AbstractIn this study, Mg/Fe-doped biochar (MFDB) was prepared using the impregnation pyrolysis method, and its preparation was optimized using response surface methodology (RSM). The competitive adsorption between dissolved organic matter (DOM) and phosphorus was also investigated. The best adsorption capacity was obtained with an Mg impregnation ratio of 3.17:1 (Mg: biomass, g:g), Fe impregnation ratio of 1.3:1 (Fe: biomass, g:g), and pyrolysis temperature of 491°C. The adsorption capacity of MFDB for phosphorus was 179.21 mg/g at 40°C, an initial phosphate concentration of 50 mg/g and pH 4. The phosphate adsorption by MFDB conformed to a pseudo-primary and secondary adsorption kinetic model, proving the coexistence of physical and chemical adsorption. The adsorption mechanisms, including ligand exchange, electrostatic interaction, and complexation reaction were revealed. As the pH increased, it weakened the electrostatic interaction of phosphate by MFDB and the ligand exchange between phosphate and OH−. When the pH was less than 3, the metal oxide dissolved. For pH values exceeding, OH− competed with phosphate for adsorptio, which also weakened the complexation of phosphate and MFDB. The DOM in domestic wastewater had a slight effect on the phosphorus adsorption. The phosphorus removal rates were closely related to lignin-like humic acid and tryptophan.
1. IntroductionPhosphorus is an essential element for the growth of animals and plants. However, high phosphorus content in water is an important cause of the eutrophication, as it can cause algae bloom when its concentration reaches 0.2 mg/L [1, 2]. The phosphorus content in domestic sewage is approximately 3–5 mg/L [3–5]. Direct discharge of sewage without treatment inevitably leads to water pollution. Therefore, the treatment of phosphorus in water bodies is necessary [6].
Both biological and chemical methods have been developed to remove phosphate from wastewater [7]. However, many problems remain. The application of biological methods to the treatment of low-phosphorus wastewater is limited. Studies have shown that the phosphorus concentration of the effluent of the enhanced biological phosphorus removal method is generally 0.5–1.0 mg/L. However, this still does not meet the wastewater discharge requirements [7]. Although chemical precipitation can reduce phosphate concentrations to < 0.5 mg/L, this method requires large amounts of metal salts and produces a large amount of chemical sludge [8]. Adsorption methods have unique advantages, owing to their simple operation and low cost. Several types of adsorbents exist, such as metal oxide/hydroxide adsorbents, mineral clay-based adsorbents, activated carbon, and biochar [9, 10]. Among these, biochar adsorbents are porous substances prepared by heating agricultural waste and sludge at high temperatures under anoxic conditions [11]. Compared with other adsorbents, biochar has a larger specific surface area, richer functional groups, and excellent adsorption performance and has been widely used in wastewater treatment [9, 12, 13].
However, biochar’s anion-removal efficiency is not satisfactory because of its electronegativity and low anion exchange rate [14]. The modification of biochars with metal compounds, such as Fe, Mg, Al, and La, can change their physical and chemical properties, and thus, improve their phosphorus adsorption capacity [15–18]. The removal rate of phosphorus by biochar is < 10%, After MgO and FeCl3 are used to modify the biochar, the removal rate can be increased to 74.6% and 85%, respectively, due to the increase in the specific surface area and zero-point potential [18, 19]. Additionally, the metal oxides generated after pyrolysis are uniformly distributed on the surface of the biochars, which produces a complexation reaction with phosphorus and improved the adsorption capacity of phosphorus [18, 20]. Peng et al. [21] optimized the preparation of Fe/Al (Hydr) oxide biochar for phosphate removal using corn straw, almond shells and cow dung as raw materials, and the results showed that the composite metal modified biochar had good phosphate adsorption capacity.
In this study, MFDB was prepared by immobilizing MgO and Fe2O3 on wheat straw using impregnation pyrolysis. To determine the favorable conditions for biochar synthesis and reveal the relationship between various factors, the response surface methodology (RSM), a model that requires a minimum number of experiments and outperforms conventional methods, was employed to optimize the preparation condition of the novel materials, and then the adsorption capacity of MFDB on phosphorus was investigated by adsorption kinetics, isotherms, and thermodynamics. The mechanisms of phosphorus adsorption by MFDB were studied using characterization. In addition, existing studies have been conducted mostly in pure solutions of phosphate without considering the effect of organic matter in water, so the effect of DOM on phosphate adsorption was also investigated, and the correlation between phosphorus and DOM in water was studied using three-dimensional fluorescence and parallel factor analysis (PARAFAC) [22].
2. Materials and Methods2.1. MaterialsThe wheat straw used in this study was collected from a rural area in Hebei Province, China. Magnesium chloride hexahydrate (MgCl2·6H2O, 98%) and ferric chloride hexahydrate (FeCl3·6H2O, 99%) were obtained from Shanghai Macklin Biochemical Co., Ltd. (China). Potassium dihydrogen phosphate (KH2PO4, 99.5%), sodium hydroxide (NaOH, 85%), nitric acid (HNO3, 65%) and sodium chloride (NaCl, 99.5%) were obtained from Beijing Chemical Co., Ltd. (China). SBBR-treated domestic wastewater was used in the experiment. The experimental solutions were prepared using deionized water.
2.2. Preparation of Mg/Fe-doped Biochars (MFDB)The wheat straw was washed with deionized water and dried at 60°C for up to 12 h. The dried straw was passed through a 100-mesh sieve and set aside. MFDB was produced by impregnation pyrolysis [19]. Further, 1 g biomass was placed into solutions containing different contents of Mg2+ and Fe3+, with ratios of Mg to biomass of 0:1, 2:1, and 4:1 (g/g) and of Fe to biomass of 0:1, 1.5:1, and 3:1 (g/g). The biomass and solutions were fully dissolved under ultrasound for 15 min, and the mixture was shaken for 24 h at 200 rpm, followed by drying at 60°C. The dried product was sieved through a 200-mesh sieve and pyrolyzed for 2 h in a tube furnace (OTF-1200X-S, Hefei Kejing Materials Technology Co., Ltd., China) at different temperatures (450°C, 550°C, and 650°C) under an N2 atmosphere (0.4 L/min) for 2 h. The biochar was washed with deionized water to remove residual salts and loose minerals. The final product was denoted as MFDB and stored in self-sealing bags.
RSM is a collection of mathematical and statistical methods, in which Box–Behnken design (BBD) experiments are used to optimize different experimental parameters and processes [23–25]. Three parameters, namely, the mass ratio between biomass and Mg (X1, 1:0–1:4), the mass ratio between biomass and Fe (X2, 1:0–1:3), and pyrolysis temperature (X3, 450–650°C), were used as the design variables of the RSM experiment. Seventeen BBD experiments were conducted, and the phosphorus adsorption (Y, mg/g) was arranged as the response factor. The validity of the model was determined by the p-value test, and analysis of variance (ANOVA) was used to further estimate the adequacy of the model [26]. The models of the experiment are shown in Supplementary Text S1.
2.3. Characterization of Different Biochars and MFDBThe morphology and surface element distribution of the MFDB were characterized using scanning electron microscope (SEM, Zeiss Sigma 300, Germany) with energy-dispersive spectrometer (EDS, Smart EDX, China). The Brunauer–Emmett–Teller–surface area (SBET) and Barrett–Joyner–Halenda (BJH) were analyzed according to nitrogen adsorption–desorption isotherms at 77 K using an Autosorb-IQ (Quantachrome Instruments, US). The contents of elements, such as C, H, O, N, and S, were tested via elemental analyzer (UNICUBE, China). Fourier-transform infrared (FT-IR) materal analysis was performed using an infrared spectroscopy (Perkin Elmer Frontier, China), and the functional groups of the adsorbent were determined by KBr. The samples were analyzed by X-ray diffraction (XRD, Rigaku Ultima IV, Japan). The chemical compositions of the samples were analyzed using X-ray photoelectron spectroscopy (XPS, Thermo Scientific, USA) with Al Kα radiation (1486.6 eV). The pH values of the zeta potential of the adsorbent were measured using a zeta potential tester (Zetasizer Nano S90, UK). The leaching concentrations of Mg and Fe ions in MFDB were tested by inductively coupled plasma emission spectrometry (iCAP 7000, Thermo Scientific, Massachusetts, USA).
