Use of tar color additives as a light filter to enhance growth and lipid production by the microalga Nannochloropsis gaditana
Article information
Abstract
The spectral composition of light can affect the growth and biochemical composition of photosynthetic microalgae. This study examined the use of light filtering through a solution of soluble colored additives, a cost-effective method to alter the light spectrum, on the growth and lipid production of an oleaginous microalga, Nannochloropsis gaditana (N. gaditana). Cells were photoautotrophically cultivated under a white light emitting diode (LED) alone (control) or under a white LED that passed through a solution of red and yellow color additive (4:1 ratio) that blocked light below 600 nm. The specific growth rate was significantly greater under filtered light than white light (0.2672 d−1 vs. 0.1930 d−1). Growth under filtered light also increased the fatty acid methyl ester (FAME) yield by 22.4% and FAME productivity by 80.0%, relative to the white light control. In addition, the content of saturated fatty acids was greater under filtered light, so the biodiesel products had better stability. These results show that passing white light through an inexpensive color filter can simultaneously enhance cellular growth and lipid productivity of N. gaditana. This approach of optimizing the light spectrum may be applicable to other species of microalgae.
1. Introduction
The widespread use of fossil fuels has caused serious environmental problems, and this has increased interest in renewable energy sources. Microalgae are a promising new source of renewable energy, and have the potential to replace fossil fuels [1–4]. However, microalgae typically have low productivity, so there are attempts to develop various strategies to overcome this limitation. Many studies have attempted to optimize cellular growth in photo-bioreactor or mass culture [5, 6], or to improve photosynthetic productivity by genetic engineering [7, 8]. There have also been attempts to increase the lipid content of microalgae [9–11]. Although some strategies have succeeded in enhancing growth rate or lipid content, biomass accumulation and lipid production are usually negatively correlated [12]. The simultaneous improvement of growth rate and lipid content must be achieved to achieve economically feasible lipid production.
Photosynthetic organisms convert light energy into chemical energy, and accumulate biomass and lipids [13]. The light conditions must be optimized to improve microalgal productivity, because light influences photosynthesis and subsequent metabolic steps. High levels of light can damage the photosynthetic apparatus and reduce microalgal growth [2]. The light spectrum also affects photosystem stoichiometry and cellular metabolism [14, 15]. Studies using monochromatic illumination indicate that light quality can improve cellular growth and lipid content at the same time [16, 17]. However, the optimal wavelength seems to depend on the species and other factors [18–26]. Moreover, it is difficult to scale-up laboratory experiments that use monochromatic light from light emitting diodes (LEDs), because of cost considerations. It is also difficult to change the spectrum when sunlight is used as the light source.
The present study examined use of an inexpensive light filter, in which simple pigments are used to change the light spectrum, on growth and lipid production in Nannochloropsis gaditana (N. gaditana), an oleaginous microalga that has high lipid productivity and can be cultivated on a large scale [27, 28].
2. Materials and Methods
2.1. Construction and Characterization of Light Filter
Diverse color additives (Red, Yellow, Green, and Pink, Chunwoo Co. Ltd., Kyungbuk, Korea) were used to make solutions to filter out specific areas of the spectrum from a white LED source. The wavelengths absorbed by the solutions were determined by UV/Vis spectrophotometry (JASCO-V-730). Fig. 1 shows the absorbance spectra of the different tar colors. The red and yellow additives were mixed at a 4:1 ratio to filter out light below 600 nm. A rectangular transparent container, with a light-path of 20 mm, was filled with this mixture (0.02% concentration) and positioned between the photobioreactor and white LEDs.
