Adsorption characteristics of strontium onto K2Ti4O9 and PP-g-AA nonwoven fabric
Article information
Abstract
This study investigated the possibility of using potassium titanate oxide (K2Ti4O9) and acrylic acid-grafted polypropylene fabric (PP-g-AA) as adsorbents capable of removing strontium from aqueous solutions. K2Ti4O9 showed the highest rate of strontium removal in the weak alkaline range, while the PP-g-AA increased strontium removal in the neutral range. Moreover, the adsorption capacity of the K2Ti4O9 was not affected by the coexistence of K and Na ions, while the adsorption capacity decreased when Ca and Mg ions were present at the same concentration as that of strontium. When coexisted at the same concentration as strontium, Na, K, Ca, and Mg ions strongly reduced the adsorption capacity of the PP-g-AA. The results also indicated that the adsorption of strontium on K2Ti4O9 was consistent with both the Langmuir and Freundlich adsorption isotherms. In contrast, the adsorption of strontium on the PP-g-AA was more consistent with the Langmuir isotherm model. Moreover, the adsorption equilibrium time of K2Ti4O9 was generally 12 h, while that of the PP-g-AA was 5 h, indicating that the adsorption rates were consistent with the pseudo-second order kinetics model. K2Ti4O9 and the PP-g-AA could be regenerated by simple washing with 0.5 N HCl.
1. Introduction
Radioactive nuclides rapidly arise from the leaching of natural hatching, development of uranium deposits, employment of nuclear energy and different nuclear fuel conversion processes [1, 2]. These nuclides also circulate in the Earth’s surface system because of various activities such as the generation and treatment of nuclear waste, leakage at nuclear power plants, nuclear tests, and the use and disposal of artificial radioactive isotopes [1, 3]. Notably, if the generated radionuclides exceed a certain concentration, the risks to humans and environmental ecosystems rise exponentially. Consequently, an improved understanding and management of the circulation system of radionuclides is of significant importance. Numerous studies have been conducted to investigate the mobility and adsorption behavior of these elements in various lipid media in an effort to facilitate proper radioactive waste storage and control their diffusion behavior on the Earth’s surface layer [4, 5]. Hitherto, various studies have been carried out to develop various ion exchangers and adsorbents for the purpose of preventing environmental inflow and diffusion of radionuclides, as well as extracting them from radioactive waste, seawater, and wastewater for further separation and recovery [4, 6–9]. In the treatment of radioactive wastes and waste-containing heavy metal ions, an adsorption method employing an organic or inorganic ion exchanger is commonly used. One of the most popularly applied compounds is potassium titanate oxide (K2Ti4O9), which is characterized by its double acid resistance and selective adsorption of strontium ions [10–12]. It also serves as an inorganic ion exchanger and has been used for removing strontium ions in strongly acidic environments. There has been a growing interest in the development of a natural polymer adsorbent (or a polymeric adsorbent with functionality imparted by a polymerization method) as an alternative agent for overcoming the limitations associated with post-use treatment of inorganic ion exchangers [13–15].
The purpose of this study was to evaluate the possibility of using K2Ti4O9 and acrylic acid-grafted polypropylene (PP-g-AA) nonwoven fabric as adsorbents for the removal, separation, and recovery of strontium. The PP-g-AA nonwoven fabric employed herein was prepared by photo-irradiation polymerization of acrylic acid onto polypropylene fiber. The sorption capacity and sorption selectivity for strontium of K2Ti4O9 and the PP-g-AA nonwoven fabric were determined and compared. In addition, their adsorption characteristics were evaluated using adsorption equilibrium experiments and the influence of pH on the removal of strontium ions using these adsorbents was investigated.
2. Materials and Methodology
2.1. Materials
2.1.1. Materials for the preparation of the PP-g-AA nonwoven fabric
Non-woven polypropylene (PP) fabric (180 g/m2, Samsung Non-Woven Fabric Co., Korea) was used as the substrate polymer for grafting. In the preparation, acrylic acid (AA) served as a monomer, benzophenone (BP) as a photosensitizer, while sulfuric acid (H2SO4) and iron sulfate (FeSO4.7H2O) were used as a graft accelerator and a homo-polymer inhibitor. The solvent used for removing the homo-polymer was methanol.
2.1.2. Materials for the preparation of K2Ti4O9
K2CO3 (99.9% purity, Sigma-Aldrich, US) and TiO2 (98.5%, purity, TaiHai, Japan) were used as the main materials for the synthesis of the adsorbent.
2.2. Methodology
2.2.1. Preparation of the PP-g-AA nonwoven fabric
A 400 W high-pressure mercury lamp (H400, Mia Electric, Korea) was used as a light source for photografting. The distance between the sample and light source was set to 10 cm. The graft polymerization was carried out by adding 30 mL of a methanol/water (30/70 vol.%) solution. A mixed solution containing AA at a final concentration of 3.6–14.4 wt.%, 0.2 wt.% benzophenone, 0.4 N H2SO4, and 5.0 × 10−3 M FeSO4 (~400 mg) was immersed in a Pyrex tube. Nitrogen gas was flushed through the mixture for 5 min to remove any dissolved oxygen. The solution was then irradiated uniformly at 60°C for 60 to 90 min. The PP nonwoven fabric grafted with AA was subjected to Soxhlet extraction through washing with hot water and methanol for 24 h to remove the homo-polymer and unreacted monomer adhered to the surface of the sample. This process was followed by drying at 60°C for 24 h. After drying, the graft yield was calculated from the weight gain. The aforementioned experimental conditions resulted in a PP-g-AA nonwoven fabric with a graft of 100 wt.%, which was then used as an adsorbent.
