AbstractDespite the severity of health impacts caused by particulate matter with an aerodynamic diameter ≤ 1.0 μm (PM1.0), corresponding data have been poorly documented compared to that of particulate matter with an aerodynamic diameter ≤ 2.5 μm (PM2.5) or ≤ 10 μm (PM10). In this study, the characteristics of PM1.0 and its chemical species (black carbon [BC], SO42−, NO3−, NH4+, and 16 metals) were investigated in the roadside area of Daejeon. The mean PM1.0 concentration was 19.9 ± 5.50 μg m−3 (range of 6.86–34.7 μg m−3) near a road with high traffic density during a 3-month period in the fall/winter of 2019. The mean concentrations (± SD and range) of BC, NO3−, and SO42− in PM1.0 were 4.89 ± 4.21 (1.26–8.23), 4.21 ± 1.39 (1.76–9.78), and 4.30 ± 1.10 (1.16–7.03) μg m−3, respectively. Strong relationships between PM1.0 and major species such as BC, SO42−,NO3−, and NH4+ were observed with correlation coefficients of 0.52–0.96. After evaluating the factors affecting PM1.0 concentrations using a positive matrix factorization model, six source types were revealed: secondary aerosol (54.1%), vehicle exhaust (21.2%), re-suspended soil-road dust (9.6%), fossil fuel combustion (9.1%), biomass burning (3.3%), and industrial activities (2.6%).
Graphical Abstract
1. IntroductionSmall air particulate matter (PM) can be deposited in the lower human respiratory tract due to its permeability. [1–4]. In particular, PM with an aerodynamic diameter ≤ 2.5 μm (PM2.5) is a well-known pollutant exhibiting potential human health effects. However, the environmental significance of PM with an aerodynamic diameter ≤ 1.0 μm (PM1.0), which are submicron particles in the air, remains poorly recognized in urban areas due to a lack of information (e.g., data distribution and characterization). As PM1.0 has a larger specific surface area than that of PM2.5, toxic pollutants can easily adsorb onto it. Thus, PM1.0 poses a greater threat to human health than that presented by PM2.5 [5–6]. According to the results of Zwozdziak et al. [7], PM1.0 has a greater impact on lung functioning than PM2.5. In addition, some epidemiological studies have indicated that fine particles are significantly associated with adverse health effects [8–9]. The adverse health effects of PM are determined by the combined effects of both the particulate mass and the chemical species. For instance, many trace metals (such as As, Be, Cd, Cr, Cu, Hg, Mn, Ni, Pb, Sb, Se, V, and Zn) have been identified as toxic components in urban air PM [10].
In recent decades, most studies on air particulates and chemical species have focused on PM2.5. Only a few studies have made efforts to describe the characteristics of PM1.0 and relevant data in urban areas [11–13], industrial areas [14–16], roadside areas [6, 17], underground parking garages [18], and rural areas [11]. There are also a few studies on the PM1.0 and constituents in Korea conducted in the early 2010s, but no data have been reported since then. Consequently, relatively little is known about the distribution and chemical species of PM1.0 in roadside areas. Hence, investigating the chemical composition and source of PM1.0 is becoming an urgent need for evaluating effective mitigation policies and conducting research on environmental, health, and climate impacts.
In many urban areas worldwide, traffic activity has been recognized as one of the most important sources of urban particulate matter pollution. Further, such pollution has been worsening due to fossil fuel consumption resulting from increased traffic volume and growth of industrial activities. According to the results of Qiu et al. [18], the total respiratory deposition dose rates of PM around roadside areas were significantly higher than those of the background. Grahame and Schlesinger [20], investigated the database of numerous ambient air studies and provided substantial evidence to associate adverse effects on cardiovascular health with vehicular emissions.
The concentration levels of airborne PM are tightly affected by meteorological conditions, long-distance transmission, and social and economic factors [21]. Previous studies in China have shown that the distribution of PM, regardless of the particle size, is affected by meteorological and geographical wind pathways [17, 21]. PM is a complex mixture of natural and anthropogenic pollutants that consists of elemental C, SO42−, NO3−, ammonium, metals, and many other organic compounds. It is also affected by various local and transboundary sources in Korea [22–25]. Secondary pollutants are likely to be formed photochemically or through other routes in the atmosphere, whereas primary pollutants are generated from numerous megacities and industrial areas located on the east coast of China and passing through the West Sea. Thus, air particulate could be flowed into the Korean Peninsula at elevated level. Further, their concentrations can substantially increase if inactive pollutants are generated from large industrial areas and coal power plants located in the air pathway. Therefore, information on PM measured at the receptor points can provide important insights into the quantitative apportionment of local pollution sources and long-range transboundary pollutants.
Receptor modeling, especially positive matrix factorization (PMF), is a suitable assessment method for PM sources based on chemical species data collected at receptor points [3, 26, 27]). PMF can be used to assign every factor loading value and the common factor to a positive value. Further, owing to its superior applicability in diverse source profiles [28], many researchers have applied PMF for source apportionment studies of PM1.0 source apportionment studies of PM1.0 [12, 26], PM2.5 [25, 27, 29], hourly PM2.5 [30], indoor PM10 and PM2.5 [31], and volatile organic compounds [32–34].
In this study, the characteristics of PM1.0 and its associated chemical species were investigated at the roadside area in Daejeon, which is a major metropolitan city in Korea. A total of 67 PM1.0 samples were collected near the road with high traffic density. Most PM concentrations in East Asia are seasonally characterized by being low in summer and high in winter due to the climatic variations between summer and winter (i.e., the degree of circulation and rainfall). Moreover, fuel-burning for space heating and agriculture-related activities (e.g., biomass burning) in fall and winter inevitably contributes to PM1.0 pollution [17]. Thus, in this study, intensive field campaigns were carried out during a three-month period in the fall and winter of 2019.
The concentrations of the chemical species in the PM1.0 samples were measured using the multi-wavelength absorption technique, ion chromatography (IC), and inductively coupled plasma–mass spectrometry (ICP–MS). Based on the data obtained, we attempted to elucidate the distribution characteristics of PM1.0 and the chemical species in the studied roadside area. The source types and relative contributions of each source in the study area were then estimated and quantified using the PMF receptor model.
2. Experimental2.1. Site Characteristics and SamplingThe PM1.0 samples were simultaneously collected with low-volume air samplers at the roadside area in Daejeon city, central Korea. The sampling site (36°21′45″ N, 127°20′42″ E) is surrounded by densely populated residential and commercial areas. As the study area is located on a roadside with heavy traffic volumes (approximately 8000 vehicles/h) and regular congestion, it represents an area with considerable traffic-related pollution in Daejeon city.
A total of 67 PM1.0 samples on a 24-h basis were collected in the fall and winter (September 30–December 15) of 2019. A vacuum air sampler (URG, 3000C model) was adopted, and a cyclone of 1 μm cut-point (URG-2000-30EHB) was used to collect PM1.0 samples on the polycarbonate filter (47 mm, 0.4-μm pore size, Nuclepore). The airflows for the sampler were adjusted to a rate of 16.7 L min−1 at the beginning of sampling. Each filter was weighed in a controlled atmosphere (20 °C and 50% relative humidity) for 24 h before and after exposure to air. These filters were weighed three times in a pre-calibrated and tared microbalance with a readability of 1 μg (XPE26, Mettler-Toledo Ltd.) and controlled electrostatic charges in order to restrain any charge forces from a filter in the balance. The hourly meteorological data (e.g., rainfall, temperature, relative humidity, wind direction, and horizontal wind velocity) were obtained from the Korea Meteorological Administration (KMA).
2.2. Species AnalysisThe PM-bound concentrations of black carbon (BC), ions, and trace elements were determined sequentially. To determine the BC concentration, a multi-wavelength absorption BC instrument (MABI, Australian Nuclear Science and Technology Organization) that functions at seven wavelengths (405, 465, 525, 639, 870, 940, and 1050 nm) was used [35, 36]. The results for the different wavelengths were used to differentiate the different BC size fractions originating from sources such as wood smoke or vehicle exhaust. The BC concentration was evaluated from its absorption value at 639 nm, which the predominant absorber wavelength. The mass concentration of BC converted using the specific mass attenuation given by the manufacturer. When the standard filter was repeatedly measured, the relative standard deviation of the intensities was < 5%.