2.4. Phosphate Adsorption ExperimentsThe phosphate was adsorbed at an optimal dosing rate of 0.4 g/L. The experimental results are shown in Fig. S1. The adsorption experiment was performed, adding 50-mL phosphate solution and 0.02 g MFDB to a 150-mL triangular flask and shaking at 150 rpm for 96 h at room temperature (25 ± 1°C). The phosphate concentration was determined after filtration through a 0.45μm membrane. The adsorption kinetics, isotherms and thermodynamics were also studied. Batch adsorption tests were performed at different adsorption times (1–96 h), phosphorus concentrations (2–240 mg/L), temperatures (10–40°C) and pH values (2–12). Additionally, 0.02 g of MFDB was added to 50 mL domestic wastewater for adsorption experiments at 150 rpm for 96 h, and the adsorption effect of MFDB on phosphorus was investigated in the actual effluent. The phosphate concentration, total phosphorus, and total organic carbon (TOC), UV254, and fluorescence values were measured after filtration through a 0.45-μm membrane. The adsorption apparent molecular weight (AMW) was also measured to investigate the adsorption performance of the material. The removal rates (R, %) and adsorption capacities (qe, mg/g) of organic pollutants and phosphorus were calculated using the following equations: [27].
where R is the removal efficiency of MFDB for phosphate (%), qe is the adsorption capacity of phosphate onto MFDB (mg/g), C0 and Ce are the initial and equilibrium concentrations of phosphate in the solutions (mg/L), V is the volume of phosphate solution (L), and M is the dosage of MFDB (g).
2.5. Analytical Methods2.5.1. Excitation emission matrix (EEM) fluorescence spectroscopy and PARAFAC analysis-modeling procedureThe fluorescence of DOM in domestic wastewater was measured using a fluorescence spectrophotometer (HITACHI F-7000, Japan) with both excitation and emission wavelengths in the range of 220–550 nm. The scan speed was set to 1,200 nm/min. Deionized water was used to eliminate the background noise of the sample.
Parallel factor analysis (PARAFAC) decomposes the data matrix of the excitation emission matrix (EEM) into a set of linear terms and an array of residuals [28]. PARAFAC modeling was performed using the EFC platform [29]. The theory and calculations of this software combine fluorescence spectral data correction, PARAFAC, and MATLAB GUI to facilitate the processing of fluorescence spectral data [30, 31]. The model was processed by data correction, and scattering peak elimination. Finally, the RSM model was tested using PARAFAC analysis optimization, sum of squared errors, core consistency and half score test [32]. The experimental results are shown in Fig. S2
2.5.2. Contaminant determination methodThe adsorbed solution was filtered through a 0.45-μm filter membrane and the phosphate concentration was determined by the molybdenum blue colorimetric method using a UV–visible spectrophotometer (HACH DR6000, USA) under 700-nm light. The AMW distribution of water was determined by high-performance size-exclusion chromatography with a UV detector (260 nm), and the separation was performed using chromatography in the 0.1 mol/L phosphate buffer solution (pH = 6.8) [33]. The TOC was analyzed using a Shimadzu TOC-VCPN TOC Analyzer.
3. Results and Discussion3.1. Optimization of the MFDB Based on RSMThe preparation condition of MFDB was optimized using RSM, and BBD comprised 17 experiments. The adsorption capacity of phosphorus by MFDB was 0.23–74.23 mg/g. The response index of phosphorus-removal efficiency fluctuated in a wide range, indicating a strong variation in phosphate adsorption by MFDB with the factors test. The experimental design of MFDB is shown in Table S1. Design-Expert 8.0 was used to simulate the phosphate adsorption behavior, and an empirical response model equation was acquired as follows:
where Y is the response value of phosphate adsorption, and X1, X2, and X3 are the impregnation ratio of Mg to biomass, impregnation ratio of Fe to biomass and biomass pyrolysis temperature, respectively [34].
The response analysis of three independent variables to the adsorption amount resulted in a three-dimensional response surface as a function of the adsorption amount to two factors, as shown in Fig. 1(a)–(c). The optimal preparation conditions derived from Design Expert 8.0 were a pyrolysis temperature of 491°C and impregnation ratios of 3.17:1 and 1.3:1 for Mg and Fe, respectively, to achieve an adsorption capacity of 77.48 mg/g after 24 h of adsorption. It was demonstrated that the adsorption capacity increases with increase in metal ratio, but decreases when the ratio of Mg or Fe exceeds 3.17:1 or 1.3:1, respectively, due to the super-saturation of the biochar attachment sites [21]. The pores of the biochar collapse as temperature increases, Which also limits the increase of adsorption capacity [35]. The estimated adsorption capacity values obtained from the model show a good correlation with the experimental results, as shown in Fig. 1(d), proving the feasibility of the regression model. The ANOVA of the model was calculated as R2 = 0.993, F-test, and p < 0.0001. The model is available within the significance level and has good correlation [36]. The F-value test indicated that the significances of each factor decreased from Mg content (FMg = 316.52) > Fe content (FFe = 10.51) > temperature (FT = 7.45), which proven in the RSM test [37, 38]. The ANOVA of the model is shown in Table S2. The following adsorption experiments were performed using the optimized MFDB.
3.2. Removal Rate of Phosphate by Biochar AdsorptionThe phosphate adsorption capacity and model of MFDB were explored via the adsorption kinetics, adsorption isotherms, and adsorption thermodynamics of MFDB, as shown in Fig. 2.
3.2.1. Adsorption kineticsThe adsorption kinetics was studied using the optimized MFDB, with phosphate concentrations of 5, 10, 25, and 50 mg/L, and adsorption times of 0–96 h, respectively. Three kinetic models were applied to analyze the phosphate adsorption process at MFDB:
where qt and qe (mg/g) are the amount of phosphorus adsorbed by MFDB at time t (h) and adsorption equilibrium, respectively; k1 (1/h), k2 (g/(mg·h)) and k3 (mg/(g·h0.5)) are the rate constants in the pseudo-first-order, pseudo-second-order and intraparticle diffusion kinetic models, respectively; and C is a constant in the intraparticle diffusion kinetic model.
Fig. 2(a) exhibited that phosphate was continuously adsorbed by MFDB in the first 60 h; further, the adsorption slowed and eventually remained constant. Because adsorption sites were abundant in the initial adsorption stage, the adsorption speed was fast. However, as the adsorption sites became occupied and gradually saturated, the phosphate adsorbed on the surface could induce repulsive forces and the adsorption rate continued slowing down to reach the adsorption equilibrium state [42]. The adsorption of phosphate by MFDB grew with concentration because higher phosphate levels could overcome the greater mass transport resistance [43]. The kinetic parameters and correlation coefficients are listed in Table S3. When the phosphate concentration was 50 mg/L, the kinetic fit of MFDB was consistent with the pseudo-primary and pseudo-secondary models (R2 = 0.998 and 0.997), which proved that the main adsorption mechanisms were chemisorption and physical adsorption [44]. The MFDB also shows a good fit for internal diffusion (R2 = 0.983). Fig. 2(b) suggests that the adsorption of phosphorus by MFDB was divided into three stages. First, the adsorption was fast because of the high initial phosphorus concentration and abundant binding sites. Furthermore, phosphate was adsorbed to the outer surface by external diffusion and transferred to the pores of MFDB, in which it was finally immobilized [45].
3.2.2. Adsorption isothermsMFDB was used for phosphate adsorption at concentrations of 2–240 mg/L at 10, 25, and 40°C to verify the adsorption isotherms and investigate the adsorption mechanisms. The following three models were used to simulate the data:
where b (L/mg) and KF are coefficients of the model; Ce (mg/L) is the equilibrium concentration; qe (mg/g) and qm (mg/g) are the equilibrium and maximum adsorption amounts, respectively; and 1/n is the heterogeneity coefficient. Fig. 2(c) shows the adsorption isotherm model, in which an increase in temperature accelerated the adsorption of phosphorus by MFDB, as the temperature increased the diffusion of sites. Further, the trend of the curve increased rapidly and gradually stabilized, indicating that the high concentration of phosphate provided the driving force for mass transfer during the initial adsorption period [48]. Based on the R2 values of the three models in Table S4, the adsorption isotherm model has a better fit to the Langmuir and Freundlich isotherms, indicating that the adsorption of phosphate by MFDB is related to the heat of adsorption [49, 50]. The maximum adsorption of phosphorus by MFDB could be as high as 179.21 mg/g at 40°C, which was better than most previous adsorbents [51, 52]. Compared with other adsorbents reported in the literature (Table 1), MFDB is very promising due to its high adsorption capacity at low phosphate concentrations.