2.2.2. Preparation of K2Ti4O9
K2O and TiO2 were mixed in a molar ratio of 1:4 and isopropyl alcohol was added to the mixture. The powder mixture was then placed in a platinum crucible and heated to a temperature of 900 to 1,150°C. The sample was taken out from the furnace at a temperature of 1,150°C and then quenched in air.
2.2.3. Adsorption experiment
In the adsorption experiment, 1 L of the experimental solution with known ionic species and concentration was placed in a 1 L high-density polyethylene (HDPE) sample bottle. Then, 1 g of adsorbent was added to the sample, after which the bottle was sealed. After a batch-wise shaking at 120 rpm and 25°C using a thermostatic stirrer, the pH of the test solution was adjusted to 2.5–11.5 using 0.1 N HCl and NaOH solutions. After shaking it for the required amount of time, the solution was immediately filtered using a 0.45-um membrane filter and the ion concentration remaining in the filtrate was measured. The adsorption of ions, q (mg/g), was calculated using the following equation:
where, C0 and Ce are the initial and post-adsorption strontium concentrations (mg/L) of the filtrate, respectively, V is the volume of the test solution (mL), and W is the dose (g) of adsorbent. After the desorption experiments, the filtered adsorbent was washed several times with distilled water and dried at 90 ± 5°C. Next, 0.1 g of dried strontium adsorbed-adsorbent was added to 100 mL of 0.5 N HCl solution. The resulting mixture was shaken at 25°C and 120 rpm for 1 h, and subsequently filtered. The concentration of strontium ions in the filtrate was measured using mass spectrometry. The desorption efficiency (%) was then calculated in relation to the adsorption amount.
2.2.4. Properties and chemical analysis
The zeta potential of each sample was measured using a flat board cell of an electrophoresis apparatus (Otsuka ELS-8000, Japan). This measurement was conducted in order to investigate the change of the surface potential according to the pH of the adsorbent. The particle size of K2Ti4O9 was analyzed using a laser particle size analyzer (Mastersizer S, Malvern Instruments Ltd., UK). The surface of the PP-g-AA nonwoven fabric and K2Ti4O9 was observed by a scanning electron microscope (SUPRA 55VP, Carl Zeiss, Germany). The PP-g-AA nonwoven fabric was subjected to Fourier-transform infrared spectroscopy (FTIR; Nicolet 6700, Thermo Scientific, USA) analysis in order to confirm the structural changes before and after the adsorption. The mineral composition of K2Ti4O9 was observed using X-ray diffraction (XRD; Panalytical xpert pro mpd, Netherlands).
3. Result and Discussion
3.1. Physical Properties of the Adsorbent
3.1.1. K2Ti4O9
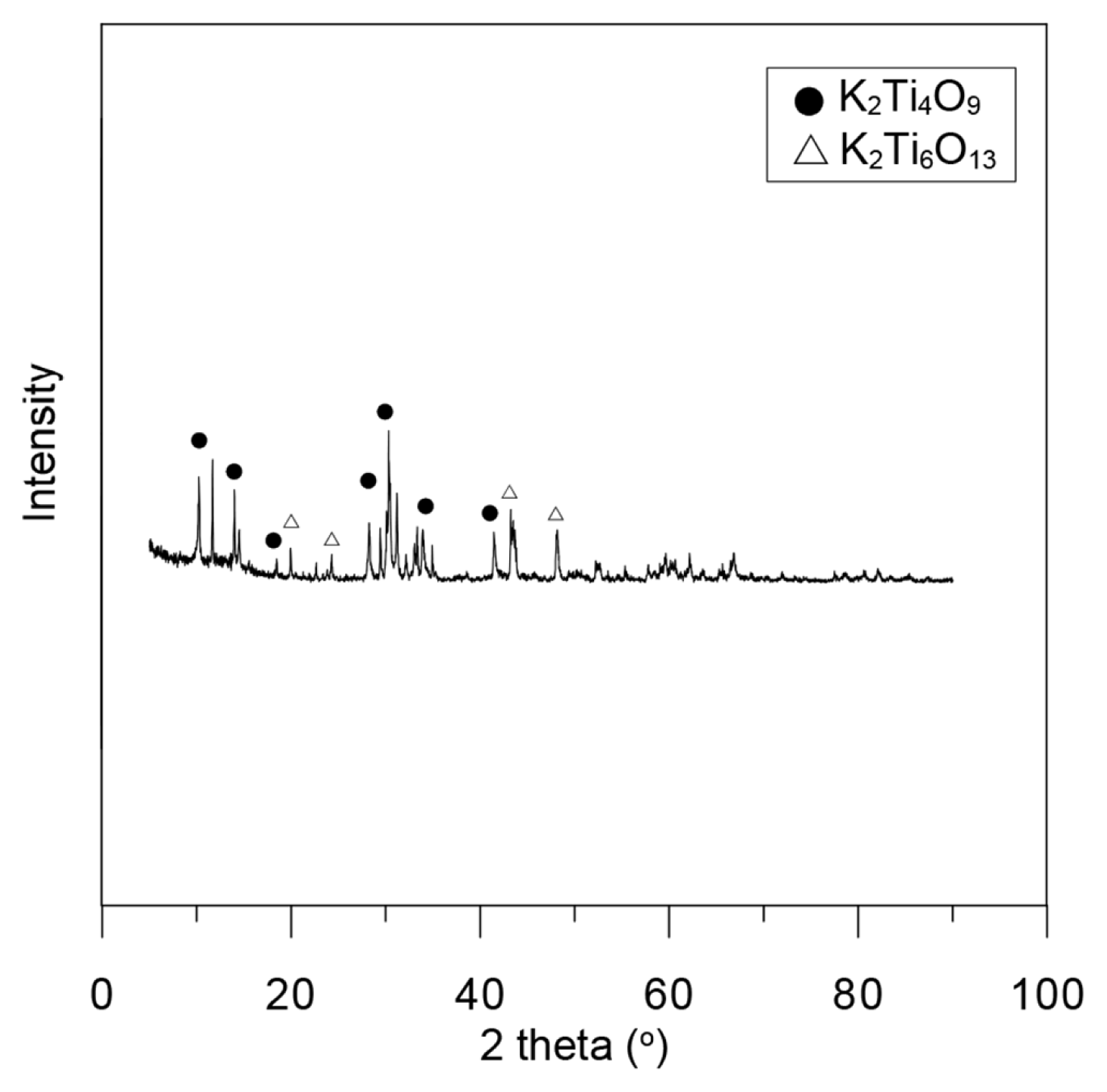
The mineral composition of K2Ti4O9 used in the adsorption experiments was observed by XRD (Fig. 1). The resulting solution from the heating of the mixture of K2O and TiO2 to 1,150°C and subsequent quenching in air contained a low amount of K2Ti6O13. However, it was confirmed that K2Ti4O9 was the main synthetic product and the material synthesized with K2Ti4O9 was observed using a scanning electron microscope (Fig. 2) [11]. The morphology of the K2Ti4O9 compound was prismatic and showed an undivided crystal, which is consistent with previous reports on the K2Ti4O9 crystal structure. The average particle size of K2Ti4O9 was found to be 2,373 nm, and the Brunauer-Emmett- Teller (BET) surface area was 2.5282 m2/g.