After measuring the gravimetric mass and multi-wavelength absorption, three-quarters of the PM filter were digested using a microwave digestion system (ETHOS EASY, Milestone). Then, the sample was loaded in a PTFE digestion vessel with 8 mL of HNO3 (65%, Optima Grade, Thermo Fisher Scientific), and the temperature was increased to 200 °C within 5 min (1200 W) and then maintained for another 20 min. After digestion, the vessels were cooled to room temperature for 1 h, and the remaining solutions were transferred to a centrifuge tube and diluted with deionized water. The centrifuged sample aliquot was introduced into the ICP-MS system (ICAP RQ, Thermo Fisher Scientific) using the kinetic energy discrimination (KED) mode (collision cell with He gas and kinetic energy discrimination). Although the ICP-MS technique is accurate for determining multiple elements in PM samples, interference from molecular overlap or polyatomic ions should also be considered. Recent developments in the ICP-MS technique have removed interfering ions by applying gas reaction/collision in the KED mode [37–40]. Sixteen elements were analyzed: Al, As, Ba, Cd, Cr, Cu, Fe, K, Mg, Mn, Ni, Pb, Se, Ti, V, and Zn. Five working standards were prepared from stock solutions (TraceCERT®, Sigma-Aldrich) in 2% HNO3. The analytical conditions for ICP-MS are shown in Table S1. The standard calibration curves for the ICP-MS analysis were generally in line with those obtained using the five working standards (r-value > 0.999). Additionally, the final concentrations were corrected using combined reagent and filter blanks. Spiked and recovered 115In concentrations were set as an internal standard for each sample. Recovery of the pretreatment steps and ICP-MS analysis was evaluated and adjusted for the measurement data. The recovery of spiked 115In ranged from 82–105%, with a mean of 93%. For analysis of ionic components (e.g., SO42−, NO3−, and NH4+) in the filter samples, ion chromatograph (IC, Thermo Scientific Dionex ICS-2100 system) was employed. IC consists of a separation column, guard column, and suppressor using sulfuric acid. The ionic components were extracted from a quarter of the filter sample with ultrapure water using an ultrasonic instrument (Branson 8210, USA) for 30 min.
For quality assurance, the NIST standard reference material was used (SRM: NIST, National Institute of Standards and Technology, USA; SRM 2783, air particulate on filter media) for the ICP-MS measurements. The relative errors (against SRM values) of all target elements were < 20%. After repetitive analysis, the relative standard deviations were determined to be < 5% for all the target elements. For the IC analysis, the pooled standard deviation for the peak area of the standard solution was approximately 3%, and the recovery of spiked species was within ± 5%.
2.3. Conditional Probability FunctionThe conditional probability function (CPF) was computed using wind direction and wind speed to help understand the characteristics of PM1.0 and the species concentrations in the study area. A CPF can be used to specifically estimate possible local sources using wind direction data [12, 41–43]. The CPF (probability value from 0 to 1) is defined by the following equation (1):
where mΔθ indicates the wind frequency blowing from the direction of Δθ on different days, with a concentration higher than the threshold criterion; whereas, nΔθ indicates the frequency of wind blowing from the direction of Δθ in the overall data. As the current domestic meteorological data can represent wind from 16 directions, Δθ was set as 22.5 in this study. The same daily concentration data were assigned to each hour of a given day to correspond to the hourly wind data. Calm wind (<1.0 m·s−1) was excluded from the analysis; the threshold criterion of the upper 25th percentile value was used.
2.4. Positive Matrix FactorizationThe details regarding PMF theory and its applications can be found in several studies [12, 17, 25–28, 30, 44]. In the present study, the EPA PMF (ver. 5.0), which is the most recent version using the multilinear engine 2 (ME-2), was used for source apportionment following the EPA PMF User Guide [45]. The selection of input data is critical for PMF-based source apportionment, and both the concentrations of the chemical components and their error estimates should be provided as PMF input data. The expanded uncertainty in 99 % confidence interval of each analytical value is evaluated from the major uncertainty factors for the analytical process such as reproducibility of the measurements, recovery ratio, and stability of efficiency calibration. Basic equation of PMF model is defined by the following equation (2):
The method has been developed to obtain the unknown matrix, G and F by the iterative treatment of a least square method by the following equation (3):
Here, X(m×n) is the data matrix consisting of the m chemical components analyzed in n samples. G(n×p) is the source contribution to each sample. F(p×m) is the matrix of source profile. E presents the residual matrix of calculation.
In this study, both terms were determined by the following methods: 1) the measured data were used directly with their error estimates at the expanded uncertainty; and 2) data below the detection limit were replaced with a half value of the detection limit for each element with 5/6 of their detection limits as error estimates [42, 44, 45]. The PMF run with the lowest Q representing the global minima was selected for obtaining an optimal solution for the number of factors. The initial solutions were iterated with random seed start and robust modes to minimize random errors and rotational ambiguities. In addition, intra-run residual analysis, bootstrapping (BS), displacement (DISP), and BS–DISP were applied to evaluate the optimal solution [27].
3. Results and Discussion3.1. PM1.0 Characteristics
Table 1 summarizes the PM1.0 and associated species concentration data measured near the roadside in sampling site. According to the summary provided in Table 1, the mean PM1.0 concentration was 19.9 ± 5.50 μg m−3, with a range of 6.86–34.7 μg m−3. During the sampling period in 2019, there was no significant trend in the PM1.0 concentrations for distinctive pollution events. The time series of temperature, rainfall, and wind speed frequency based on a 1-h resolution of the meteorological data during the study period are presented in Fig. S1. As shown in Fig. S1, rainfall was 227 mm (only 5 days), the mean temperature was 9.6 °C, and the frequency of calm wind (defined as < 1.0 m s−1) was 21.6%. Owing to the cumulative effect of stagnant movements of the atmosphere and low rainfall, particulate concentrations in the air increased in the fall and winter in Korea.
The distribution characteristics of PM1.0 concentrations (N = 67) observed at the roadside area are presented in Fig. 2; 70.1% of the PM1.0 concentrations were between 15–25 μg m−3 and 92.5% were < 30 μg m−3. The results for the relatively steady PM1.0 concentration revealed the absence of a transboundary invasion event with significant influence in the sampling period.
According to previous studies, the PM1.0 concentration distribution trend is consistent with that of PM2.5. The mass concentration ratios of PM1.0 and PM2.5 were 0.64 [46] and 0.90 [14] in an industrial area, 0.61–0.91 in an urban area [6, 16], 0.80 in a rural area [22], 0.82 at the roadside in a commercial and industrial area [17], and 0.84–0.92 in an underground parking garage [18]. These ratios suggest that submicron particles < 1.0 μm are the major components of PM2.5, regardless of the characteristics of the area. Currently, there are no legislative criteria to regulate PM1.0 concentrations in Korea; however, annual PM2.5 standard was set to < 15 μg m−3. It is noteworthy that the field campaign was conducted over three months, and caution is needed in comparison with annual standard. However, even upon comparison with PM2.5 standard, when considering the PM1.0/PM2.5 ratio, it is clear that the PM1.0 level in the study area was significantly high and needs to be managed.
As a simple means to evaluate the pollution levels of the study area, the PM1.0 concentration data in this study can be compared to those from comparable studies (see Table 2). The mean PM1.0 concentrations measured in this study were comparable to those in rural [22] and industrial areas in Korea [46] and the UK [11], residential areas with heavy traffic in Italy [26], urban areas with heavy traffic in Vietnam [16], and industrial areas in Poland [15]. However, the PM1.0 values in the present study were considerably lower than those of other Asian cities, such as Wuhan (22–128 μg m−3: [14]), Harbin (58–429 μg m−3: [18]; 11.2–125.3 μg m−3: [6]), Beijing (6.40–216.32 μg m−3: [17]), and Delhi (31.5–568.8 μg m−3: [12]).