3.2.3 Adsorption thermodynamicsA thermodynamic study on phosphate adsorption by MFDB was also carried out using the thermodynamic equations below, and the results are illustrated in Fig. 2(d) [56].
where ΔS0 (J/(mol·K)), ΔH0 (KJ/mol) and ΔG0 (KJ/mol) are the change in entropy, change in enthalpy, and Gibbs free energy, respectively; R is the ideal gas constant (8.314 J/(K·mol)); T is the absolute temperature (K); and Kd is thermodynamic constant. The thermodynamic parameters are listed in Table S5. ΔG0 is negative, suggesting that the adsorption of phosphate by MFDB is spontaneous; ΔH0 is positive, indicating that the adsorption reaction is an endothermic process and becomes more favorable with increasing temperature; and ΔS0 is positive, which suggests that the adsorption may increase disorder and be dominated by irreversible chemical reactions [57].
3.3. Adsorption MechanismsThe physicochemical properties of the prepared samples are listed in Table S6. Because of the addition of Mg and Fe elements, the C content of MFDB was significantly reduced, while the O content was greatly increased. The fragrance and polarity of biochar are also estimated by H/C and O/C contents. The aromaticity of MFDB decreased and the polarity increased, which indicated that the hydrophilicity of the modified MFDB rose [58]. The Fe and Mg contents increased from 0.55% and 0.54% to 13.6% and 29.1%, respectively, and the phosphorus content decreased by 0.225%. The SBET and total pore volume (TPV) results increased from 10.5 m2/g and 0.015 cm3/g to 112.810.5 m2/g and 0.14 cm3/g, respectively, indicating FeCl3, MgCl2, and ZnCl2 can serve as carbon activators, producing more pores and higher surface area. The decrease in APR may be due to the microporosity produced by oxidative metal attack [59].
Fig. 3 shows the morphology and elemental distribution of SBC and MFDB before and after adsorption. Fig. 3(a) illustrates the presence of sharp corners and edges with furrow-like pores on the surface of SBC. The surface of MFDB had Mg and Fe oxides with a diameter of approximately 100 μm, and the particles were uniformly attached to the biochar, as shown in Fig. 3(b), (c), taking on a similar morphology to that observed in previous studies [16]. The MFDB metal oxides become coarse and inhomogeneous after adsorption of phosphorus, as illustrated in Fig. 3(d), suggesting that MFDB may have reacted with phosphate. Fig. 3(e) shows that C, O, Mg, Fe, and P were evenly distributed on the surface of MFDB before adsorption, but the P content was low, indicating that Fe and Mg were successfully loaded on the biochar via impregnation pyrolysis. Fig. 3(f) and Fig. S3 show an increase in P and a decrease in Fe and Mg elements on MFDB after adsorption, indicating the reaction of metal oxides and P.
3.3.1. Ligand exchange and electrostatic interactionThe adsorption of phosphate by MFDB at pH 2–12 is shown in Fig. 4(a). The adsorption amount increased rapidly at pH 2–4 and decreased gradually. Fig. 4(b) shows the stability of Mg and Fe in MFDB at different pH values over time, indicating that MFDB was less stable at pH 2 for Mg and more stable at pH 3–12. The maximum adsorption quantity at pH 4 might be related to the metal stability of MFDB loading and the competition between hydroxide and phosphate adsorption [60]. Hydroxide and phosphate were exchanged on MFDB—the phosphate was fixed and the hydroxide was released, making the solution alkaline. This adsorption mechanism is a ligand exchange [61], and the corresponding reaction mechanism is expressed by Eqs. (12)–(14):
Fig. 4(c) shows that the zeta potential of the material decreases continuously with pH, and the zeta potential of MFDB was greater than that of SBC. The zero potential (pHPZC) of the modified biochar increased from 5.21 to 5.74 mV. MFDB underwent protonation under acidic conditions to produce MFDB-OH2 +, which positively charged the adsorbent. Under alkaline conditions, the adsorbent deprotonated to generate negatively charged MFDB-O− [62]. It was observed that, for pH less than 5.74, MFDB was positively charged and could adsorb the negatively charged phosphate via electrostatic adsorption. Meanwhile, as the pH value increased, the electrostatic adsorption weakened and the adsorption force decreased [63]. The adsorption mechanism of MFDB on phosphate was shown to be dominated by electrostatic adsorption at low pH values, whereas the ligand exchange accompanied the whole process at different pH values. The reactions are as follows:
3.3.2. Complexation reactionThe structures of SBC and MFDB before and after adsorption are analyzed in Fig. 5. The XRD, and FTIR results of SBC and MFDB before and after adsorption are shown in Fig. 5(a), (b). The FTIR results showed Fe–O and Mg–O peaks of MFDB at 400–1,000 cm−1 [51]. The introduction of Mg and Fe led to a significant increase in 1,090 cm−1 C–O and 1,640 cm−1 C=C content, and the adsorbed MFDB had a significant P-O bond at 1,050 cm−1 [64]. The XRD results showed the difference in the material crystals. The typical diffraction peaks at 25.4°, 49.8°, 28.5°, 40.4°, 66.5° indicated the presence of SiO2, and KCl in SBC after pyrolysis [65], whereas those at 36.8°, 42.8°, 62.0°, 74.6°, 78.4°, and 35.5° demonstrated the formation of MgO and Fe2O3 in MFDB after pyrolysis [19, 66]. The peaks of MFDB at 10.9°, 12.1°, 26.6°, and 30.8° after adsorption indicated the presence of Mg3(PO4)2 [67]. The corresponding weakening of both MgO and Fe2O3 proved the formation of MgO and Fe2O3 on the surface of MFDB and the reaction with phosphate to form Mg3(PO4)2. Thus, the FTIR and XRD analyses demonstrated that MFDB reacted with PO43− to form a stable compound.
Fig. 5(c)–(h) shows the full spectrum before and after MFDB adsorption and the fine spectrum after MFDB adsorption. Fig. 5(c) shows the presence of C, O, Mg, and Fe in MFDB. The adsorption demonstrated the introduction of P, which in turn indicated a complexation reaction between PO43− and MFDB. The C spectrum of MFDB was corrected to three peaks of 284.4 eV (C=C), 285.9 eV (C–O), and 289 eV (C–C) [68]. Fig. 5(e) shows a peak at 530.0 eV indicating metal and O bonding, and Fig. 5(f) illustrates the presence of MgO and Mg3(PO4)2 with peaks at 1,304.9eV and 1,306.3 eV [69, 70]. Fig. 5(g) shows characteristic peaks at 710.5, 711.8 and 714.3 eV for MFDB after adsorption, indicating that Fe(II)(III) was still present in MFDB, whereas the XRD results did not show divalent iron, probably because of its reduced content after being oxidation [71]. Fig. 5(h) shows that the phosphorus in MFDB after adsorption existed as PO43− and HPO42−, indicating that part of the phosphorus was still adsorbed through electrostatic and ligand exchange [69]. The adsorption of phosphorus by MFDB mainly occurred through a complexation reaction, as indicated by FTIR, XRD, and XPS analysis. The reactions are as follows:
Li et al. [71] found that under different pH values, the phosphate adsorption mechanism varied. With increasing pH, for pH > PHpzc (5.74), the MFDB electronegativity increases, generating a repulsive force with phosphate and the weakening the electrostatic interaction. Under high-pH conditions, OH− and phosphate competed for adsorption, which also weakened the ligand exchange. For complexation, when pH < 3, metal oxides would dissolve, and under strong alkaline conditions, OH− and phosphate competed for adsorption, which also weakened the complexation. Shan [72] demonstrated the adsorption mechanism, including electrostatic adsorption, internal complexation, and ion exchange by measuring the zeta potential, XPS, and D partition rate of PR/MgAl2O4. Xu [35] prepared the La-biochar using the impregnation pyrolysis method and similarly demonstrated that the adsorption of phosphorus included electrostatic adsorption, internal layer complexation and ion exchange, all of which confirmed the experimental results of this study. Therefore, with the support of the above theory, the adsorption mechanisms of phosphate by MFDB is summarized in Fig. 6.
3.4. Removal of Phosphorus from Domestic Sewage3.4.1. Effect of organic matter on phosphorus removal
Fig. 7(a) shows the region divided according to the fluorescence area integration method, where Regions I and II respond to aromatic proteins with tyrosine (Ex/Em = 200–250 nm/280–330 nm) and tryptophan (Ex/Em = 220–250 nm/330–380 nm), respectively; Region III is composed of fulvic-acid-like substances, such as fulvic acid (Ex/Em = 220–250 nm/380–550 nm); Region IV consists of soluble microbial metabolites (Ex/Em = 250–280 nm/280–380 nm), such as tyrosine and phenyl ring proteins; and Region V is dominated by hydrophobic substances, such as humic-acid-like substances (Ex/Em = 250–400 nm/380–550 nm) [73]. Fig. 7 reveals the EEM fluorescence spectra of the actual water after MFDB adsorption from 0–96 h, with Ex/Em = 275 nm/350 nm (Peak T), Ex/Em = 250 nm/420 nm (Peak A), and Ex/Em = 230 nm/340 nm (Peak B). Peaks T, A, and B in the domestic wastewater were microbial metabolites and humic acids, fullerenes, and aromatic proteins, respectively, as found by previous researchers [74]. Fig. S6(c) shows that, after the fluorescence regions were integrated at different times, the volume of the five integrations decreased continuously with the change in time, and the volume decreased rapidly in the first hour. This indicated that the adsorption of organic substances mainly occurred in the first hour, with the most obvious effect on the adsorption of humic-acid-like substances.