3.1.2. The PP-g-AA nonwoven fabric
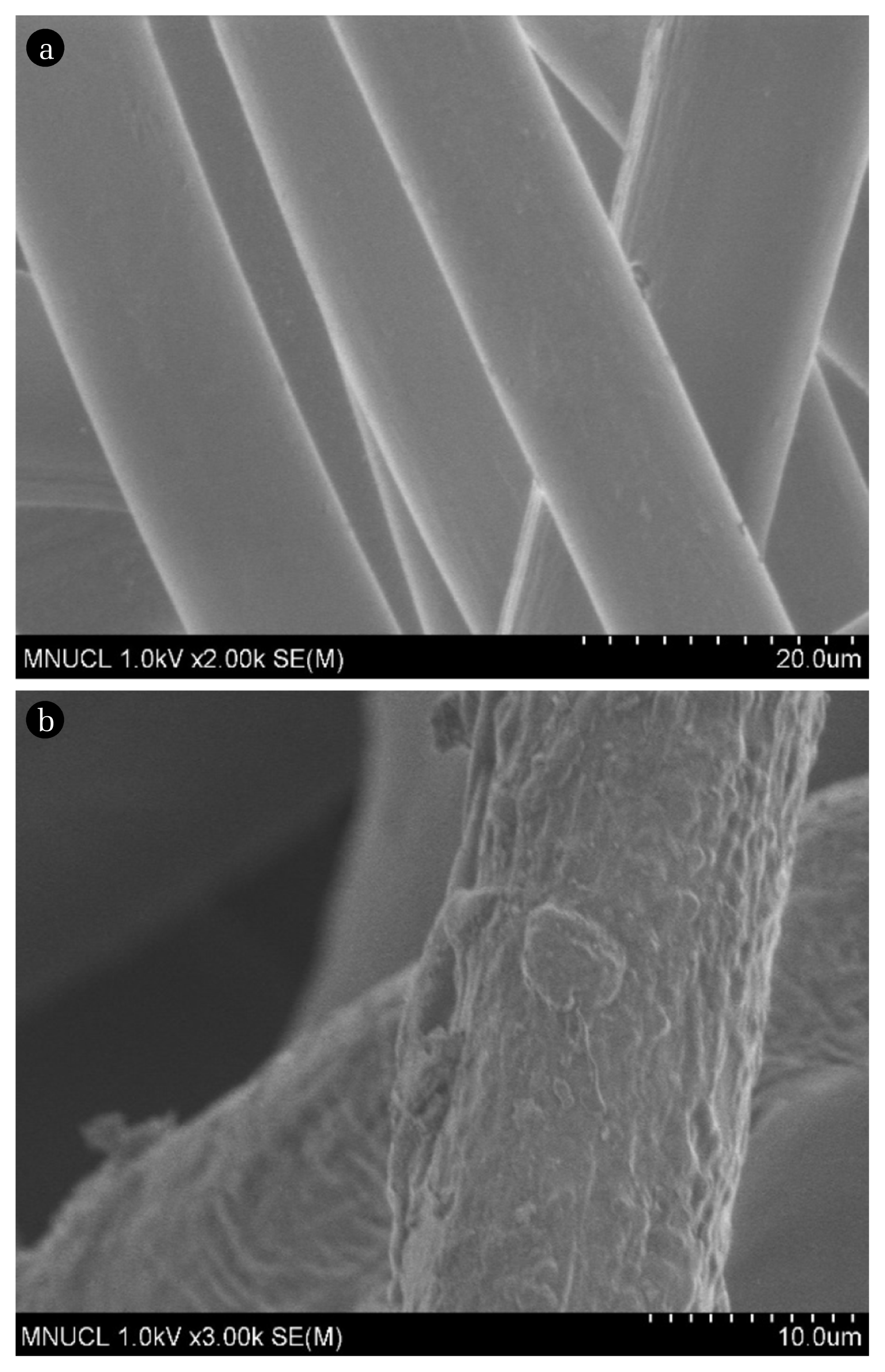
Fig. 3 shows the FTIR spectrum of the PP-g-AA nonwoven fabric, in which the peak ~1,740 cm−1 was attributed to the C=O double bond stretching of the AA grafting. Moreover, a peak arising from the −OH group was also detected at around 3,200–3,600 cm−1. Fig. 4 shows a comparison of the scanning electron microscope (SEM) photographs of raw PP nonwoven fabric and the PP-g-AA nonwoven fabric with a graft ratio of 100 wt.%. The original PP nonwoven fabric had a thin and smooth surface, whereas the PP grafted with AA showed increased thickness and became rough and inhomogeneous simultaneously [16].
