive removal. It was concluded that IDS-ATP composites have the potential to be used for the treatment of HMs in water due to their efficiency, environmental friendliness, and low cost. It can be used in a complex water environment. In a word, an eco-friendly and promising water treatment agent was successfully prepared.
AcknowledgmentsThis work was supported by the Fundamental Research Funds for the Central Universities, and the World-Class Universities (Disciplines), and the Characteristic Development Guidance Funds for the Central Universities; and the National Natural Science Foundation of China [grant numbers 51979077]; the Fundamental Research Funds for the Central Universities [grant numbers 2019B42414]; the Jiangsu Science and Technology Department [grant numbers SBA2018030430 and BE2019121]; the Research on compound improvement and ecological utilization technology of power transmission and transformation project backfill soil [grant numbers SGJSHY00XMJS2100174]; and the Characteristic Development Guidance Funds for the Central Universities.
NotesAuthor contribution Q.Z.Z. (PhD student) and M.S. (Master) performed the batch experiments and initial data analysis and wrote the original draft; C.S.W. (PhD student) provided reviewing and editing; J.Z.Z. (Professor) provided reviewing and editing; L.L. (Master) provided formal analysis; D.L.J. (Associate Professor) provided funding acquisition; W.J.B. (Doctor) provided reviewing; and W.T.S. (Master) provided editing and project administration. All authors have given approval to the final version of the manuscript. Reference1. Feng Z, Ji S, Ping J, Cui D. Recent advances in metabolomics for studying heavy metal stress in plants. TrAC Trends Anal Chem. 2021;143:116402.
https://doi.org/10.1016/j.trac.2021.116402
2. Zhou P-F, Adeel M, Shakoor N, et al. Application of nano-particles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials. 2021;11(1)26.
https://doi.org/10.3390/nano11010026
3. Kul S. Removal of Cu(II) from aqueous solutions using modified sewage sludge ash. Int. J. Environ. Sci Technol. 2021;18:3795–3806.
https://doi.org/10.1007/s13762-021-03419-7
4. Gupta N, Yadav KK, Kumar V, Krishnan S, Alam J. Evaluating heavy metals contamination in soil and vegetables in the region of North India: Levels, transfer and potential human health risk analysis. Environ. Toxicol Phar. 2021;82:103563.
https://doi.org/10.1016/j.etap.2020.103563
5. Ali H, Khan E, Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem. 2019;2019:67300305.
https://doi.org/10.1155/2019/6730305
6. Naccarato A, Tassone A, Cavaliere F, et al. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci Total Environ. 2020;749:141438.
https://doi.org/10.1016/j.scitotenv.2020.141438
7. US EPA. Ground water and drinking water [Internet]. Washington: US EPA; c2021. [cited 26 October 2021]. Available from: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganic
8. Mateen QS, Khan SU, Islam DT, Khan NA, Farooqi IH. Copper (II) removal in a column reactor using electrocoagulation: Parametric optimization by response surface methodology using central composite design. Water Environ Res. 2020;92(9)1350–1362.
https://doi.org/10.1002/wer.1332
9. Ambiado K, Bustos C, Schwarz A, Bórquez R. Membrane technology applied to acid mine drainage from copper mining. Water Sci Technol. 2017;75(3)705–715.
https://doi.org/10.2166/wst.2016.556
10. Su Y-N, Lin W-S, Hou C-H, Den W. Performance of integrated membrane filtration and electrodialysis processes for copper recovery from wafer polishing wastewater. J Water Process Eng. 2014;4:149–158.
https://doi.org/10.1016/j.jwpe.2014.09.012
11. Caprarescu S, Corobea MC, Purcar V, et al. San copolymer membranes with ion exchangers for Cu(II) removal from synthetic wastewater by electrodialysis. J. Environ Sci. 2015;35:27–37.
https://doi.org/10.1016/j.jes.2015.02.005
12. Kim J, Bae S. Fabrication of Ti/Ir-Ru electrode by spin coating method for electrochemical removal of copper. Environ. Eng Res. 2019;24(4)646–653.