The removal of phosphate and total phosphorus from actual water by MFDB demonstrated in Fig. S6(a). The initial concentration of phosphate decreased from 3.56 to 0.23 mg/L, and the total phosphorus concentration declined from 5.83 to 0.72 mg/L after adsorption. The total phosphorus adsorption by MFDB reached 127.6 mg/g. The phosphorus adsorption rate accelerated during the first 10 h and then reached equilibrium, proving the feasibility of MFDB for phosphorus removal in real bodies of water. Fig. S6(b) shows the adsorption of MFDB on TOC and UV254 in water, which were used to indicate the total organic carbon, humus-like macromolecular organic matter, and aromatic compounds in bodies of water [75, 76]. MFDB also quickly adsorbed organic matter within the first 10 h, and the removal rates of TOC and UV254 were 40% and 20%, respectively. The EEM fluorescence spectra, phosphorus removal, and organic carbon removal results indicated good removal of both phosphate and organic matter by MFDB, although the phosphate adsorption effect varied less in the presence of organic matter. This may be due to the removal of phosphorus by MFDB occurring through complexation, ligand exchange, and electrostatic interaction, with complexation as the main mechanism [67, 69]. The removal of organic matter by MFDB was mainly through electrostatic interaction and hydrophobic interaction [12, 77]. This difference in sorption mechanisms may have contributed to the lesser effect on phosphate sorption in the presence of DOM.
3.4.2. Correlation analysisBecause the EEM fluorescence spectra comprised overlapping peaks of multiple substances, the EEM fluorescence could be decomposed into a single fluorescence for analysis using PARAFAC [78]. To investigate the relationship between phosphorus and organic matter adsorption in real water, EEM fluorescence spectra during adsorption were analyzed using PARAFAC, and four independently varying fluorescence components are identified in Fig. S4. Component 1 (C1) was a humic acid associated with lignin, and Component 2 (C2) was also a humic acid which is mainly derived from terrestrial plants [79, 80]. Component 3 (C3) was tryptophan-like and obtained from microorganisms [81]. Component 4 (C4) was protein-, polyphenol-, and tryptophan-like [82]. Fig. S5 (a) shows the correlation analysis of parallel factors and various indicators obtained by exploring the correlations among C1–C4, phosphate, total phosphorus, TOC, and UV254. Phosphate and total phosphorus were shown to have greater correlation with C1, C3, C4, and UV254, whereas the correlation of C2 with phosphate and total phosphorus was weaker. This indicated that MFDB used for simultaneous removal of phosphate and total phosphorus can also achieve good removal of organic matter, as the two processes had a synergistic effect that was not found in previous research. The apparent molecular weight (AWM) of the actual water at different adsorption times was also measured, which suggested that there were a large number of substances with low molecular weight (400–2,000 Da) in domestic wastewater, and the adsorption of organic matter by MFDB mainly applied to small molecular substances. The study showed that MFDB was synergistic for phosphorus and DOM adsorption, and DOM in domestic wastewater had less effect on phosphate.
4. ConclusionsIn this study, the optimal MFDB was prepared and optimized using the impregnation pyrolysis and RSM methods. The best preparation conditions comprised a pyrolysis temperature of 491°C; impregnation ratios of 3.17:1 and 1.3:1 for Mg and Fe, respectively; and an adsorption capacity of 77.48 mg/g after 24 h of adsorption. The adsorption of phosphate by MFDB was consistent with the pseudo-primary and pseudo-secondary processes, indicating both chemical and physical adsorption. MFDB’s adsorption of phosphate was consistent with the Langmuir and Freundlich model. The adsorption was analyzed thermodynamically as an exothermic reaction, and the adsorption mechanism of phosphate adsorption onto MFDB consisted of ligand exchange, electrostatic interaction, and complexation. It was found that in wastewater containing DOM, MFDB had less effect on phosphate adsorption in actual domestic wastewater.
AcknowledgmentsThis research was funded by the National Key Research and Development Project (No. 2019YFD1100204) and the Fundamental Research Funds for the Central Universities of China (FRF-MP-20-33). The experimental supporting by National Environmental and Energy Science and Technology International Cooperation Base in University of Science and Technology Beijing were greatly appreciated.
NotesAuthor Contributions Mr. H.W. (M.Sc. Student) and Ms. J.D. (M.Sc. Student) conducted all the experiments and wrote the manuscript. Mr. H.C. (Professor), Mr. F.W. (Professor), Ms. Y.Z. (M.Sc. Student), and Mr. J.L. (M.Sc. Student) revised the manuscript. Ms. R.Y. (Associate Professor) and Mr. B.Z. (Professor) are the corresponding authors who had supervised the work and made all the possible corrections in the manuscript. References1. Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries Coast. 2002;25:704–726.
https://doi.org/10.1007/BF02804901
2. Kilpimaa S, Runtti H, Kangas T, et al. Physical activation of carbon residue from biomass gasification: Novel sorbent for the removal of phosphates and nitrates from aqueous solution. J Ind Eng Chem. 2015;21:1354–1364.
https://doi.org/10.1016/j.jiec.2014.06.006
3. Saleh AAS, Ibrahim N, Awang NR. Characteristics study of ammonia-n and phosphorus in sewage wastewater effluent: a case study of Alkhumrah, Jeddah Wastewater Treatment Plant. IOP Conf Ser Earth Environ Sci. 2021;842:012034.
https://doi.org/10.1088/1755-1315/842/1/012034
4. Hylander LD, Kietlińska A, Renman G, et al. Phosphorus retention in filter materials for wastewater treatment and its subsequent suitability for plant production. Bioresour Technol. 2006;97:914–921.
https://doi.org/10.1016/j.biortech.2005.04.026
5. Wang HJ, Wang HZ. Mitigation of lake eutrophication: Loosen nitrogen control and focus on phosphorus abatement. Prog Nat Sci. 2009;19:1445–1451.
https://doi.org/10.1016/j.pnsc.2009.03.009
6. Novais SV, Zenero MDO, Barreto MSC, et al. Phosphorus removal from eutrophic water using modified biochar. Sci Total Environ. 2018;633:825–835.
https://doi.org/10.1016/j.scitotenv.2018.03.246
7. Mayer BK, Gerrity D, Rittmann BE, et al. Innovative Strategies to Achieve Low Total Phosphorus Concentrations in High Water Flows. Crit Rev Environ Sci Technol. 2013;43:409–441.
https://doi.org/10.1080/10643389.2011.604262
8. Zhang ZJ, Li H, Zhu J, et al. Improvement strategy on enhanced biological phosphorus removal for municipal wastewater treatment plants: Full-scale operating parameters, sludge activities, and microbial features. Bioresour Technol. 2011;102:4646–4653.
https://doi.org/10.1016/j.biortech.2011.01.017
9. Huang WY, Zhang YM, Li D. Adsorptive removal of phosphate from water using mesoporous materials: A review. J Environ Manage. 2017;193:470–482.
https://doi.org/10.1016/j.jenvman.2017.02.030
10. Bacelo H, Pintor AMA, Santos SCR. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem Eng J. 2020;381:122566–122566.
https://doi.org/10.1016/j.cej.2019.122566
11. Luo MK, Lin H, Li B, et al. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour Technol. 2018;259:312–318.
https://doi.org/10.1016/j.biortech.2018.03.075
12. Almanassra IW, Mckay G, Kochkodan V, et al. A state of the art review on phosphate removal from water by biochars. Chem Eng J. 2021;409:128211.
https://doi.org/10.1016/j.cej.2020.128211
13. Cui XQ, Dai X, Khan KY, et al. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour Technol. 2016;218:1123–1132.
https://doi.org/10.1016/j.biortech.2016.07.072
14. Liu R, Chi L, Wang X, et al. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water. J Environ Chem Eng. 2018;6:5269–5286.
https://doi.org/10.1016/j.jece.2018.08.008
15. Li R, Wang JJ, Gaston LA, et al. An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon. 2018;129:674–687.
https://doi.org/10.1016/j.carbon.2017.12.070
16. Loganathan P, Vigneswaran S, Kandasamy J. Removal and Recovery of Phosphate From Water Using Sorption. Crit Rev Environ Sci Technol. 2014;44:847–907.