Z-statistics were applied to the PM1.0 datasets to assess the significance of quantitative differences in the mean concentrations with variation in days of the week. Pairwise comparison between PM1.0 concentrations (through Z-statistics tests) indicated that there was no statistically significant difference between the mean PM1.0 concentrations on different days of the week.
As shown in Fig. 3, wind direction was not a distinct contributing factor when determining the pollution sources according to the CPF calculations for PM1.0, BC, SO42−, and NO3−. Similar probabilities reflected widespread pollution in the study area. However, the comparatively high values in the southeastern region of the study area may reflect the impact of the large residential area combined with a relatively higher traffic volume and heating oil combustion during winter.
3.2. Characteristics of Chemical Species
Table 1 shows that the BC, SO42−, and NO3− concentrations in PM1.0 were substantially higher than those of the metal species. BC, SO42−,and NO3−, which are predominant in fine particles with NH4+ in air particulates, are produced as a result of the reaction and transformation between the pollutants emitted from various combustion processes [24]. Thus, it is generally difficult to accurately determine their unique sources. The mean concentrations ± standard deviation (and ranges) of BC, NO3−, and SO42− in PM1.0 were 4.89 ± 4.21 (1.26–8.23), 4.21 ± 1.39 (1.76–9.78), and 4.30 ± 1.10 (1.16–7.03) μg m−3, respectively. The BC, NO3−, and SO42− concentrations were added and compared with the PM1.0 mass concentrations; each fraction accounted for approximately 24.6%, 21.2%, and 21.6% of the total content, respectively. Their total amount accounted for a mean of 13.1 μg m−3 (65.8%) in PM1.0 in this area during the study period.
It has been well recognized that BC is a marker element of air particulates in combustion processes (e.g., vehicle exhaust, biomass burning, and fossil fuel combustion) regardless of the particle size fraction. BC is likely an important causal agent intrinsically and many carbonaceous species co-emitted from diesel and other vehicles can be adsorbed onto the surface of BC [20]. BC, which is an important contributor to global warming, is also considered an effective additional indicator for estimating human health effects [47]. The light absorption by BC in the filter samples was measured at seven different wavelengths from 405–1050 nm. As the characteristics (e.g., shape and absorption sensitivity) of BC vary with different emission sources, the BC data from each wavelength can be used to distinguish BC source contributions from vehicles and smoke events such as biomass burning [35, 48]). Thus, more information on possible sources of BC could be derived from the difference between the BC values at 405 nm and 1050 nm [49]. The variations in BC concentrations are depicted in Fig. S2, with the differences ranging between 405–1050 nm. As shown in Fig. S2, there was no significant trend in BC concentrations during distinctive pollution events. In addition, there was a distinct spike in the daily BC (405–1050 nm) value. These results imply that the major fraction of BC concentrations originated from the vehicular exhaust in the study area. Further, the CPF result for BC (Fig. 3b) supports the widely distributed traffic volume within the study area.
As a certain portion of SO2 and NO2 diffuse to Korea from the Chinese continent with westerly winds, the SO42− and NO3− in air particulates of the study area, which is located in the heartland of the Korean Peninsula, could increase during the fall and winter due to northwesterly winds. Hence, it is surmised that NO3− and SO42− from various regional sources were transported into the study area, which contributed to the formation of secondary aerosols. The CPF results of similarly distributed probabilities with wind direction for NO3− and SO42− (Fig. 3) support this interpretation.
To evaluate the characteristics of the secondary ionic species, the measured NH4+ concentrations were compared with the corresponding NH4+ concentrations calculated based on the stoichiometric ratios of the major compounds, such as ammonium sulfate ((NH4)2SO4), ammonium bisulfate (NH4HSO4), and ammonium nitrate (NH4NO3). This was carried out under the assumption that NO3− exists in the form of NH4NO3 and that SO42− is in the form of either (NH4)2SO4 or NH4HSO4 [26, 50]. Sulfate aerosols containing mainly NH4HSO4 can be considered as moderately aged aerosols, whereas sulfate aerosols containing mainly (NH4)2SO4 can be viewed as highly aged aerosols [51]. As shown in Fig. S3, the ratios between the calculated NH4+ (assuming (NH4)2SO4 or NH4HSO4) and measured NH4+ were 1.2 and 0.84, respectively. This indicates that SO42− in PM1.0 at the study area was well mixed with freshly emitted gas and aged particles. Furthermore, from the correlation analysis between BC and SO42− of PM1.0 measured in a rural area of Korea, Lim et al. [22] stated that BC is likely to mix with SO42− sufficiently under the influence of substantial anthropogenic sources. As SO2 emissions have dramatically decreased in Korea, it could be surmised that the considerable SO42− concentrations were affected by long-range transported SO2. Thus, the compound forms of SO42− and the higher correlation (r = 0.81) between BC and SO42− in PM1.0 at the study area suggested that the SO4−2 concentrations were influenced by pollutants emitted in the study area and trans-boundary pollutants from outside the study area.
A higher correlation coefficient suggests a strong relationship between the pollution sources. As shown in Fig. 4, strong relationships between PM1.0 and the major species (e.g., BC, SO42−, NO3−, and NH4+) were observed with correlation coefficients between 0.52–0.96. In particular, the correlation coefficients between PM1.0 and BC, NO3−, and SO42− were 0.96, 0.82, and 0.84, respectively. Hence, it may be concluded that the PM1.0 concentrations in the study area were strongly ruled by secondary ionic species and BC concentrations. According to a statistical summary of the data in Table 1, the concentrations of major species (e.g., BC, SO42−,NO3−, and NH4+) were apportioned by 79.7 ± 6.3% (66.1–98.2%) of the PM1.0 concentration.
However, the concentrations of the 16 analyzed elements (Al, As, Ba, Cd, Cr, Cu, Fe, K, Mg, Mn, Ni, Pb, Se, Ti, V, and Zn) were approximately 5.5 ± 3.8% (1.6–26.5%), according to the results of the quantitative elemental analysis for airborne PM1.0 samples by ICP-MS. Although the elements constitute only approximately 5% of the total particulate mass, they can play a critical role in the identification of diverse source processes. As large amounts of C, ionic components, and major crustal elements are commonly bound to each other in most sources, each source type can be specifically assigned to its tracer [3]. The concentrations of metals associated with crustal sources (such as Al, Fe, and K) were much higher than those of any other toxic metals (As, Cd, Cr, Cu, Ni, Pb, Se, and Zn). The mean concentrations (± standard deviation) of As, Cd, Cr, Cu, Ni, Pb, Se, and Zn in PM1.0 were averaged as 1.91 (± 0.56), 2.51 (± 0.74), 19.0 (± 6.22), 18.8 (± 16.6), 14.8 (± 13.1), 21.6 (± 9.05), 1.62 (± 0.49), and 41.1 (± 117) ng m−3, respectively. Based on a simple comparison between the metal concentrations and their magnitudes, the datasets can be arbitrarily grouped into three different categories: 1) < 101 ng m−3: As, Cd, Se, V, and Ti; 2) 101–102 ng m−3: Ba, Cr, Cu, Mn, Ni, Pb, and Zn; and 3) > 102 ng m−3: Al, Fe, and K. These distribution characteristics of quantitatively measured elemental concentrations are comparable with those observed in residential areas with heavy traffic [26].