https://doi.org/10.4491/eer.2018.229
13. Zhang L-J, Tao H-C, Wei X-Y, Lei T. Wu W-M. Bioelectrochemical recovery of ammonia-copper(II) complexes from wastewater using a dual chamber microbial fuel cell Chemosphere. 2012;89(10)1177–1182.
https://doi.org/10.1016/j.chemosphere2012.08.011
14. Bal DK, Bhasarkar JB. Adsorptive degradation of hexavalent chromium from aqueous solution using coconut shell as a green adsorbent. Environ. Prog Sustainable Energy. 2021;40(4)e13594.
https://doi.org/10.1002/ep.13594
15. Malsawmdawngzela R, Lalhmunsiama , Tiwari D. Novel and highly efficient functionalized bentonite for elimination of Cu2+ and Cd2+ from aqueous wastes. Environ. Eng. Res. 2022;27(6)210355.
https://doi.org/10.4491/eer.2021.355
16. Uddin MK. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng J. 2017;308:438–462.
https://doi.org/10.1016/j.cej.2016.09.029
17. Nefzi H, Abderrabba M, Ayadi S, Labidi J. Formation of palygorskite clay from treated diatomite and its application for the removal of heavy metals from aqueous solution. Water. 2018;10(9)1257.
https://doi.org/10.3390/w10091257
18. Mu B, Wang A. Adsorption of dyes onto palygorskite and its composites: A review. J. Environ. Chem Eng. 2016;4(1)1274–1294.
https://doi.org/10.1016/j.jece.2016.01.036
19. Zhang W, Qian L, Ouyang D, Chen Y, Han L, Chen M. Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization. Chemosphere. 2019;221:683–692.
https://doi.org/10.1016/j.chemosphere.2019.01.070
20. Yang R, Li D, Li A, Yang H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018;151:20–28.
https://doi.org/10.1016/j.clay.2017.10.016
21. Potgieter JH, Potgieter-Vermaak SS, Kalibantonga PD. Heavy metals removal from solution by palygorskite clay. Miner Eng. 2006;19(5)463–470.
https://doi.org/10.1016/j.mineng.2005.07.004
22. Bennett RH, Hulbert MH, Curry KJ, Curry A, Douglas J. Organic matter sequestered in potential energy fields predicted by 3-D clay microstructure model: Direct observations of organo-clay micro- and nanofabric. Mar Geol. 2012;315–318:108–114.
https://doi.org/10.1016/j.margeo.2012.04.009
23. Huang J, Ye M, Qu Y, et al. Pb (II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J Colloid Interface Sci. 2012;385(1)137–146.
https://doi.org/10.1016/j.jcis.2012.06.054
24. Wang J, Zhang D, Liu S, Wang C. Enhanced removal of chromium(III) for aqueous solution by EDTA modified attapulgite: Adsorption performance and mechanism. Sci Total Environ. 2020;720:137391.
https://doi.org/10.1016/j.scitotenv.2020.137391
25. Kumar KV, Porkodi K. Modelling the solid-liquid adsorption processes using artificial neural networks trained by pseudo second order kinetics. Chem. Eng J. 2009;148(1)20–25.
https://doi.org/10.1016/j.cej.2008.07.026
26. Wang Y, Zhou R, Wang C, Zhou G, Song Z. Novel environmental-friendly nano-composite magnetic attapulgite functionalized by chitosan and EDTA for cadmium (II) removal. J Alloys Compd. 2020;817:153286.
https://doi.org/10.1016/j.jallcom.2019.153286
27. Lv L, Chen N, Feng C, Gao Y, Li M. Xanthate-modified magnetic chitosan/poly (vinyl alcohol) adsorbent: Preparation, characterization, and performance of Pb(II) removal from aqueous solution. J. Taiwan Inst. Chem Eng. 2017;78:485–492.
https://doi.org/10.1016/j.jtice.2017.06.009
28. Zhang W, Meng L, Mu G, Zhao M, Zou P, Zhang Y. A facile strategy for fabrication of nano-ZnO/yeast composites and their adsorption mechanism towards lead (II) ions. Appl. Surf Sci. 2016;378:196–206.