https://doi.org/10.1080/10643389.2012.741311
17. Jung KW, Hwang MJ, Ahn KH, et al. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int J Environ Sci Te. 2015;12:3363–3372.
https://doi.org/10.1007/s13762-015-0766-5
18. Min L, Zhang ZS, Li Z. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol Eng. 2020;149:105792.
https://doi.org/10.1016/j.biortech.2016.07.072
19. Liu JW, Jiang JG, Aihemaiti A. Removal of phosphate from aqueous solution using MgO-modified magnetic biochar derived from anaerobic digestion residue. J Environ Manage. 2019;250:109438.
https://doi.org/10.1016/j.jenvman.2019.109438
20. Haddad K, Jellali S, Jeguirim M. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust. J Environ Manage. 2018;216:305–314.
https://doi.org/10.1016/j.jenvman.2017.06.020
21. Peng YT, Sun YQ, Sun RZ. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model. J Clean Prod. 2019;237:117770.
https://doi.org/10.1016/j.jclepro.2019.117770
22. Genz A, Baumgarten B, Goernitz M. NOM removal by adsorption onto granular ferric hydroxide: Equilibrium, kinetics, filter and regeneration studies. Water Res. 2008;42:238–248.
https://doi.org/10.1016/j.watres.2007.07.005
23. Behbahani M, Moghaddam MRA, Arami M. Techno-economical evaluation of fluoride removal by electrocoagulation process: Optimization through response surface methodology. Desalination. 2011;271:209–218.
https://doi.org/10.1016/j.desal.2010.12.033
24. Rahimi T, Kahrizi D, Feyzi M, et al. Catalytic performance of MgO/Fe2O3-SiO2 core-shell magnetic nanocatalyst for biodiesel production of Camelina sativa seed oil: Optimization by RSM-CCD method. Ind Crops Prod. 2021;159:113065.
https://doi.org/10.1016/j.indcrop.2020.113065
25. Karimifard S, Moghaddam MRA. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci Total Environ. 2018;640:772–797.
https://doi.org/10.1016/j.scitotenv.2018.05.355
26. Singh KP, Gupta S, Singh AK. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J Hazard Mater. 2011;186:1462–1473.
https://doi.org/10.1016/j.jhazmat.2010.12.032
27. Su M, Fang YL, Li B. Enhanced hexavalent chromium removal by activated carbon modified with micro-sized goethite using a facile impregnation method. Sci Total Environ. 2019;647:47–56.
https://doi.org/10.1016/j.scitotenv.2018.07.372
28. Bro R; PARAFAC. Tutorial and applications. Chemometr Intell Lab Syst. 1997;38:147–171.
https://doi.org/10.1016/S0169-7439(97)00032-4
29. He W, Hur J. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic matter. Water Res. 2015;83:217–226.
https://doi.org/10.1016/j.watres.2015.06.044
30. Zepp RG, Sheldon WM, Moran MA. Dissolved organic fluorophores in southeastern US coastal waters: correction method for eliminating Rayleigh and Raman scattering peaks in excitation-emission matrices. Mar Chem. 2004;89:15–36.
https://doi.org/10.1016/j.marchem.2004.02.006
31. Stedmon CA, Bro R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. LIMNOL OCEANOGR-METH. 2008;6:572–579.
https://doi.org/10.4319/lom.2008.6.572
32. Wang SN, Yuan RF, Chen HL, et al. Effect of sulfonamides on the dissolved organic matter fluorescence in biogas slurry during anaerobic fermentation according to the PARAFAC analysis. Process Saf Environ Prot. 2020;144:253–262.
https://doi.org/10.1016/j.psep.2020.07.033
33. Feng Z, Chen H, Li H, et al. Preparation, characterization, and application of magnetic activated carbon for treatment of biologically treated papermaking wastewater. Sci Total Environ. 2020;713:136423.
https://doi.org/10.1016/j.scitotenv.2019.136423
34. Li JH, Lv GH, Bai WB, et al. Modification and use of biochar from wheat straw (Triticum aestivum L) for nitrate and phosphate removal from water. Desalin Water Treat. 2016;57:4681–4693.
https://doi.org/10.1080/19443994.2014.994104
35. Xu QY, Chen ZB, Wu ZS, et al. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration. Bioresour Technol. 2019;289:121600.
https://doi.org/10.1016/j.biortech.2019.121600
36. Yetilmezsoy K, Saral A. Stochastic modeling approaches based on neural network and linear-nonlinear regression techniques for the determination of single droplet collection efficiency of countercurrent spray towers. Environ Model Assess. 2007;12:13–26.
https://doi.org/10.1007/s10666-006-9048-4
37. Sen R, Swaminathan I. Response surface modeling and optimization to elucidate and analyze the effects of inoculum age and size on surfactin production. Biochem Eng J. 2004;21:141–148.
https://doi.org/10.1016/j.bej.2004.06.006
38. Yetilmezsoy K, Demirel S, Vanderbei RJ. Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L.: Box–Behnken experimental design. J Hazard Mater. 2009;171:551–562.
https://doi.org/10.1016/j.jhazmat.2009.06.035
39. Mohammed NAS, Abu-Zurayk RA, Hamadneh I, et al. Phenol adsorption on biochar prepared from the pine fruit shells: Equilibrium, kinetic and thermodynamics studies. J Environ Manage. 2018;226:377–385.
https://doi.org/10.1016/j.jenvman.2018.08.033
40. Ho YS. Review of second-order models for adsorption systems. J Hazard Mater. 2006;136:681–689.
https://doi.org/10.1016/j.jhazmat.2005.12.043
41. Liu JW, Jiang JG, Aihemaiti A, et al. Removal of phosphate from aqueous solution using MgO-modified magnetic biochar derived from anaerobic digestion residue. J Environ Manage. 2019;250:109438.
https://doi.org/10.1016/j.jenvman.2019.109438
42. Peng XM, Hu XJ, Fu DF. Adsorption removal of acid black 1 from aqueous solution using ordered mesoporous carbon. Appl Surf Sci. 2014;294:71–80.
https://doi.org/10.1016/j.apsusc.2013.11.157
43. Cheung WH, Szeto YS, McKay G, et al. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol. 2007;98:71–80.
https://doi.org/10.1016/j.biortech.2006.09.045
44. Jung KW, Lee S, Lee YJ. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour Technol. 2017;245:2897–2904.
https://doi.org/10.1016/j.biortech.2017.09.035
45. Li RH, Wang JJ, Zhou BY. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J Clean Prod. 2017;147:96–107.
https://doi.org/10.1016/j.jclepro.2017.01.069
46. Wang Z, Wang H, Li Q. pH effect on Re(VII) and Se(IV) diffusion in compacted GMZ bentonite. Appl Geochemistry. 2016;73:1–7.
https://doi.org/10.1016/j.apgeochem.2016.07.015
47. Altintig E, Onaran M, Sarı A, et al. Preparation, characterization and evaluation of bio-based magnetic activated carbon for effective adsorption of malachite green from aqueous solution. Mater Chem Phys. 2018;220:313–321.
https://doi.org/10.1016/j.matchemphys.2018.05.077
48. Liu JY, Zhou Q, Chen JH, et al. Phosphate adsorption on hydroxyl-iron-lanthanum doped activated carbon fiber. Chem Eng J. 2013;215:859–867.
https://doi.org/10.1016/j.cej.2012.11.067
49. Yu Y, Chen JP. Key factors for optimum performance in phosphate removal from contaminated water by a Fe-Mg-La tri-metal composite sorbent. J Colloid Interface Sci. 2015;445:303–311.
https://doi.org/10.1016/j.jcis.2014.12.056
50. Shimizu Y, Ateia M, Yoshimura C. Natural organic matter undergoes different molecular sieving by adsorption on activated carbon and carbon nanotubes. Chemosphere. 2018;203:345–352.
https://doi.org/10.1016/j.chemosphere.2018.03.197
51. Li RH, Wang JJ, Zhou BY, et al. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour Technol. 2016;215:209–214.
https://doi.org/10.1016/j.biortech.2016.02.125
52. Sheng T, Zhang Z, Hu YC, et al. Adsorption of phosphorus by using magnetic Mg-Al-, Zn-Al- and Mg-Fe-layered double hydroxides: comparison studies and adsorption mechanism. Environ Sci Pollut Res. 2019;26:7102–7114.
https://doi.org/10.1007/s11356-019-04191-5
53. Rahman S, Navarathna CM, Das NK, et al. High capacity aqueous phosphate reclamation using Fe/Mg-layered double hydroxide (LDH) dispersed on biochar. J Colloid Interface Sci. 2021;597:182–185.