3.3. Sources Affecting PM1.0 ConcentrationsAlthough various constituents of particles (e.g., C, ionic components, and major crustal elements) are produced from and are related to diverse source activities, these sources can be distinctly classified by the relative composition of specific trace elements [3]. In this study, the PMF analysis from four to ten factors along with the input of data for PM1.0 and 20 species were tested. Subsequently, the possible sources of PM1.0 were classified into six categories that provided physically meaningful source profiles and contributions. The number of factors was determined based on the Q-value. The initial solutions were iterated with random seed start and robust modes to minimize random errors and rotational ambiguities. The residuals could be examined to evaluate the number of factors that corresponded to the optimized Q-value. If the scaled residuals followed a symmetrical distribution with a range of −3–3, then the number of factors could be considered appropriate. The reliability of PMF modeling can be estimated by correlation analysis between the measured and predicted mass concentrations. According to the modeling results optimized in this study, the determination coefficient between the observed and predicted PM1.0 concentrations was 0.95, and six sources explained 99% of the measured PM1.0 concentration. The source profiles are shown in Fig. 5, where the bars and circles represent the absolute number of factors generated in a source and the percentage of species for each source, respectively. To estimate possible regions of local source, the CPF plots for the source contributions from PMF were presented in Fig. S4.
The relative contributions of these categories (with marker species) decreased in the following order: secondary aerosols (SO42−, NO3−, and NH4+), vehicle exhaust (BC, V, Cr, and Ti) [52,53], re-suspended soil-road dust (Ti, Al, Mg, K, Fe, Ba, and Zn) [27, 41, 42], fossil fuel combustion (Se, Cd, As, Ni, and Cr) [15, 54,55], biomass burning (K and Pb) [56–59], and industrial activities (Fe, Ni, Cu, and Al) [60–62].
The first source was estimated to be a secondary pollutant, as the resolved factors accounted for 56%, 42%, and 43% of the total NO3−, SO42−, and NH4+ concentrations, respectively. As secondary aerosols are produced as a result of the reaction and transformation between pollutants emitted in the air, it is generally difficult to allocate their sources accurately. Thus, it was surmised that NO3−, SO42−, and NH4+ from various regional sources were transported into the study area and contributed to secondary aerosol formation. Secondary pollutants in this study area showed the highest contribution among all sources, with a mean of 10.7 μg m−3 (54.1%). The quantitative NO3−, SO42−, and NH4+ concentrations in the first factor agreed with the previous results of source apportionment for the PM10 concentrations in this study area [42]. This finding reveals that secondary pollutants (e.g., NO3−, SO42−, and NH4+) were major contributors to air PM regardless of the particle size. The second source was estimated to be vehicle exhaust because it is dominated by elements such as BC (47%), V (38%), Cr (33%), and Ti (32%). Carbonaceous species, such as EC, OC, and BC, have been widely used as representative markers for vehicular sources [52,53]. Vehicle exhaust constituted approximately 21% of PM1.0 with a mean of 4.2 μg m−3. The relatively higher contribution of vehicle exhaust to PM1.0 in the study area reflected the on-site characteristics of the roadside area. Furthermore, because the study was conducted in the fall and winter, the contribution of vehicle exhaust emissions could be explained by the stimulated mechanism of transforming vehicle exhaust into PM at low temperatures [25]. The third source was typical soil dust. This was elucidated by the high percentages of major crustal elements, such as Ti (43%), Al (41%), Mg (39%), K (26%), and Fe (27%). Due to the relatively higher loadings of Ba (29%) and Zn (24%), this source was also estimated to be re-suspended road dust by mobile transportation from paved or non-paved roads. Further, Ba is added to the lubricating oil of diesel vehicles to prevent smoke and engine abrasion [41 and references therein). Zn is a well-known road dust marker element which is generated by tires and brake wear of mobile vehicles [27]. The fourth source was assumed to be fossil fuel combustion, such as coal and heavy oil, reflected by the high loadings of Se (51%), Cd (48%), As (40%), Ni (29%), and Cr (28%). Cd is known to occur at high temperatures during fuel combustion [54]. Ni and V are widely used as pair markers for the combustion of heating fuel, whereas Se is a representative marker species for oil-fired power plants and coal combustion [55]. The fifth source was surmised to be biomass burning due to the high percentages of K (47%) and Pb (42%); a large proportion of K could be explained by biomass burning [56–58]. The relatively high percentage of Pb in the fifth source profile could be explained that the emission of particulate matter from illegal incineration in the plain ground increased with biomass burning activities in the fall season [59]. The sixth source was assigned to industrial activities such as metal smelting [60, 61], refuse incineration [62], and mechanical abrasion [61], reflected by the high loading of Fe (38%), Ni (34%), Cu (28%), and Al (27%).
Estimated mean source contributions (SCE ± standard deviation; relative SCE) for the following are shown in Fig. 6: secondary aerosols (10.7 ± 3.1 μg m−3; 54.1%), vehicle exhaust (4.2 ± 1.4 μg m−3; 21.2%), re-suspended soil-road dust (1.9 ± 0.8 μg m−3; 9.6%), fossil fuel combustion (1.8 ± 0.7 μg m−3; 9.1%), biomass burning (0.7 ± 0.3 μg m−3; 3.3%), and industrial activities (0.5 ± 0.3 μg m−3; 2.6%). As shown Fig. S5, there was no significant variation in the estimated source contribution by distinctive pollution events. The relatively increasing trend in contributions of fossil fuel combustion source in winter seasons was reflected increasing consumption of the heating fuel.
5. ConclusionsIn this study, the characteristics of PM1.0 and its chemical species were investigated at a roadside area in Daejeon, which is a major metropolitan city in Korea. Based on our data, we attempted to elucidate the distribution characteristics of PM1.0 and the chemical species in the study area. The source types and relative contributions of each source in the study area were then estimated and quantified using the PMF receptor model.
The mean PM1.0 concentration was 19.9 ± 5.50 μg m−3 (range of 6.86–34.7 μg m−3) in close vicinity of a road with a high traffic density during the three-month study period in the fall and winter of 2019. The concentrations of major species (e.g., BC, SO42−, NO3−, and NH4+) were apportioned by 79.7 ± 6.3% (66.1–98.2%) of the PM1.0 concentration. The strong relationships between PM1.0 and the major species (e.g., BC, SO42−, NO3−, and NH4+) were indicated by the correlation coefficients. The compound forms of SO42− and the higher correlation (r = 0.81) between BC and SO42− in PM1.0 at the study area suggest that the concentrations of species were influenced by pollutants emitted in the study area and trans-boundary pollutants from outside the study area. The dominant sources of PM1.0 concentrations were identified and quantified by PMF using a positive matrix factorization model. Six important source types were identified: secondary aerosol (54.1%), vehicle exhaust (21.2%), re-suspended soil-road dust (9.6%), fossil fuel combustion (9.1%), biomass-burning (3.3%), and industrial activities (2.6%).
Although there was no significant trend during distinctive pollution events in the PM1.0 concentrations during the study period, the PM1.0 levels in the study area are concerning and need to be managed. It is hoped that the findings for the characteristics of airborne PM1.0 and associated chemical constituents help to determine policies and strategies for a regional air quality management at a roadside area in Korea.
NotesAuthor Contributions All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by L.C.Y. (Master’s student), L.Y.J. (Researcher), K.H.C. (Principle researcher), and L.J.H. (Professor). The first draft of the manuscript was written by L.J.M. (Principle researcher) and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. References1. Miller FJ. Dosimetry of particles: critical factors having risk assessment implications. Inhal. Toxicol. 2000;12:389–395.
https://doi.org/10.1080/08958378.2000.11463250
2. See SW, Balasubramanian R. Risk assessment of exposure to indoor aerosols associated with Chinese cooking. Environ. Res. 2006;102:197–204.
https://doi.org/10.1016/j.envres.2005.12.013
3. Lim JM, Jeong JH, Lee JH, Moon JH, Chung YS, Kim KH. The analysis of PM2.5 and associated elements and their indoor/outdoor pollution status in an urban area. Indoor Air. 2011;21:145–155.
https://doi.org/10.1111/j.1600-0668.2010.00691.x
4. Nie D, Chen M, Wu Y, et al. Characterization of fine particulate matter and associated health burden in Nanjing. Int. J. Environ. Res. Pub. He. 2018;15:602.
https://doi.org/10.3390/ijerph15040602
5. Agudelo-Castaneda D, Teixeira E, Schneider I, Lara S, Silva L. Exposure to polycyclic aromatic hydrocarbons in atmospheric PM1.0 of urban environments: carcinogenic and mutagenic respiratory health risk by age groups. Environ. Pollut. 2017;224:158–170.