https://doi.org/10.1016/j.apsusc.2016.03.215
29. Nurchi VM, Cappai R, Crisponi G, Sanna G, Gama S. Chelating agents in soil remediation: A new method for a pragmatic choice of the right chelator. Front Chem. 2020;8:597400.
https://doi.org/10.3389/fchem.2020.597400
30. Wei M, Chen J, Wang Q. Remediation of sandy soil contaminated by heavy metals with Na2EDTA washing enhanced with organic reducing agents: element distribution and spectroscopic analysis. Eur. J Soil Sci. 2018;69(4)719–731.
https://doi.org/10.1111/ejss.12560
31. Rui D, Wu Z, Ji M, Liu J, Wang S, Ito Y. Remediation of Cd- and Pb-contaminated clay soils through combined freeze-thaw and soil washing. J. Hazard Mater. 2019;369:87–95.
https://doi.org/10.1016/j.jhazmat.2019.02.038
32. Zhen H-b, Xu Q, Hu Y-y, Cheng J-h. Characteristics of heavy metals capturing agent dithiocarbamate (DTC) for treatment of ethylene diamine tetraacetic acid-Cu (EDTA-Cu) contaminated wastewater. Chem. Eng J. 2012;209(1)547–557.
https://doi.org/10.1016/j.cej.2012.08.045
33. Palden T, Machiels L, Onghena B, Regadío M, Binnemans K. Selective leaching of lead from lead smelter residues using EDTA. Rsc Adv. 2020;10:42147–42156.
https://doi.org/10.1039/D0RA08517K
34. Wu Q, Cui Y, Li Q, Sun J. Effective removal of heavy metals from industrial sludge with the aid of a biodegradable chelating ligand GLDA. J. Hazard Mater. 2015;283:748–754.
https://doi.org/10.1016/j.jhazmat.2014.10.027
35. Gusiatin ZM, Kulikowska D, Klik B. Suitability of humic substances recovered from sewage sludge to remedy soils from a former As mining area - a novel approach. J. Hazard Mater. 2017;338:160–166.
https://doi.org/10.1016/j.jhazmat.2017.05.019
36. Kaurin A, Gluhar S, Tilikj N, Lestan D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere. 2020;260:127673.
https://doi.org/10.1016/j.chemosphere.2020.127673
37. Xiao R, Ali A, Wang P, Li R, Tian X, Zhang Z. Comparison of the feasibility of different washing solutions for combined soil washing and phytoremediation for the detoxification of cadmium (Cd) and zinc (Zn) in contaminated soil. Chemosphere. 2019;230:510–518.
https://doi.org/10.1016/j.chemosphere.2019.05.121
38. Wang G, Zhang S, Zhong Q, et al. Effect of soil washing with biodegradable chelators on the toxicity of residual metals and soil biological properties. Sci Total Environ. 2018;625:1021–1029.
https://doi.org/10.1016/j.scitotenv.2018.01.019
39. Zeng M, Yang B, Zhang H, Jia F. A green depressant iminodisuccinic acid (IDS) for apatite-dolomite separation and its interaction mechanism. Miner Eng. 2022;175:107276.
https://doi.org/10.1016/j.mineng.2021.107276
40. Kołodyńska D. Adsorption characteristics of chitosan modified by chelating agents of a new generation. Chem. Eng J. 2012;179:33–43.
https://doi.org/10.1016/j.cej.2011.10.028
41. Cokesa Z̆, Knackmuss HJ, Rieger PG. Biodegradation of all stereoisomers of the EDTA substitute iminodisuccinate by Agrobacterium tumefaciens BY6 requires an epimerase and a stereoselective C-N lyase. Appl. Environ Microbiol. 2004;70(7)3941–3947.
https://doi.org/10.1128/AEM.70.7.3941-3947.2004
42. Wang G, Pan X, Zhang S, Zhong Q, Peijnenburg W. Remediation of heavy metal contaminated soil by biodegradable chelator-induced washing: Efficiencies and mechanisms. Environ Res. 2020;186:109554.