https://doi.org/10.1016/j.jcis.2021.03.114
54. Tao XF, Huang T, Bo L. Synthesis of Fe/Mg-Biochar Nanocomposites for Phosphate Removal. Materials. 2020;13:816.
https://doi.org/10.3390/ma13040816
55. Ajmal Z, Muhmood A, Dong RJ. Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal. J Environ Manage. 2019;253:109730.
https://doi.org/10.1016/j.jenvman.2019.109730
56. Amin F, Talpur FN, Balouch A, et al. Biosorption of mercury(II) from aqueous solution by fungal biomass Pleurotus eryngii: Isotherm, kinetic, and thermodynamic studies. Environ Prog Sustain. 2016;35:1274–1282.
https://doi.org/10.1002/ep.12342
57. Jung KW, Choi BH, Ahn KH. Synthesis of a novel magnetic Fe3O4/gamma-Al2O3 hybrid composite using electrode-alternation technique for the removal of an azo dye. Appl Surf Sci. 2017;423:383–393.
https://doi.org/10.1016/j.apsusc.2017.06.172
58. Wang YT, Xin ZB, Peng F. Influence of Pyrolysis Temperature on Characteristics and Nitrobenzene Adsorption Capability of Biochar Derived from Reed and Giant Reed. Sci Adv Mater. 2019;11:1523–1530.
https://doi.org/10.1166/sam.2019.3463
59. Liu WJ, Tian K, Jiang H. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci Rep. 2013;3:2419.
https://doi.org/10.1038/srep02419
60. Nodeh HR, Sereshti H, Afsharian EZ. Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite. J Environ Manage. 2017;197:265–274.
https://doi.org/10.1016/j.jenvman.2017.04.004
61. Liao TW, Li T, Su XD, et al. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour Technol. 2018;263:207–213.
https://doi.org/10.1016/j.biortech.2018.04.108
62. Xiong WP, Tong J, Yang ZH, et al. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J Colloid Interface Sci. 2017;493:17–23.
https://doi.org/10.1016/j.jcis.2017.01.024
63. Gimenez J, Martinez M, Rovira M, et al. Arsenic sorption onto natural hematite, magnetite, and goethite. J Hazard Mater. 2007;141:575–580.
https://doi.org/10.1016/j.jhazmat.2006.07.020
64. Sowmya A, Meenakshi S. Phosphate uptake studies on different types of lanthanum-loaded polymeric materials. Environ Prog Sustainble Energy. 2015;34:146–154.
https://doi.org/10.1002/ep.11978
65. Zhao SS, Wang B, Gao Q, et al. Adsorption of phosphorus by different biochars. Spectrosc Lett. 2017;50:73–80.
https://doi.org/10.1002/ep.11978
66. Yang Q, Wang XL, Luo W, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour Technol. 2018;247:537–544.
https://doi.org/10.1016/j.biortech.2017.09.136
67. Xu Y, Liu TJ, Huang YK, et al. Role of phosphate concentration in control for phosphate removal and recovery by layered double hydroxides. Environ Sci Pollut Res. 2020;27:16612–16623.
https://doi.org/10.1007/s11356-020-08102-x
68. Koilraj P, Sasaki K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: Batch and column studies. Chem Eng J. 2017;317:1059–1068.
https://doi.org/10.1016/j.cej.2017.02.075
69. Zhu D, Chen YQ, Yang HP, et al. Synthesis and characterization of magnesium oxide nanoparticle-containing biochar composites for efficient phosphorus removal from aqueous solution. Chemosphere. 2020;247:125847.
https://doi.org/10.1016/j.chemosphere.2020.125847
70. Geng HH, Wang F, Yan CC, et al. Leaching behavior of metals from iron tailings under varying pH and low-molecular-weight organic acids. J Hazard Mater. 2020;383:121136.
https://doi.org/10.1016/j.jhazmat.2019.121136
71. Li MX, Liu JY, Xu YF, et al. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ Rev. 2016;24:319–332.
https://doi.org/10.1139/er-2015-0080
72. Shan XC, Yang LY, Zhao YM, et al. Biochar/Mg-Al spinel carboxymethyl cellulose-La hydrogels with cationic polymeric layers for selective phosphate capture. Environ Rev. 2016;24:319–332.
https://doi.org/10.1016/j.jcis.2021.08.078
73. Zhou J, Wang JJ, Baudon A. Improved Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Fluorescent Dissolved Organic Matter. J Environ Qual. 2013;42:925–930.
https://doi.org/10.2134/jeq2012.0460
74. Andrade-Eiroa A, Canle M, Cerda V. Environmental Applications of Excitation-Emission Spectrofluorimetry: An In-Depth Review II. Appl Spectrosc Rev. 2013;48:77–141.
https://doi.org/10.1080/05704928.2012.692105
75. Altmann J, Massa L, Sperlich A, et al. UV254 absorbance as real-time monitoring and control parameter for micropollutant removal in advanced wastewater treatment with powdered activated carbon. Water Res. 2016;94:240–245.
https://doi.org/10.1016/j.watres.2016.03.001
76. Catalkaya EC, Kargi F. Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: a comparative study. J Hazard Mater. 2007;139:244–253.
https://doi.org/10.1016/j.jhazmat.2006.06.023
77. Nam SW, Choi DJ, Kim SK, et al. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J Hazard Mater. 2014;270:144–152.
https://doi.org/10.1016/j.jhazmat.2014.01.037
78. Li WT, Chen SY, Xu ZX, et al. Characterization of dissolved organic matter in municipal wastewater using fluorescence PARAFAC analysis and chromatography multi-excitation/emission scan: a comparative study. Environ Sci Technol. 2014;48:2603–2609.
https://doi.org/10.1021/es404624q
79. Osburn CL, Stedmon CA. Linking the chemical and optical properties of dissolved organic matter in the Baltic-North Sea transition zone to differentiate three allochthonous inputs. Mar Chem. 2011;126:281–294.
https://doi.org/10.1016/j.marchem.2011.06.007
80. Fellman JB, Petrone KC, Grierson PF. Source, biogeochemical cycling, and fluorescence characteristics of dissolved organic matter in an agro-urban estuary. Limnol Oceanogr. 2011;56:243–256.
https://doi.org/10.4319/lo.2011.56.1.0243
81. Kothawala DN, Koehler B, Tranvik LJ. Selective loss and preservation of lake water dissolved organic matter fluorescence during long-term dark incubations. Sci Total Environ. 2012;433:238–246.
https://doi.org/10.1016/j.scitotenv.2012.06.029
82. Yamashita Y, Maie N, Briceno H. Optical characterization of dissolved organic matter in tropical rivers of the Guayana Shield, Venezuela. J Geophys Res-Biogeo. 2010;115:1–15.