3.2. Effect of pH
In order to investigate the effect of pH on the strontium adsorption capacity of PP-g-AA nonwoven fabric and K2Ti4O9, adsorption experiments were carried out by adjusting the pH of the test solution using NaOH and HCl. Measurements of the final pH of the test solution indicated that the final pH tended to be lower than that the initial value. Correspondingly, it was difficult to properly determine the effect of pH. Therefore, this study investigated the effect of pH on the adsorption capacity, using a buffer solution suitable for each pH range in an attempt to maintain a constant pH, by minimizing the changes in pH that arise from release of hydrogen ions. The following buffers were employed for this aim: primary phosphate (pH = 2), secondary phosphate (pH = 7), tertiary phosphate (pH = 12), secondary carbonate (pH = 10), and acetate (pH = 4). In order to minimize the effect of cation-forming possible salts, bases of weak acids (in the form of Na) were used as cations. A 0.01 M buffer solution for each pH range was prepared such that the acid to base ratio was 1:1. Also, a solution with a strontium concentration of 50 mg/L was prepared using the buffer solution. The adsorption experiment was conducted by mixing 100 mg of the PP-g-AA fabric with a graft ratio of 100 wt.% and 100 mg of K2Ti4O9, and the results are shown in Fig. 5.
The results confirmed that the precipitation of insolubilized strontium increased with the rise in pH. More specifically, a blank test system that did not contain any adsorbent was prepared with the same solution as the one used in the adsorption experiments, and the same procedure was employed. Then, the solutions were filtered through a 0.45-μm filter paper and their concentrations were measured. After the adsorption was completed, the solution was subjected to the same procedure as the solution of the blank test system in order to determine the final residual concentration. The results showed that strontium tends to precipitate strongly in the alkaline region; hence, it is difficult to precisely investigate the effect of pH unless the experiment is conducted at a concentration that can exist in a dissolved phase in all pH regions. Therefore, the results shown in Fig. 5 were not compared at a constant concentration. In addition, since the buffer system differed depending on the pH, it was difficult to completely exclude the effect. However, it is generally shown that strontium has excellent adsorption capacity in the neutral region. These results suggested that the adsorption competitiveness of the ion can be changed according to the pH of the solution. The findings also confirmed the possibility of using PP-g-AA nonwoven fabric and K2Ti4O9 as adsorbents for the separation and purification of strontium.
3.3. Adsorption Competitiveness
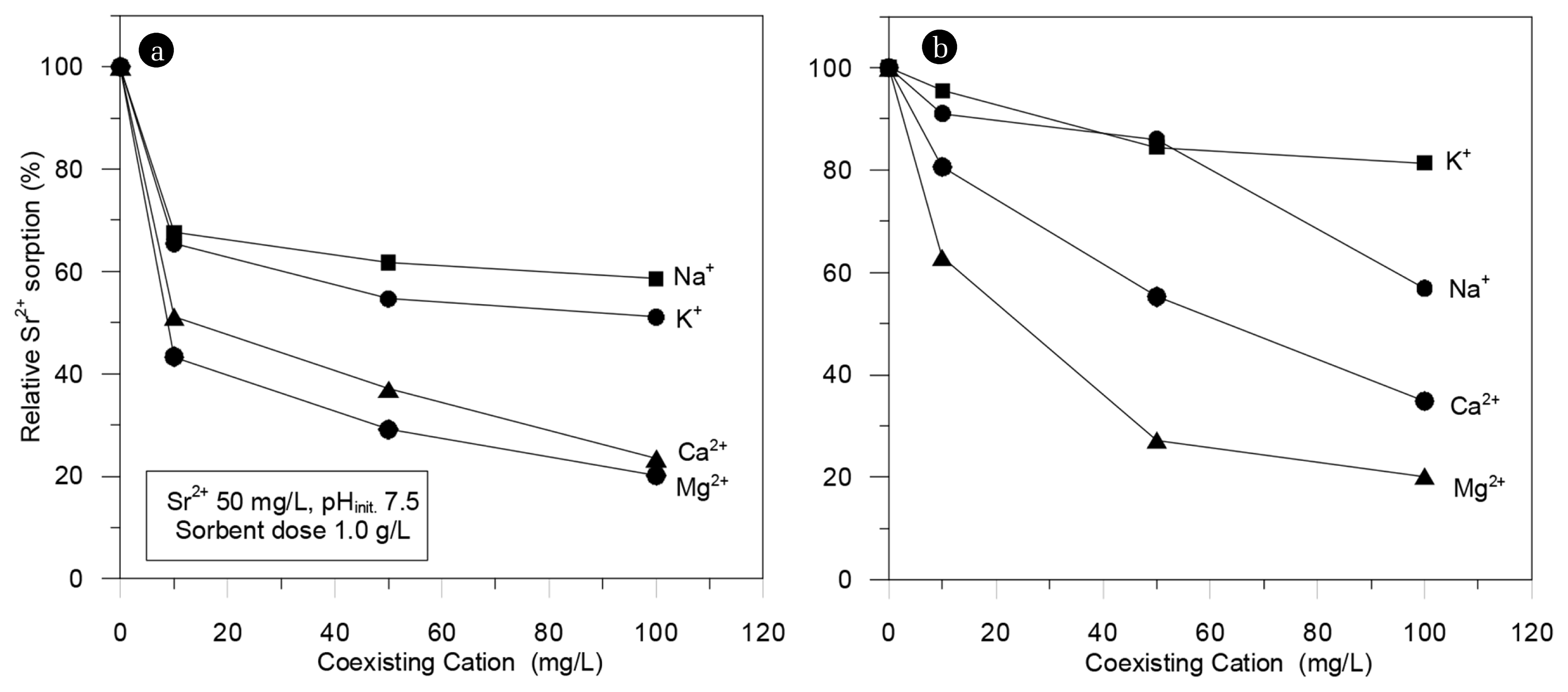