https://doi.org/10.1016/j.envres.2020.109554
43. Wu Q, Duan G, Cui Y, Sun J. Removal of heavy metal species from industrial sludge with the aid of biodegradable iminodisuccinic acid as the chelating ligand. Environ. Sci. Pollut Res. 2015;22:1144–1150.
https://doi.org/10.1007/s11356-014-3365-y
44. Kołodyńska D. Iminodisuccinic acid as a new complexing agent for removal of heavy metal ions from industrial effluents. Chem. Eng J. 2009;152(1)277–288.
https://doi.org/10.1016/j.cej.2009.05.002
45. Mohammed AA, Moamen OAA, Metwally SS, El-Kamash AM, Ashour I, Al-Geundi MS. Utilization of modified attapulgite for the removal of Sr(II), Co(II), and Ni(II) ions from multicomponent system, part I: Kinetic studies. Environ. Sci. Pollut Res. 2020;27:6824–6836.
https://doi.org/10.1007/s11356-019-07292-3
46. Repo E, Warchoł JK, Bhatnagar A, Mudhoo A, Sillanpää M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013;47(14)4812–4832.
https://doi.org/10.1016/j.watres.2013.06.020
47. Chen B, Zhang N, Li C, Li R, Fan A. Synthesis and chromatographic characteristics of iminodisuccinic acid-functionalized silica stationary phase. J Chromatogr A. 2017;1530:120–128.
https://doi.org/10.1016/j.chroma.2017.11.032
48. Deb A, Kanmani M, Debnath A, Bhowmik KL, Saha B. Ultrasonic assisted enhanced adsorption of methyl orange dye onto polyaniline impregnated zinc oxide nanoparticles: Kinetic, isotherm and optimization of process parameters. Ultrason Sonochem. 2019;54:290–301.
https://doi.org/10.1016/j.ultsonch.2019.01.028
49. Khamizov RK. A pseudo-second order kinetic equation for sorption processes. Russ. J. Phys Chem. 2020;94:171–176.
https://doi.org/10.1134/S0036024420010148
50. Youcef LD, Belaroui LS, López-Galindo A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019;179:105145.
https://doi.org/10.1016/j.clay.2019.105145
51. Ezzeddine Z, Batonneau-Gener I, Pouilloux Y, Hamad H, Saad Z, Kazpard V. Divalent heavy metals adsorption onto different types of EDTA-modified mesoporous materials: Effectiveness and complexation rate. Microporous Mesoporous Mater. 2015;212:125–136.
https://doi.org/10.1016/j.micromeso.2015.03.013
52. Zhou X, Zhou X. The unit problem in the thermodynamic calculation of adsorption using the langmuir equation. Chem. Eng Commun. 2014;201(11)1459–1467.
https://doi.org/10.1080/00986445.2013.818541
53. Li H, Cao X, Zhang C, et al. Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. RSC Adv. 2017;27:16273–16281.
https://doi.org/10.1039/C7RA01647F
54. Eltaweil AS, El-Monaem EMA, Mohy-Eldin MS, Omer AM. Fabrication of attapulgite/magnetic aminated chitosan composite as efficient and reusable adsorbent for Cr (VI) ions. Sci Rep. 2021;11:16598.