https://doi.org/10.1016/j.envpol.2017.01.075
6. Wang K, Wang W, Li L, et al. Seasonal concentration distribution of PM1.0 and PM2.5 and a risk assessment of bound trace metals in Harbin, China: Effect of the species distribution of heavy metals and heat supply. Sci. Rep. 2020;10:8160.
https://doi.org/10.1038/s41598-020-65187-7
7. Zwozdziak A, Sówka I, Willak-Janc E, Zwozdziak J, Kwiecińska K, Balińska-Miśkiewicz W. Influence of PM1 and PM2.5 on lung function parameters in healthy schoolchildren-a panel study. Environ. Sci. Pollut. Res. 2016;23:23892–23901.
https://doi.org/10.1007/s11356-016-7605-1
8. Anderson JO, Thundiyil JG, Stolbach A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012;8:166–175.
https://doi.org/10.1007/s13181-011-0203-1
9. Chen G, Li S, Zhang Y, et al. Effects of ambient PM1 air pollution on daily emergency hospital visits in China: an epidemiological study. The Lancet Planet Health. 2017;1:e221–e229.
https://doi.org/10.1016/S2542-5196(17)30100-6
10. Kwon HO, Park MK, Kim SJ, et al. Size distributions of atmospheric particulate matter and associated trace metals in the multi-industrial city of Ulsan, Korea. Environ. Eng. Res. 2019;24:331–338.
https://doi.org/10.4491/eer.2018.226
11. Yin J, Harrison RM. Pragmatic mass closure study for PM1.0, PM2.5 and PM10 at roadside, urban background and rural sites. Atmos. Environ. 2008;42:980–988.
https://doi.org/10.1016/j.atmosenv.2007.10.005
12. Jaiprakash , Singhai A, Habib G, Raman RS, Gupta T. Chemical characterization of PM1.0 aerosol in Delhi and source apportionment using positive matrix factorization. Environ. Sci. Pollut. Res. 2017;24:445–462.
https://doi.org/10.1007/s11356-016-7708-8
13. Jakovljevic I, Pehnec G, Vadic V, Cacjovic M, Tomasic V, Jelinic JD. Polycyclic aromatic hydrocarbons in PM10, PM2.5 and PM1 particle fractions in an urban area. Air Qual. Atmos. Health. 2018;11:843–854.
https://doi.org/10.1007/s11869-018-0603-3
14. Gong W, Zhang T, Zhu Z, Ma Y, Ma X, Wang W. Characteristics of PM1.0, PM2.5, and PM10, and Their Relation to Black Carbon in Wuhan, Central China. Atmosphere. 2015;6:1377–1387.
https://doi.org/10.3390/atmos6091377
15. Zajusz-Zubek E, Kaczmarek K, Mainka A. Trace Elements Speciation of Submicron Particulate Matter (PM1) Collected in the Surroundings of Power Plants. Int. J. Environ. Res. Pub. He. 2015;12:13085–13103.
https://doi.org/10.3390/ijerph121013085
16. Hien PD, Bac VT, Thinh NTH, Anh HL, Thang DD, Nghia NT. A Comparison Study of Chemical Compositions and Sources of PM1.0 and PM2.5 in Hanoi. Aerosol. Air. Qual. Res. 2021;21:210056.
https://doi.org/10.4209/aaqr.210056
17. Zhang Y, Lang J, Cheng S, et al. Chemical composition and sources of PM1 and PM2.5 in Beijing in autumn. Sci. Total. Environ. 2018;630:72–82.
https://doi.org/10.1016/j.scitotenv.2018.02.151
18. Zhao Y, Song X, Wang Y, Zhao J, Zhu K. Seasonal patterns of PM10, PM2.5, and PM1.0 concentrations in a naturally ventilated residential underground garage. Build. Environ. 2017;124:294–314.
https://doi.org/10.1016/j.buildenv.2017.08.014
19. Qui Z, Xu X, Liu W, Li X. Investigation into pedestrian exposure to traffic PM around grade separations: a case study in Xi’an, China. Air Qual. Atmos. Health. 2018;11:431–443.
https://doi.org/10.1007/s11869-018-0548-6
20. Gramame TJ, Schlesinger RB. Cardiovascular health and particulate vehicular emissions: a critical evaluation of the evidence. Air Qual. Atmos. Health. 2010;3:3–27.
https://doi.org/10.1007/s11869-009-0047-x
21. Li R, Wang J, Xue K, Fang C. Spatial and temporal distribution characteristics and influencing factors analysis of particulate matter pollution in Jinan City. Air Qual. Atmos. Health. 2021;14:1267–1278.
https://doi.org/10.1007/s11869-021-01015-9
22. Lim S, Lee M, Lee G, Kim S, Yoon S, Kang K. Ionic and carbonaceous compositions of PM10, PM2.5 and PM1.0 at Gosan ABC Superstation and their ratios as source signature. Atmos. Chem. Phys. 2012;12:2007–2024.
https://doi.org/10.5194/acp-12-2007-2012
23. Son SC, Park SS. Evaluating the applicability of a semi-continuous aerosol sampler to measure Asian dust particles. Environ. Sci-Proc. Imp. 2015;17:561–569.
https://doi.org/10.1039/C4EM00404C
24. Park SS, Choa SY, Jung CH, Lee KH. Characteristics of water-soluble inorganic species in PM10 and PM2.5 at two coastal sites during spring in Korea. Atmos. Pollut. Res. 2016;7:370–383.
https://doi.org/10.1016/j.apr.2015.10.018
25. Kim S, Kim TY, Yi SM, Heo J. Source apportionment of PM2.5 using positive matrix factorization (PMF) at a rural site in Korea. J. Environ. Manage. 2018;214:325–334.
https://doi.org/10.1016/j.jenvman.2018.03.027
26. Squizzato S, Masiol M, Agostini C, et al. Factors, origin and sources affecting PM1 concentrations and composition at an urban background site. Atmos. Res. 2016;180:262–273.
https://doi.org/10.1016/j.atmosres.2016.06.002
27. Karnaea S, John K. Source apportionment of PM2.5 measured in South Texas near U.S.A. - Mexico border. Atmos. Pollut. Res. 2019;10:1663–1676.
https://doi.org/10.1016/j.apr.2019.06.007
28. Paatero P, Tapper U. Positive Matrix Factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics. 1994;5:111–126.
https://doi.org/10.1002/env.3170050203
29. Vossler T, Cernikovský L, Novak J, Williams R. Source apportionment with uncertainty estimates of fine particulate matter in Ostrava, Czech Republic using Positive Matrix Factorization. Atmos. Pollut. Res. 2016;7:503–512.
https://doi.org/10.1016/j.apr.2015.12.004
30. Park MB, Lee TJ, Lee ES, Kim DS. Enhancing source identification of hourly PM2.5 data in Seoul based on a dataset segmentation scheme by positive matrix factorization (PMF). Atmos. Pollut. Res. 2019;10:1042–1059.
https://doi.org/10.1016/j.apr.2019.01.013
31. Bennetta J, Davy P, Trompetter B, et al. Sources of indoor air pollution at a New Zealand urban primary school; a case study. Atmos. Pollut. Res. 2019;10:435–444.
https://doi.org/10.1016/j.apr.2018.09.006
32. Saeaw N, Thepanondh S. Source apportionment analysis of airborne VOCs using positive matrix factorization in industrial and urban areas in Thailand. Atmos. Pollut. Res. 2015;6:644–650.
https://doi.org/10.5094/APR.2015.073
33. Chen CH, Chuang YC, Hsieh CC, Lee CS. VOC characteristics and source apportionment at a PAMS site near an industrial complex in central Taiwan. Atmos. Pollut. Res. 2019;10:1060–1074.
https://doi.org/10.1016/j.apr.2019.01.014
34. Zheng S, Xu X, Zhang Y, et al. Characteristics and sources of VOCs in urban and suburban environments in Shanghai, China, during the 2016 G20 summit. Atmos. Pollut. Res. 2019;10:1766–1779.