https://doi.org/10.1029/2009JG000987
Fig. 1Three-dimensional response surfaces for the effects of (a) Mg ratio and Fe impregnation ratios, (b) Mg impregnation ratio and pyrolysis temperature, and (c) Fe impregnation ratio and pyrolysis temperature, (d) Correlation between predicted and actual values of adsorption capacity. 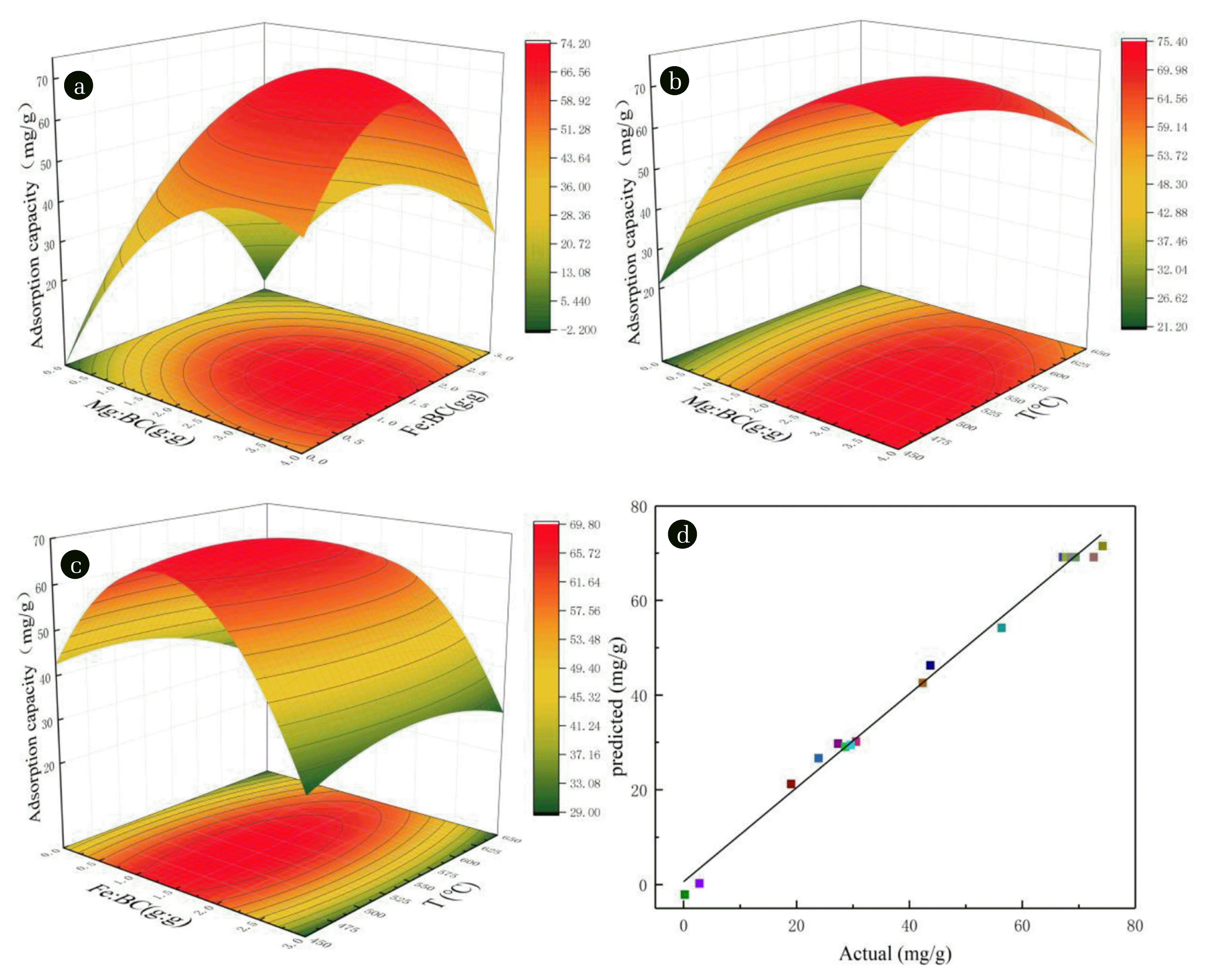
Fig. 2The effect of biochar adsorption on phosphate. (a) Adsorption kinetics of phosphate adsorption by MFDB, (b) fitted intraparticle diffusion model for phosphate adsorption, (c) adsorption isotherms of phosphate adsorption by MFDB, and (d) thermodynamic analysis of phosphate adsorption by MFDB. 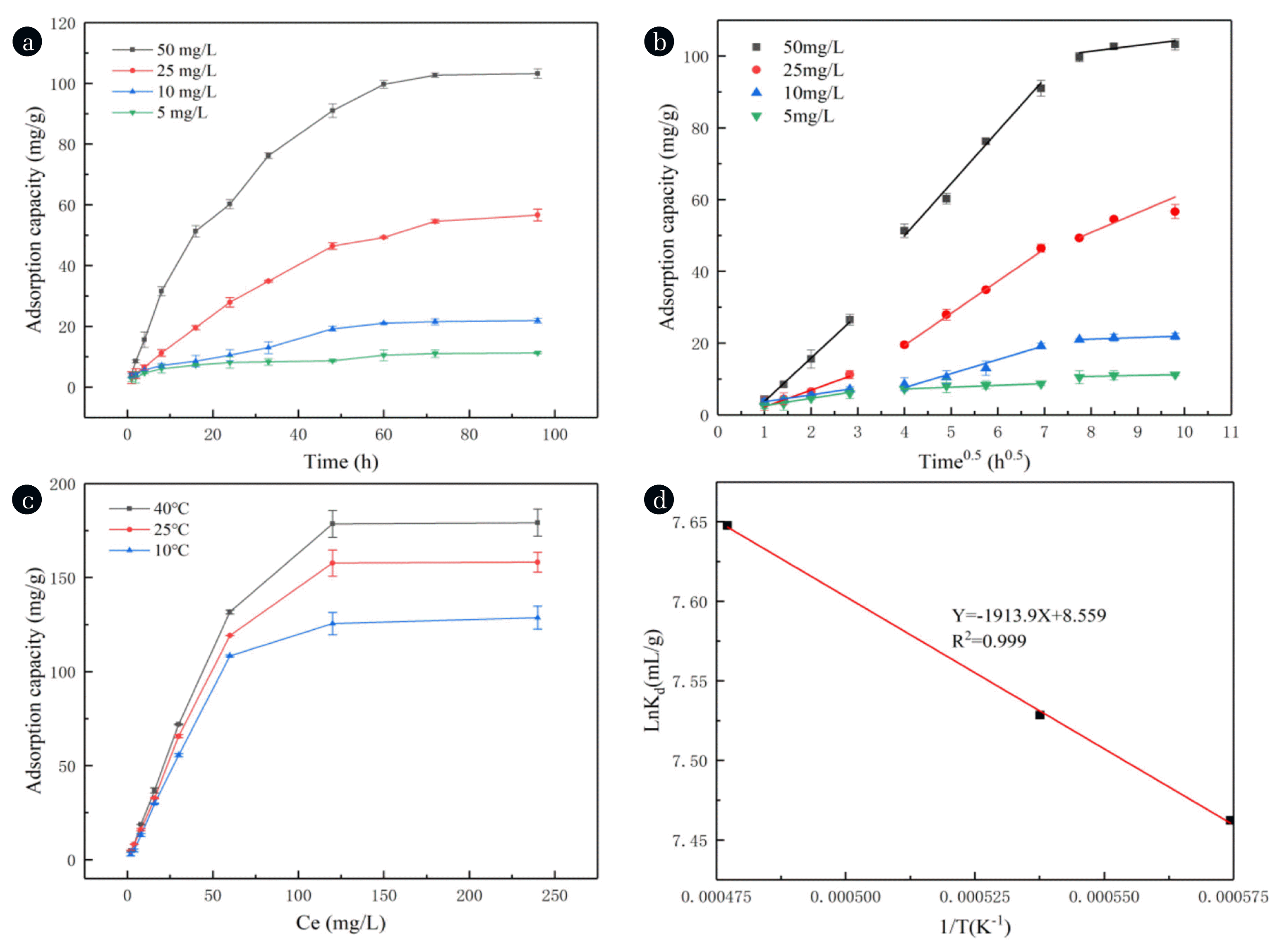
Fig. 3(a) SEM image of SBC. (b,c) MFDB before adsorption. (d) MFDB after adsorption. Element mapping image (e) before and (f) after MFDB adsorption. 