https://doi.org/10.1038/s41598-021-96145-6
Fig. 1SEM images of ATP, APTES-ATP, IDS-ATP, and EDTA-ATP (a–d); XRD patterns of ATP, APTES-ATP, IDS-ATP, and EDTA-ATP (e); FTIR spectra of ATP, APTES-ATP, IDS-ATP, and EDTA-ATP (f). 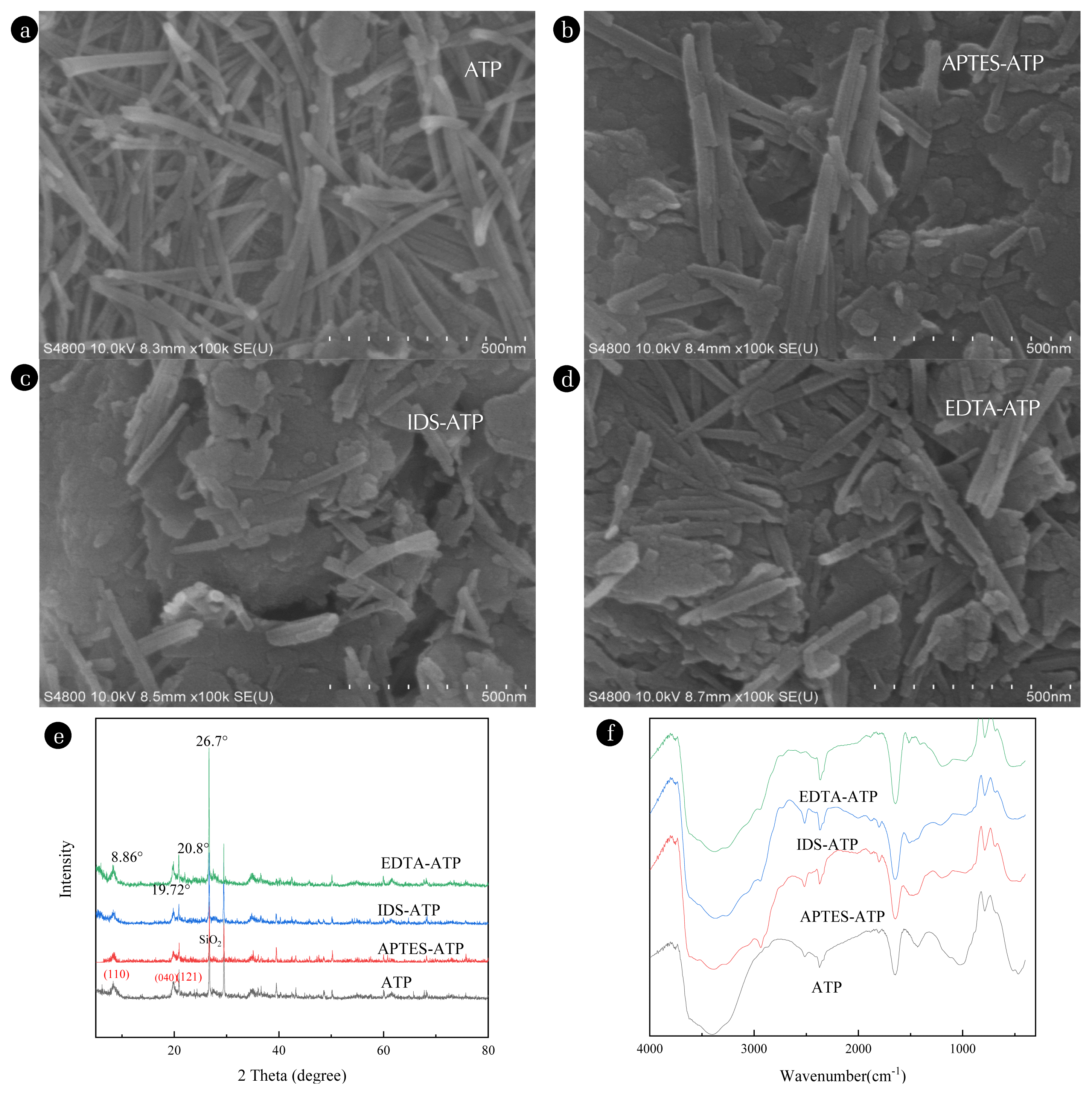
Fig. 2Effect of attapulgite functionalization on adsorption of Cu(II) at pH 5.0, 25°C, dosage is 1600mg/L and initial Cu(II) concentration of 64 mg/L (a); The effect of absorbent dosage on the adsorption of Cu(II) at pH 5.0, 25°C and initial Cu(II) concentration of 64 mg/L (b); The effect of pH on the adsorption of Cu(II) at 25°C, dosage is 1600mg/L and initial Cu(II) concentration of 64 mg/L (c); Adsorption isotherms of Cu(II) on EDTA-ATP and IDS-ATP at pH 5.0, 25 °C and dosage is 1600mg/L (d). 