Sodium (Na), magnesium (Mg), calcium (Ca), and potassium (K), which are the most abundant elements in seawater along with strontium, were selected to investigate the adsorption competitiveness of the adsorbents. Hence, the adsorption capacities of the PP-g-AA nonwoven fabric and K2Ti4O9 were investigated when these ions coexisted. In order to evaluate the ionic adsorption competitiveness, the pH was adjusted to 7.5 and the concentration of each ion was adjusted to 0–100 mg/L. The adsorption amount of each ion was determined by immersing 100 mg of the adsorbent in 100 mL of the solution (Fig. 6). The adsorption competitiveness of the PP-g-AA nonwoven fabric when each ion was presented at the same concentration was found to be the following order: magnesium > calcium > strontium > sodium. Moreover, it was found that the adsorption capacity of the fabric was affected by the coexisting cations. When the concentration of Sr and other ions are the same, the adsorption capacity for Na ions was decreased to 35%, while the corresponding values for K, Ca, and Mg ions were 40%, 60%, and 70%, respectively. Notably, when all ions competed at a concentration of 50 mg/L, the capacity of K2Ti4O9 was not considerably affected by the sodium and potassium ions. However, when Ca and Mg ions were presented at the same concentration with strontium, the adsorption capacity of K2Ti4O9 decreased to 40% and 70%, respectively.
3.4. Adsorption Isotherm
The adsorption mechanisms of the PP-g-AA nonwoven fabric and the K2Ti4O9 compound of strontium were determined by the change in the pH of the solution as the adsorption progressed. Since the adsorbing ability of the adsorbent was influenced, the adsorption characteristics of the adsorption isotherms were limited only by the change in initial concentration. Therefore, in order to investigate the adsorption characteristics under conditions of constant adsorption capacity, the adsorption isotherms of the strontium ions (5–200 mg/L) were prepared after the initial pH was maintained at a constant level of 7–7.5 (Fig. 7). The results were applied to the Langmuir and Freundlich adsorption isotherms. The Langmuir adsorption isotherm assumed monolayer adsorption, thereby also proposing various types of line forms. Weber’s equation (Eq. (2)) was used to further prove this theory [16, 17].

(a) Adsorption isotherms. (b) The Langmuir and (c) Freundlich isotherm plots of strontium adsorption by adsorbents.
where, q is the equilibrium concentration (mg/L), qe is the equilibrium adsorption (mg/g), qm is the maximum monolayer adsorption (mg/g), and b is the Langmuir constant (L/mg) associated with the adsorption energy.
The Freundlich adsorption isotherm is a multilayer adsorption model, expressed in the following linear form (Eq. (3)):
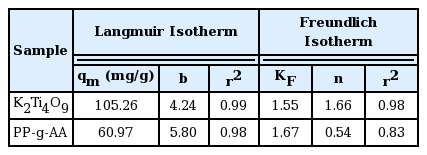
where, KF and n are the Freundlich constants. KF is a measure (mg1− (1/n)L1/ng−1) of the adsorbent’s adsorption capacity. The high value indicates the larger adsorption capacity. In turn, n represents the magnitude of the adsorption power. In general, adsorption is easy when n ≥ 2, and adsorption is difficult when n ≤ 1 [16, 18–19]. As shown in Fig. 7, the adsorption by the PP-g-AA nonwoven fabric and K2Ti4O9 compound were consistent with the Langmuir adsorption behavior rather than the Freundlich adsorption behavior. In other words, above a certain concentration, the adsorption mechanism was similar to the single-layer adsorption mechanism, where the active adsorption sites become saturated. Moreover, the maximum amount of adsorption from the Langmuir adsorption isotherm with K2Ti4O9 (104 mg/g) was higher than that with the PP-g-AA nonwoven fabric (60.97 mg/g) as shown in Table 1.