https://doi.org/10.1016/j.apr.2019.07.008
35. Bernardoni V, Pileci RE, Caponi L, Massabò D. The Multi-Wavelength Absorption Analyzer (MWAA) Model as a Tool for Source and Component Apportionment Based on Aerosol Absorption Properties: Application to Samples Collected in Different Environment. Atmosphere. 2017;8:218–236.
https://doi.org/10.3390/atmos8110218
36. Stahl C, Cruz MT, Bañaga PA, et al. Sources and characteristics of size-resolved particulate organic acids and methanesulfonate in a coastal megacity: Manila, Philippines. Atmos. Chem. Phys. 2020;20:15907–15935.
https://doi.org/10.5194/acp-20-15907-2020
37. Ari A, Ari PE, Gaga EO. Chemical characterization of size-segregated particulate matter (PM) by inductively coupled plasma -Tandem mass spectrometry (ICP-MS/MS). Talata 2020;. 208:120–350.
https://doi.org/10.1016/j.talanta.2019.120350
38. Rovelli S, Nischkauer W, Cavallo DM, Limbeck A. Multi-element analysis of size-segregated fine and ultrafine particulate via Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Anal. Chim. Acta. 2018;104:11–19.
https://doi.org/10.1016/j.aca.2018.10.026
39. Thomas R. A beginner’s guide to ICP-MS; Part IX-mass analyzer: collision/reaction cell technology. Spectroscopy. 2005;17:42–48.
40. Tanner S, Baranov VI, Volkopf U. A dynamic reaction cell for inductively coupled plasma mass spectrometry (ICP-DRC-MS). J. Anal. At. Spectrom. 2000;15:1261–1270.
https://doi.org/10.1039/B002604M
41. Kim E, Hopke PK. Comparison between conditional probability function and nonparametric regression for fine particle source directions. Atmos. Environ. 2004;38:4667–4673.
https://doi.org/10.1016/j.atmosenv.2004.05.035
42. Lim JM, Lee JH, Moon JH, Chung YS, Kim KH. Source apportionment of PM10 at a small industrial area using Positive Matrix Factorization. Atmos. Res. 2010;95:88–100.
https://doi.org/10.1016/j.atmosres.2009.08.009
43. Kanchanasuta S, Sooktawee S, Patpai A, Vatanasomboon P. Temporal Variations and Potential Source Areas of Fine Particulate Matter in Bangkok, Thailand. Air Soil Water Res. 2020;13:1–10.
https://doi.org/10.1177/1178622120978203
44. Polissar AV, Hopke PK, Paatero P, Malm WC, Sisler JF. Atmospheric aerosol over Alaska - 2. Elemental composition and sources. J. Geophys. Res. 1998;103:19045–19057.
https://doi.org/10.1029/98JD01212
45. US EPA (U.S. Environmental Protection Agency). EPA Positive Matrix Factorization (PMF) 50 Fundamentals and User Guide; EPA/600/R-14/108. Washington, DC: 2014.
46. Hieu NT, Lee BK. Characteristics of particulate matter and metals in the ambient air form a residential area in the largest industrial city in Korea. Atmos. Res. 2010;98:526–537.
https://doi.org/10.1016/j.atmosres.2010.08.019
47. Janssen NAH, Hoek G, Simic-Lawson M, et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5
. Environ. Health Persp. 2011;119:1691–1699.
https://doi.org/10.1289/ehp.1003369
48. Ran L, Deng ZZ, Wang PC, Xia XA. Black carbon and wavelength-dependent aerosol absorption in the North China Plain based on two-year aethalometer measurements. Atmos. Environ. 2016;142:132–144.
https://doi.org/10.1016/j.atmosenv.2016.07.014
49. Duc HN, Shingles K, White S, et al. Spatial-Temporal Pattern of Black Carbon (BC) Emission from Biomass Burning and Anthropogenic Sources in New South Wales and the Greater Metropolitan Region of Sydney, Australia. Atmosphere. 2020;11:570.
https://doi.org/10.3390/atmos11060570
50. Cheng Y, Zou SC, Lee SC, et al. Characteristics and source apportionment of PM1 emissions at a roadside station. J. Hazard. Mat. 2011;195:82–91.
https://doi.org/10.1016/j.jhazmat.2011.08.005
51. Hazi Y, Heikkinen MSA, Cohen BS. Size distribution of acidic sulfate ions in fine ambient particulate matter and assessment of source region effect. Atmos. Environ. 2003;37:5403–5413.
https://doi.org/10.1016/j.atmosenv.2003.08.034
52. Murillo JH, Marin JF, Roman SR, et al. Temporal and spatial variations in organic and elemental carbon concentrations in PM10/PM2.5 in the metropolitan area of Costa Rica, Central America. Atmos. Pollut. Res. 2013;4:53–63.
https://doi.org/10.5094/APR.2013.006
53. Xu Z, Wen T, Li X, Wang J, Wang Y. Characteristics of carbonaceous aerosols in Beijing based on two–year observation. Atmos. Pollut. Res. 2015;6:202–208.
https://doi.org/10.5094/APR.2015.024
54. Uberoi M, Shadman F. High-temperature removal of cadmium compounds using solid sorbents. Environ. Sci. Pollut. Tech. 1991;25:1285–1289.
https://doi.org/10.1021/es00019a009
55. Vallius M, Janssen NAH, Heirich J, et al. Sources and elemental composition of ambient PM2.5 in three European cities. Sci. Total. Environ. 2005;337:147–162.
https://doi.org/10.1016/j.scitotenv.2004.06.018
56. Marcotte S, Castilla C, Morin C, et al. Particulate inorganic salts and trace element emissions of as domestic boiler fed with five commercial brands of wood pellets. Environ. Sci. Pollt. R. 2020;27:18221–18231.
https://doi.org/10.1007/s11356-020-08329-8
57. Chantara S, Thepnuan D, Wiriya W, Prawan S, Tsai Y. Emissions of pollutant gases, fine particulate matters and their significant tracers from biomass burning in an open-system combustion chamber. Chemosphere. 2019;224:407–416.
https://doi.org/10.1016/j.chemosphere.2019.02.153
58. Yang W, Pudasainee D, Gupta R, Li W, Wang B, Sun L. An overview of inorganic particulate matter emission from coal/biomass/MSW combustion: Sampling and measurement, formation, distribution, inorganic composition and influencing factors. Fuel Process. Technol. 2021;213:106657.
https://doi.org/10.1016/j.fuproc.2020.106657
59. Wadge A, Hutton M. The cadmium and lead content of suspended particulate matter emitted from a U.K. refuse incinerator. Sci. Total. Environ. 1987;67:91–95.
https://doi.org/10.1016/0048-9697(87)90068-4
60. Chen Z, Ding Y, Jiang X, et al. Combination of UNMIX, PMF model and Pb-Zn-Cu isotopic compositions for quantitative source apportionment of heavy metals in suburban agricultural soils. Ecotox. Environ. Safe. 2022;234:113369.
https://doi.org/10.1016/j.ecoenv.2022.113369
61. Han JS, Moon KJ, Lee SJ, et al. Size-resolved source apportionment of ambient particles by positive matrix factorization at Gosan background site in East Asia. Atmos. Chem. Phys. 2006;6:211–223.
https://doi.org/10.5194/acp-6-211-2006
62. Wang G, Deng J, Ma Z, Hao J, Jiang J. Characteristics of filterable and condensable particulate matter emitted from two waste incineration power plants in China. Sci. Total. Environ. 2018;639:695–704.
https://doi.org/10.1016/j.scitotenv.2018.05.105
Fig. 1Areal maps are presented to show the study area: (a) Daejeon city is located in the middle region of Korea; and (b) the sampling site is on the roadside in the huge residential and commercial area. 