Fig. 3Adsorption of Cu(II) (a) and Cr(VI) (b) by functionalized ATP under single and combined HMs pollution systems at pH 5.0, 25 °C and dosage is 1600mg/ 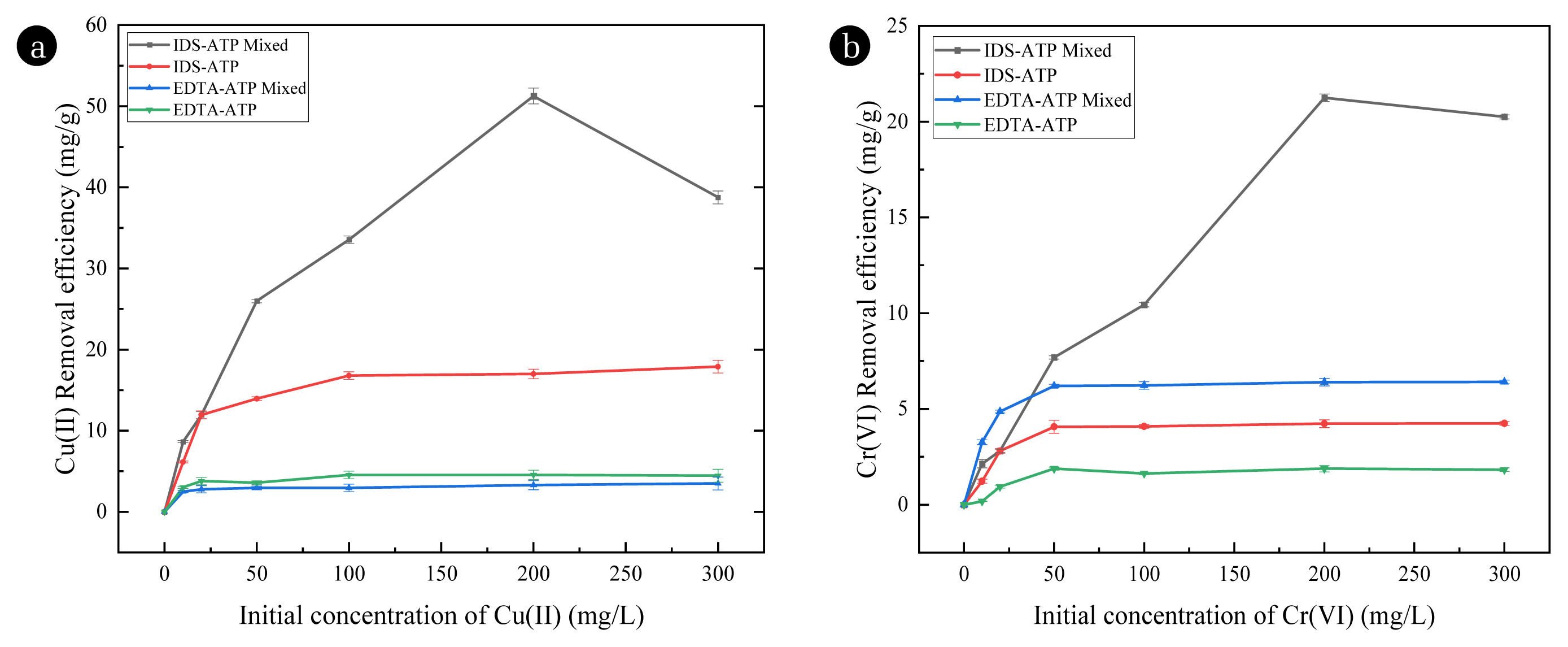
Fig. 5FT-IR spectra of IDS-ATP before and after Cu(II) adsorption (a); XPS spectra of IDS-ATP before and after Cu(II) adsorption(b–d): full-scan