3.5. Adsorption Kinetics
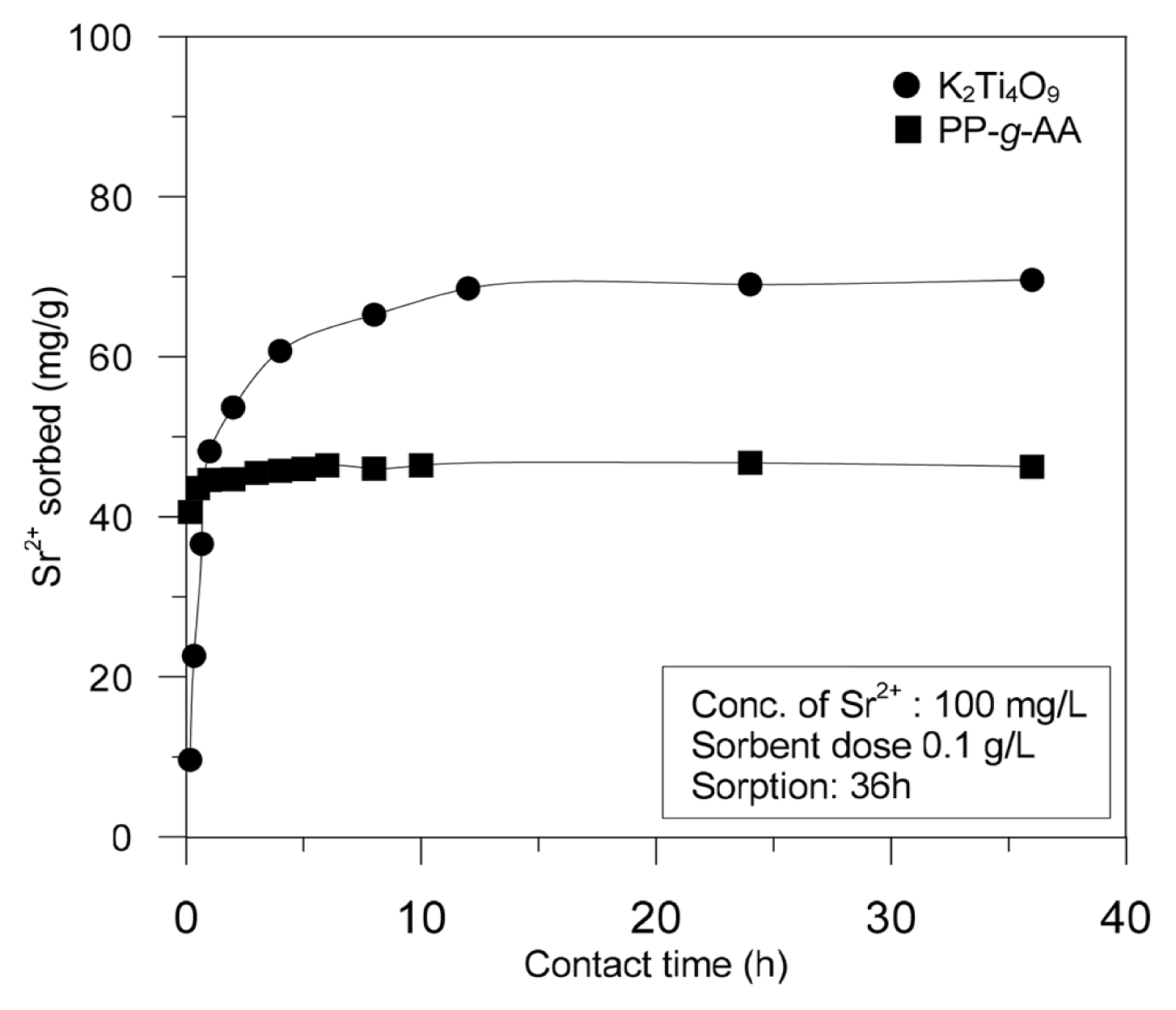
In order to investigate the adsorption kinetics of the PP-g-AA nonwoven fabric and K2Ti4O9 compound, 100 mL of a solution containing 100 mg/L of strontium was immersed and stirred at 120 rpm at room temperature for 24 h. As shown in Fig. 8, the adsorbed amount of strontium increased significantly for up to 3 h for the PP-g-AA nonwoven fabric, and the amount levelled off after 3 h. The corresponding value with the K2Ti4O9 compound continuously increased up to 10 h and then levelled off. Furthermore, the strontium adsorption reached equilibrium after about 5 h for the PP-g-AA nonwoven fabric and at least 12 h for the K2Ti4O9 compound. The adsorption was analyzed using Eq. (4) and Eq. (5), which represent the first adsorption kinetics equation and second adsorption kinetics equation, respectively [20, 21].
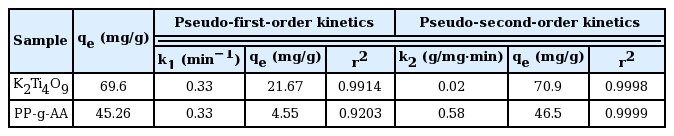
where, qe and qt are the equilibrium adsorption amount and the adsorption amount (mg/g) at time t, respectively, and k1 and k2 are the first adsorption rate constant (min−1) and the second adsorption rate constant (g/mg·min), respectively. As shown in Fig. 9, the strontium adsorption on the K2Ti4O9 compound and PP-g-AA nonwoven fabric could not be explained by the first adsorption rate equation. According to this equation, a straight line can be seen for the K2Ti4O9 compound after 60 min, while that for the PP-g-AA nonwoven fabric appears 360 min earlier. The theoretical qe values of the K2Ti4O9 compound and PP-g-AA nonwoven fabric obtained from the straight lines are shown in Table 2. The kinetic data for both the K2Ti4O9 compound and PP-g-AA nonwoven fabric have well-converged into linear line at the second adsorption kinetics equation, resulting in a high linear regression constant (r2 > 0.99) (Fig. 9(b)). The theoretical qe values of K2Ti4O9 and the PP-g-AA nonwoven fabric obtained from the linear equation were comparable to the experimental qe value. This indicated that the strontium adsorption by the K2Ti4O9 compound and PP-g-AA nonwoven fabric can be explained better by the second adsorption rate equation.