Fig. 2Graphical summary of PM1.0 concentration at study area: (a) temporal variation, (b) distribution function, and (c) Box-Whisker plots with day of the week. 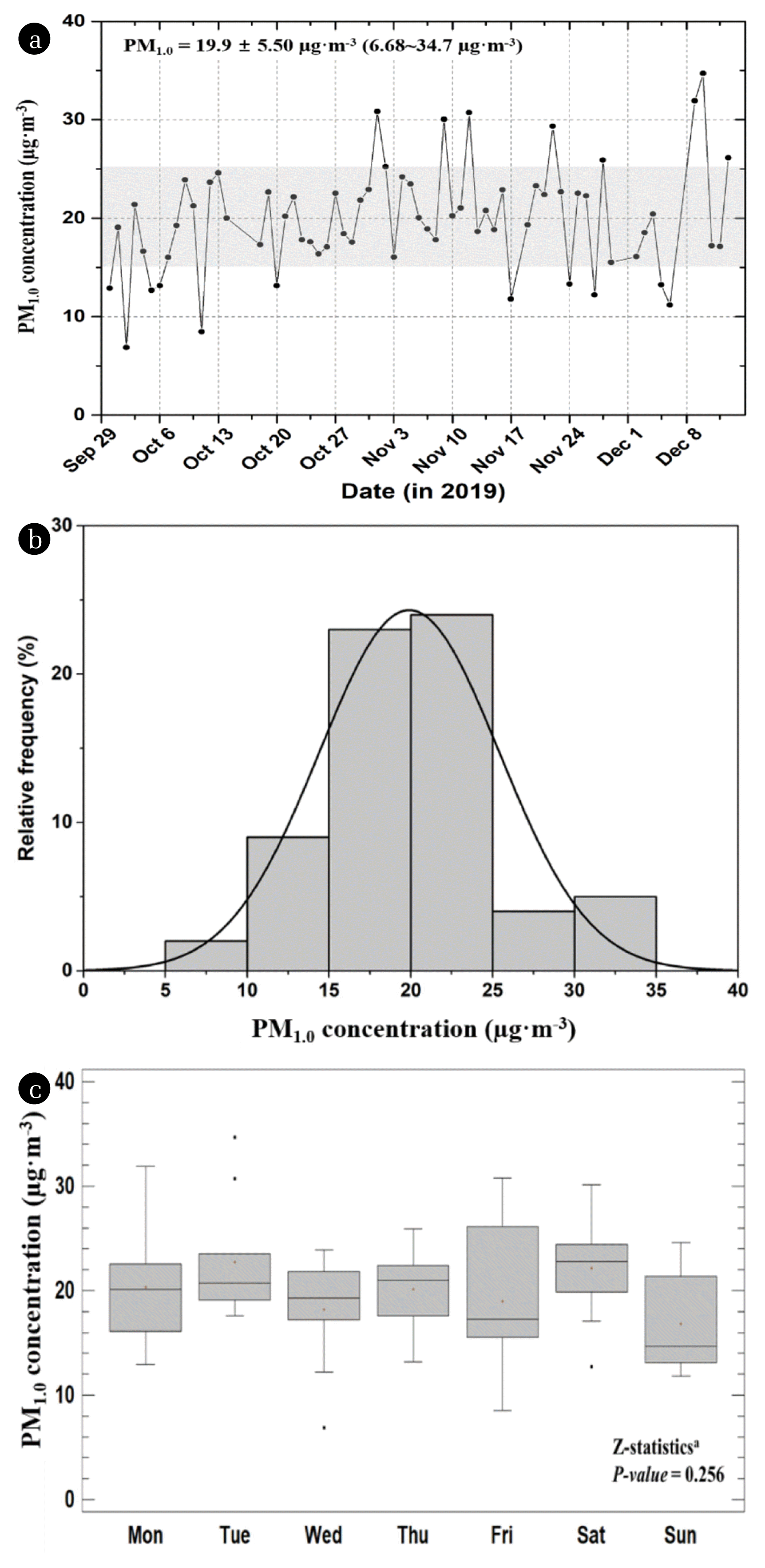
Fig. 3The CPF plots of (a) PM1.0, (b)black carbon, (c) NO3−, and (d) SO4−2 at study area. The threshold criterion of upper 25th percentile value was used to show clear directionality. 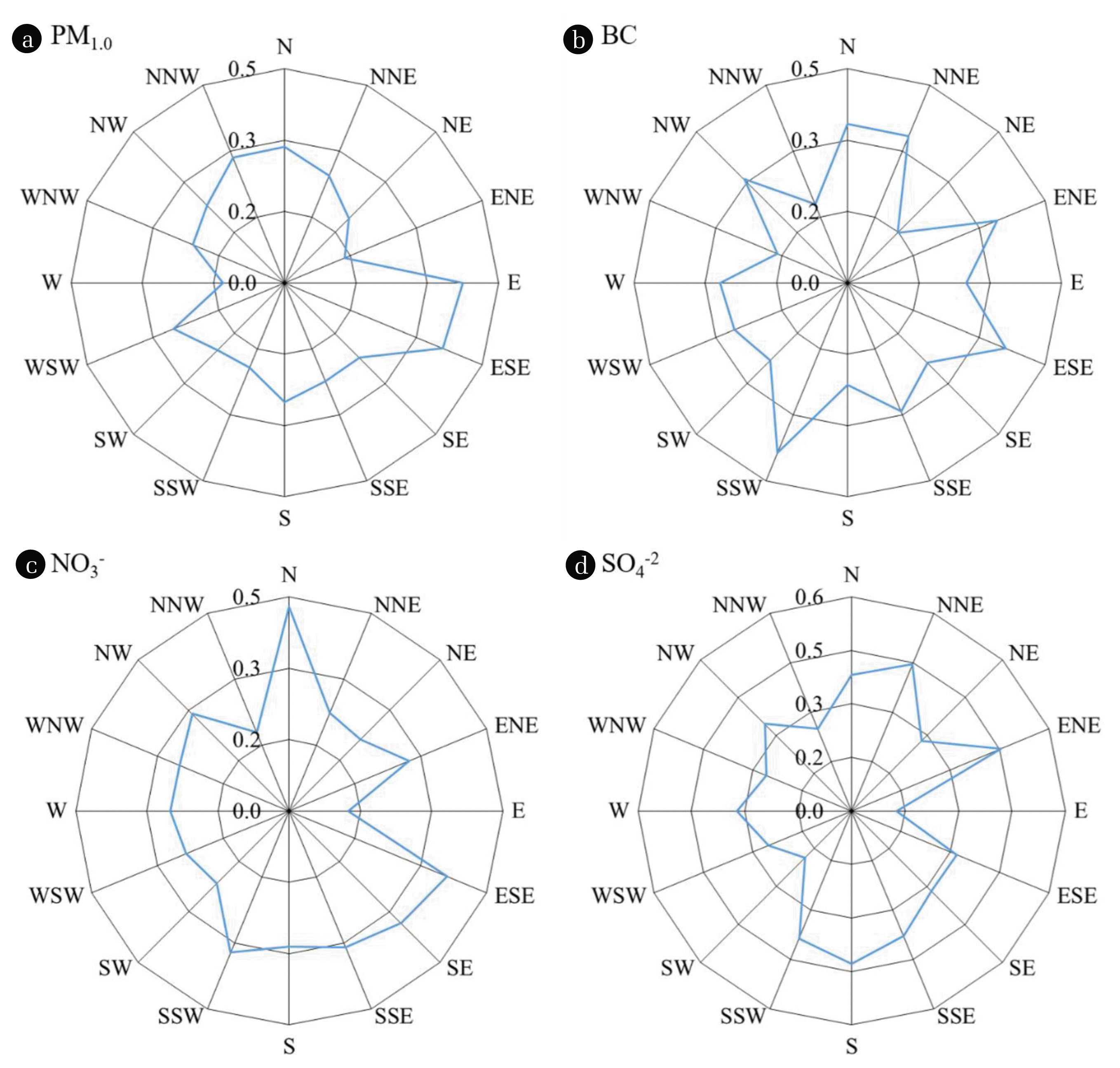
Fig. 4Matrix plots of correlation between PM1.0 and major chemical species. number in the box means the correlation coefficients. 