XPS spectra of IDS-ATP and Cu 2p spectra of IDS-ATP (b); C 1s spectra of IDS-ATP before and after Cu(II) adsorption (c); N 1s spectra of IDS-ATP before and after Cu(II) adsorption (d). 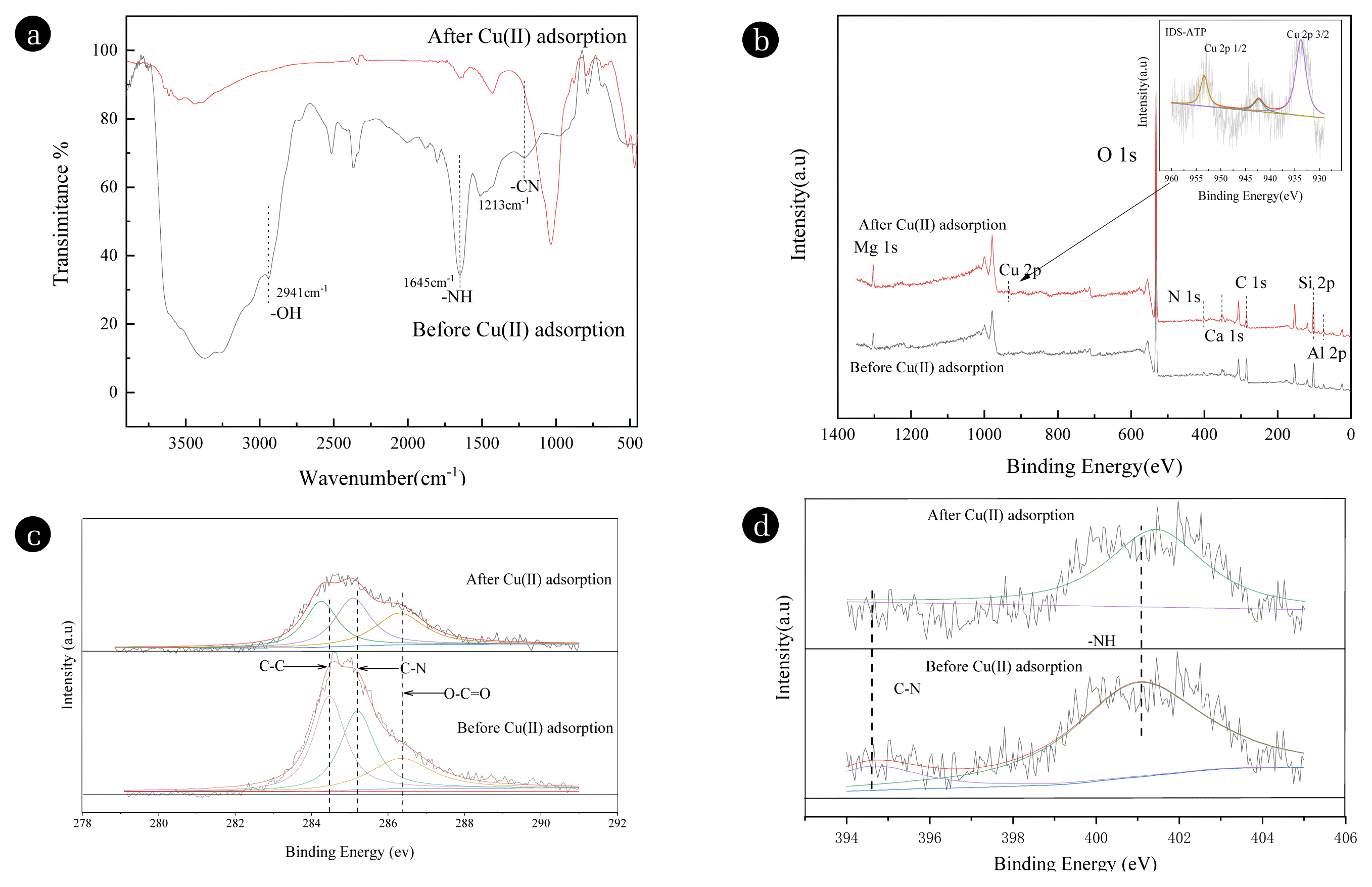
Table 1Element composition of functional ATP
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||