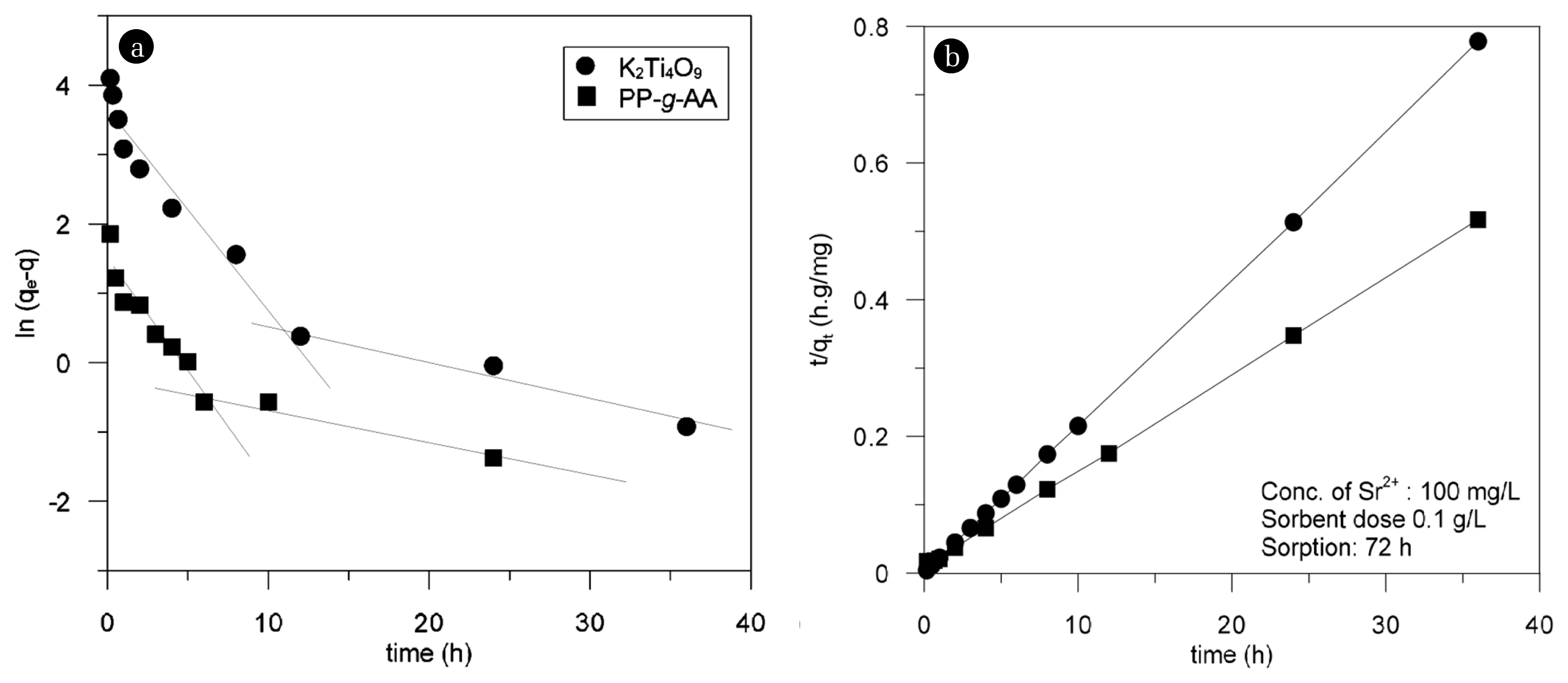
(a) Pseudo first-order and (b) pseudo second-order kinetics plots for strontium adsorption by adsorbents.
3.6. Regeneration Efficiency and Durability
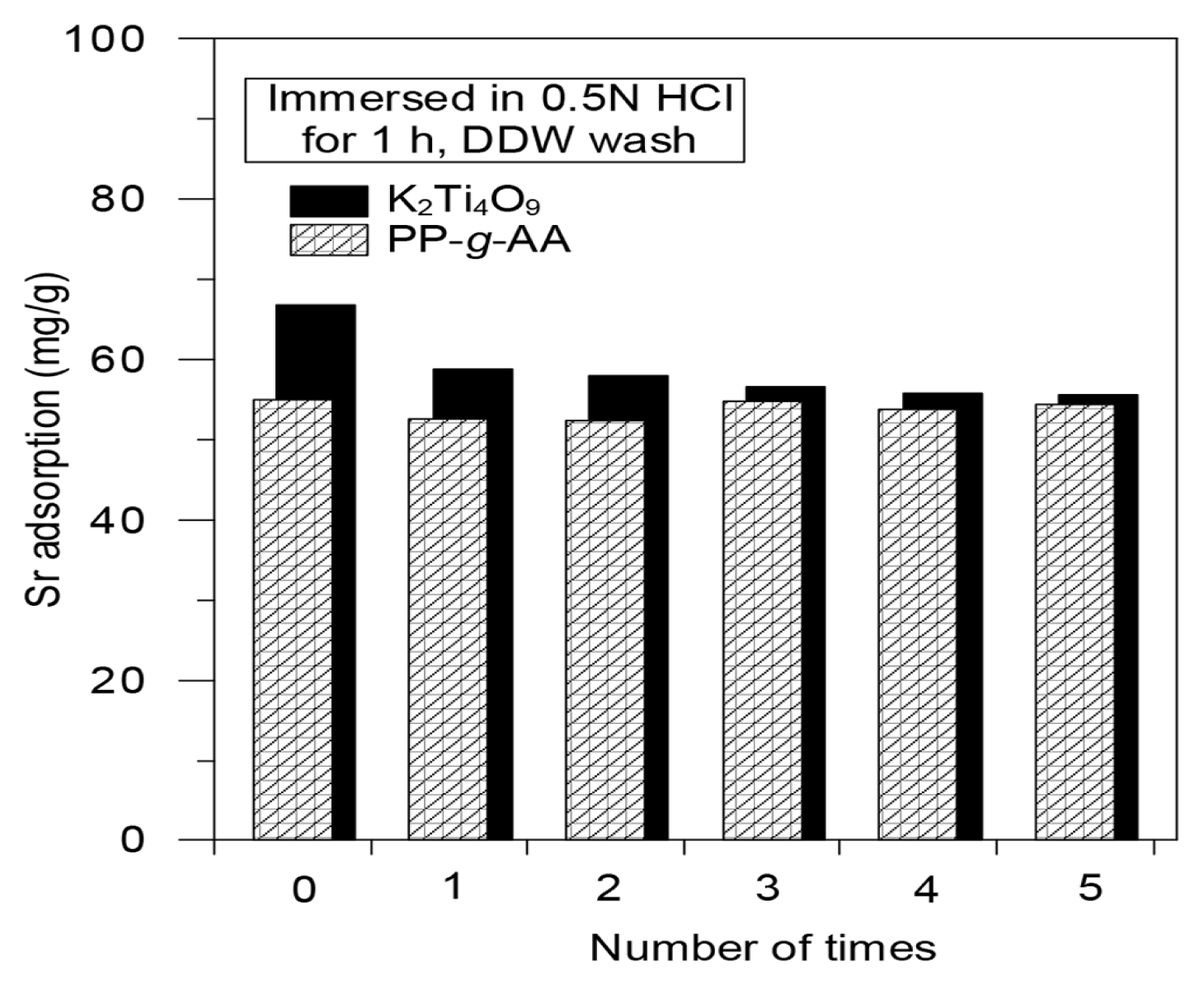
The strontium adsorption and regeneration experiments were repeated in order to investigate the regeneration efficiency of the PP-g-AA nonwoven fabric and K2Ti4O9 compound. The regeneration experiment was carried out via an acid washing method, in which the adsorbent was immersed and stirred in 100 mL of a 0.5 N HCl solution for 1 h. The desorbed adsorbent was regenerated by repeated washing with distilled water until its pH became neutral and subsequent drying at low temperature. The adsorption-regeneration process was repeated five times, and the amount of adsorbed strontium was measured (Fig. 10).
The difference in strontium adsorption capacity according to the regeneration number was calculated as a result of five repeated adsorption-desorption experiments. Although the PP-g-AA nonwoven fabric was repeatedly regenerated through the acid-washing process, the adsorption efficiency of strontium remained almost constant regardless of the repetition frequency. This result suggests that as a strontium adsorbent, the PP-g-AA nonwoven fabric could be regenerated without severe damage via a simple acid-washing process. Thus, it can be repeatedly used while maintaining the same adsorption efficiency. In the case of the K2Ti4O9 compound, the amount of adsorption decreased by 15% in the first regeneration. However, the adsorption amount each time tended to decrease by about 1.5% as the number of regenerations increased (1.5% reduction after the second regeneration, 2.3% after the 3rd, 1.3% after the 4th, and 0.5% after the 5th). As a result, using a simple pickling process, the PP-g-AA nonwoven fabric and K2Ti4O9 can be regenerated without any significant decrease in their adsorption capacity of strontium. Thus, these materials can be regenerated and used repeatedly. The SEM images of the PP-g-AA nonwoven fabric and K2Ti4O9 compound are shown in Fig. 11. In the case of the K2Ti4O9 compound, the thin film was changed to a multilayer structure in the process of adsorbing and desorbing the strontium onto and from the polyhedral structure, respectively. Nonetheless, the effects before and after the adsorption and desorption found to be similar. In contrast, the surface of PP-g-AA nonwoven fabric before and after the adsorption and desorption was found to be no significant difference.