Fig. 4(a) Effect of initial pH on phosphorus adsorption by MFDB and the final pH of the solution, (b) metal leaching rate, and (c) zeta potential at different pH values. 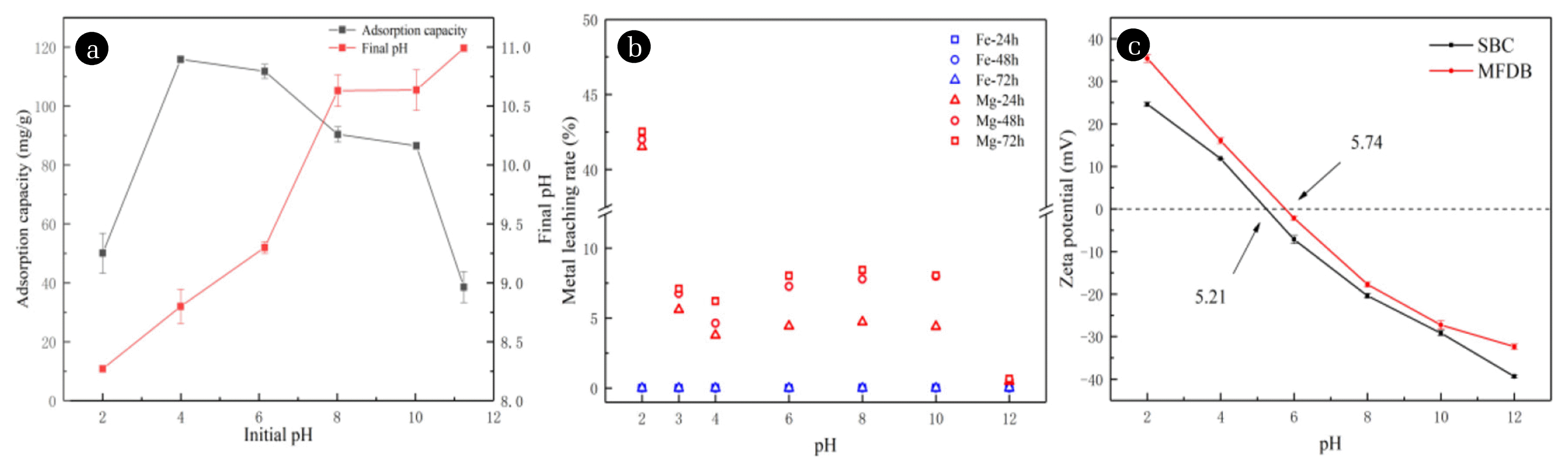
Fig. 5(a) FTIR and (b) XRD before and after the adsorption of BC and MFDB; (c) The surveyed XPS spectrum before and after the adsorption of MFDB. (d)–(h) C1s, O1s, Mg1s, Fe2p, and P2p high-resolution spectra after the adsorption of MFDB. 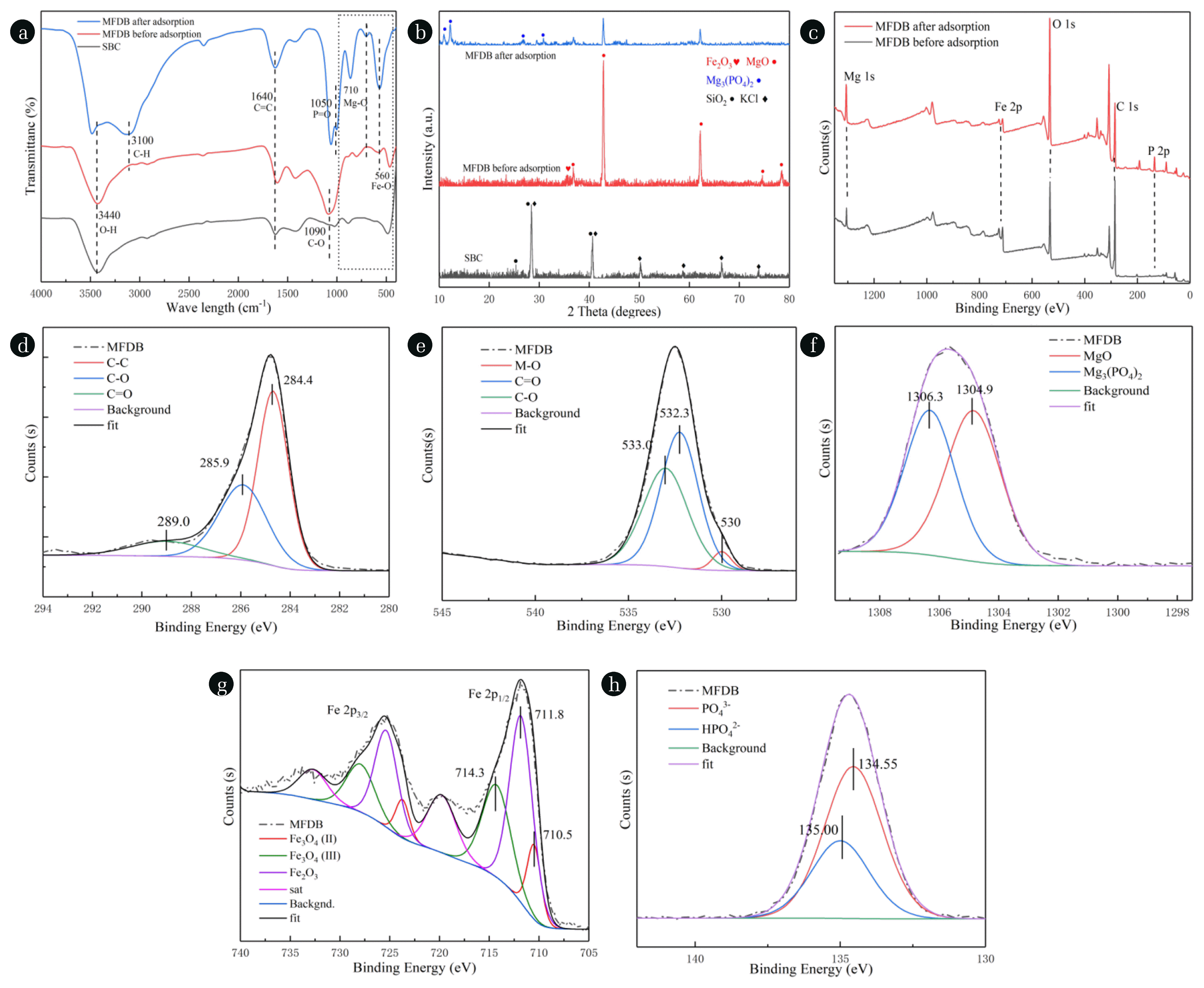
Fig. 7EEM fluorescence spectra of actual water adsorbed by MFDB at (a)–(f) 0, 1, 4, 24, 72, and 96 h. 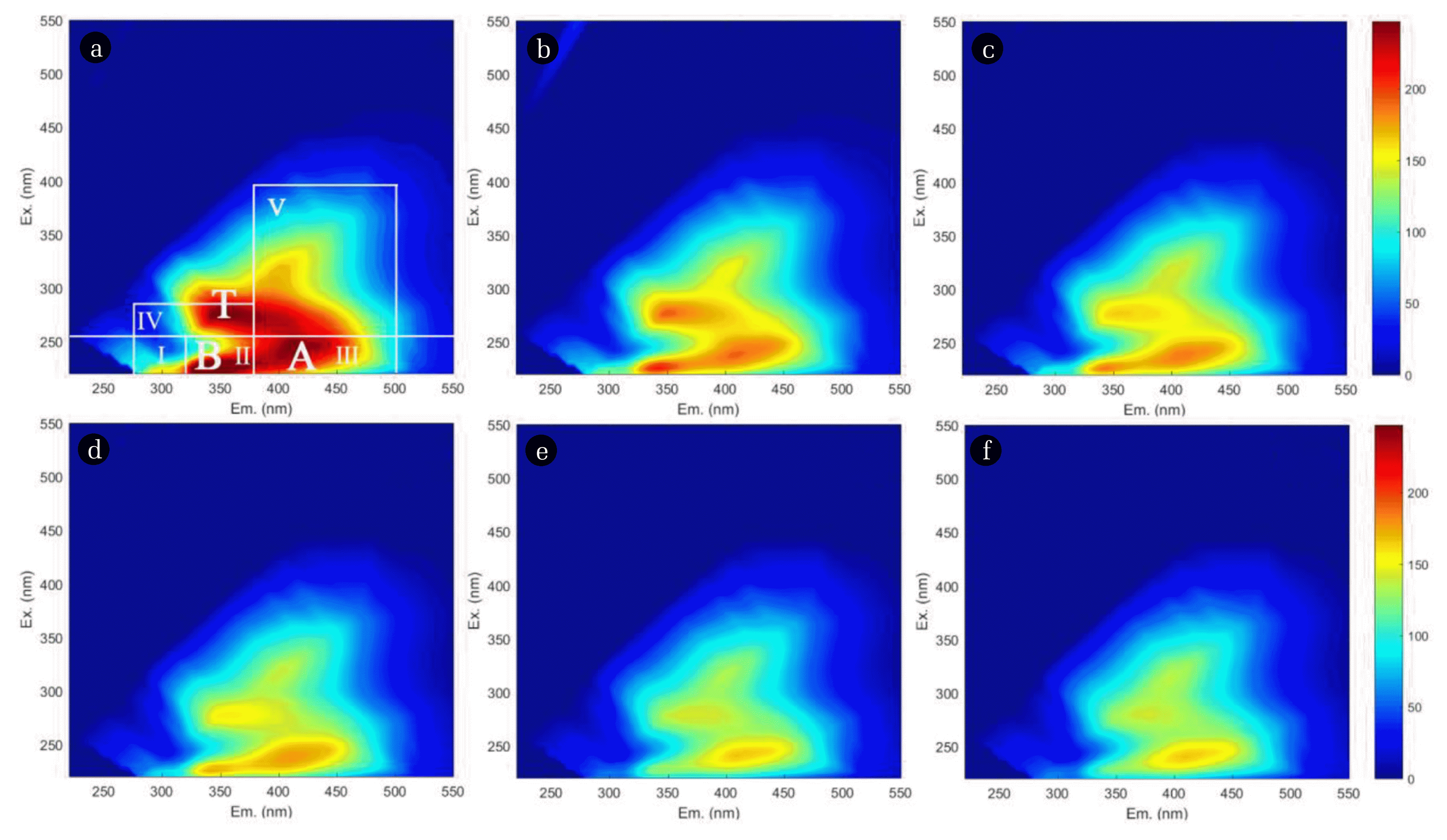
Table 1Comparison of Different Adsorbents on Phosphate Adsorption Capacity
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||