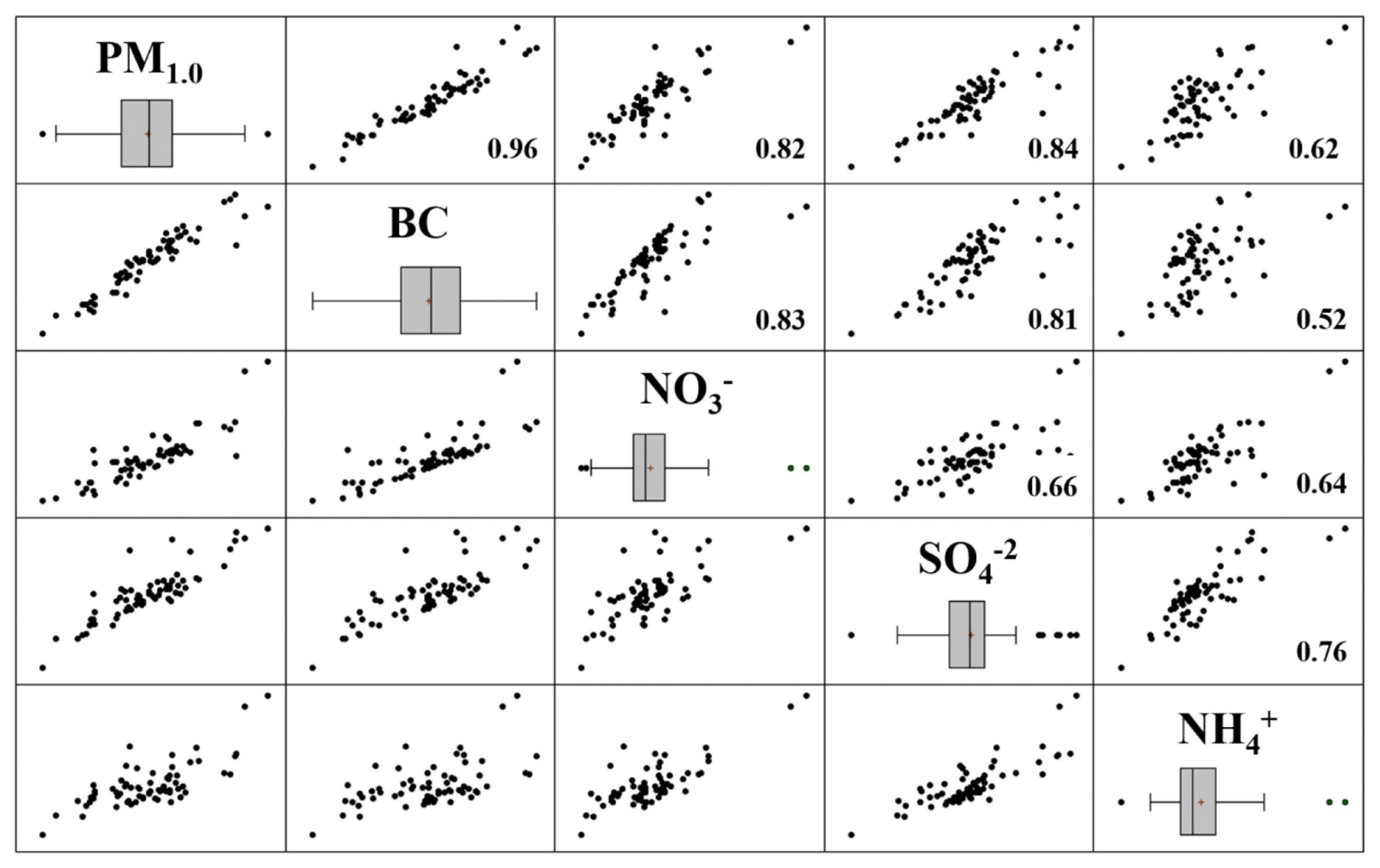
Fig. 5Source profiles from PMF; the bar represents the absolute amount of factors generated in a source, while a circle indicates the percentage of species for each source. 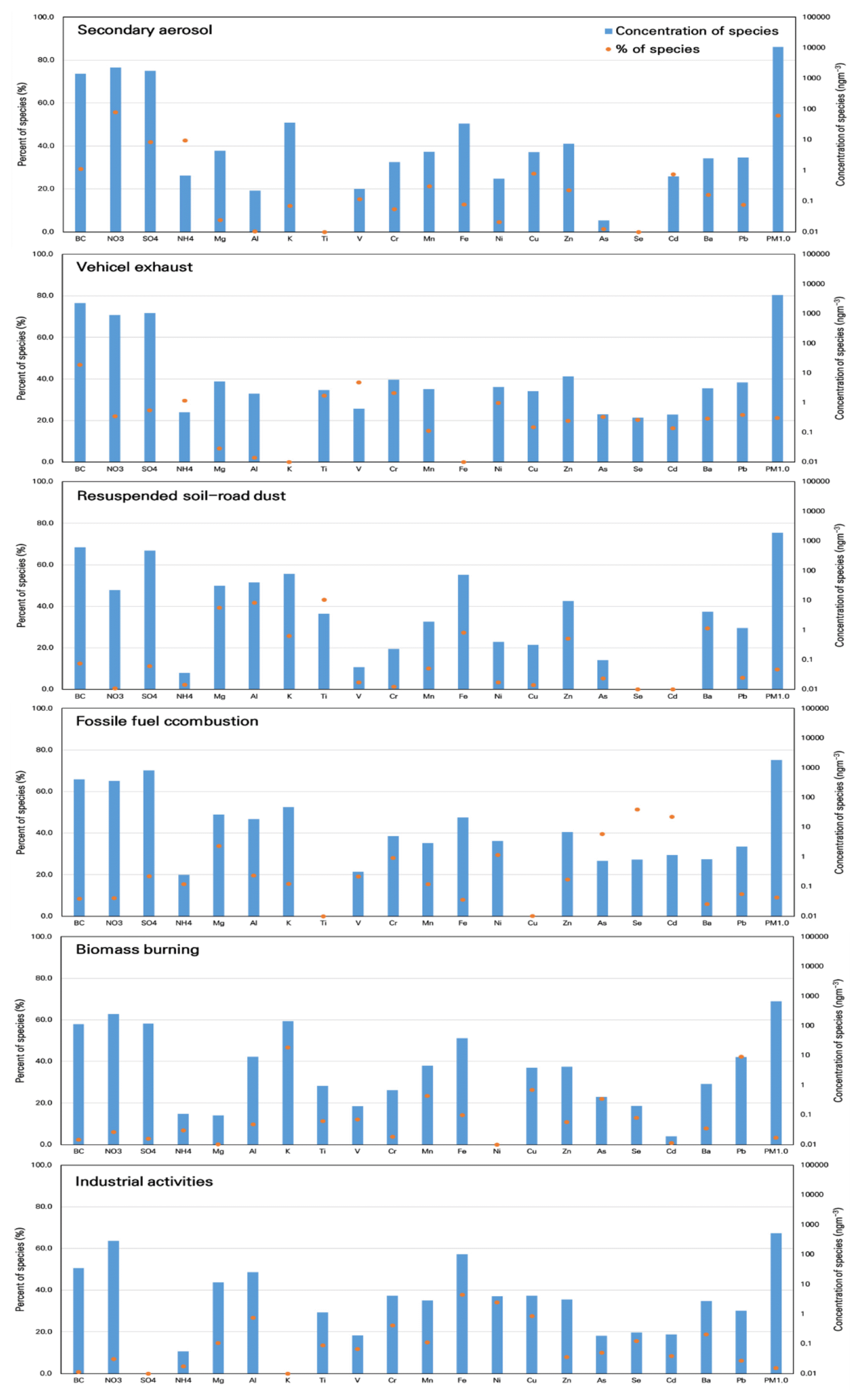
Table 1Statistical Summary of PM1.0 and Species Components at Study Area. Table 2Comparison of PM1.0 Concentrations in This Study and Previous Studies.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||