4. Conclusions
In this study, the adsorption characteristics of K2Ti4O9 and PP-g-AA nonwoven fabric, as adsorbents for strontium removal, were investigated through batch experiments. The results are summarized as follows:
The K2Ti4O9 compound showed the highest strontium removal in the weak alkaline range of pH 7–8, while the PP-g-AA nonwoven fabric exhibited increased strontium removal in the neutral range of pH 5–8.
The strontium adsorption capacity of K2Ti4O9 was not influenced by the coexistence of the K and Na ions. However, when Ca and Mg ions were present at the same concentration of strontium, the adsorption capacity decreased to 40% and 70%, respectively. In the case of the PP-g-AA nonwoven fabric, the capacity was affected by the coexisting cations. For the same concentration of strontium, the adsorption capacity of Na ions was lowered to 35%, while the corresponding values for K ions, Ca ions, and Mg ions were 40%, 60%, and 70%, respectively.
The strontium adsorption on K2Ti4O9 satisfied both the Langmuir and Freundlich adsorption isotherms. In contrast, the strontium adsorption on the PP-g-AA nonwoven fabric was more consistent with the Langmuir isotherm model. The result indicated that the adsorption mechanisms of strontium on K2Ti4O9 and the PP-g-AA nonwoven fabric were mostly dominated by ionic shifts.
The adsorption equilibrium time was generally 12 h for the K2Ti4O9 compound and 5 h for the PP-g-AA nonwoven fabric. The adsorption rates were consistent with the pseudo-second order kinetics model.
A simple washing process can help regenerate the PP-g-AA nonwoven fabric more than five times without deteriorating the adsorptivity potential and its physical properties. In the case of K2Ti4O9, the adsorption efficiency decreased by about 15% after one round of regeneration. However, the decrease in adsorption capacity was considerably smaller after two rounds of the regeneration process. In conclusion, both K2Ti4O9 and the PP-g-AA nonwoven fabric could be regenerated by simple washing with 0.5 N HCl.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1C1A2A01052334).