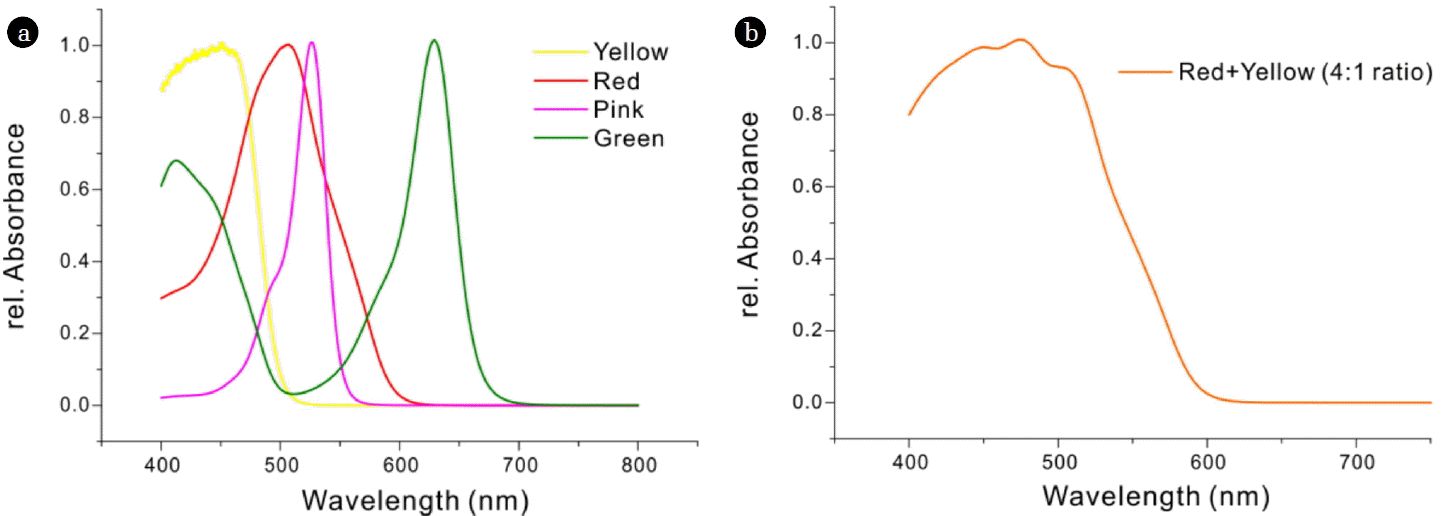
a) Absorption spectra of 4 different pigments (yellow, red, pink, and green). The red pigment had maximal absorption at 506 nm (red line) and the yellow pigment at 455 nm (yellow line). b) A mixture of the red and yellow pigments (4:1 ratio) had broad absorption in the blue region, with half-maximal absorbance at about 550 nm.
2.2. Microalgal Strain and Culture Conditions
The microalga N. gaditana CCMP526 (National Center for Marine Algae and Microbiota) was cultured at 25ºC in modified f/2 medium. This medium consisted of 30 g L−1 sea salts, 375 mg L−1 NaNO3, 5 mg L−1 NaH2PO4·9H2O, 3.15 mg L−1 FeCl3·6H2O, 4.36 mg L−1 Na2EDTA·2H2O, 9.8 μg L−1 CuSO4·5H2O, 6.3 μg L−1, Na2MoO4·2H2O, 22 μg L−1 ZnSO4·7H2O, 10 μg L−1 CoCl2·6H2O, 180 μg L−1 MnCl2·4H2O, 0.5 μg L−1 vitamin B12, 0.5 μg L−1 biotin, and 100 μg L−1 thiamine·HCl. Cell cultures were maintained in transparent 500 mL photo-bioreactors with a diameter of 50 mm, and were mixed by aeration with a constant flow of 20 mL min−1 air enriched with 3% CO2 (v/v) via a bubble tube (Fig. S1). Prior to cultivation, the prepared medium was sterile-filtered, and the reactor setup was chemically sterilized by incubation with 2 mM peroxyacetic acid for 1 h. The incident light intensity in front of the bioreactor was adjusted to the same value (50 μmol photons m−2 s−1) for samples with and without the color filter.
2.3. Cell Growth
Microalgal cells were harvested and washed twice with deionized water by centrifugation at 2,744 g-force for 5 min. The microalgal suspensions were filtered through pre-dried and pre-weighed 0.45 μm cellulose nitrate membranes (Whatman, USA), and the cells were dried in an oven at 80ºC for 24 h before measuring dry cell weight.
2.4. Analysis of FAME Yield and Composition
The lipid content was determined using a modified version of the Folch method. The 10 mg freeze-dried biomass sample was mixed with 2 mL of a chloroform-methanol solution (2:1 v/v) in a screw-cap tube and vortexed for 20 min. Then, 1 mL of the internal standard solution (C19:0, Sigma; 0.5 mg/1 mL) was added, and 1 mL of methanol and a 300 μL solution of H2SO4 were added, and the tube was incubated at 100°C for 20 min. The reaction mixture was cooled to room temperature, then 1 mL of deionized water was added. After centrifugation at 896 g-force for 10 min, the organic phase was separated and filtered with a 0.2 μm RC-membrane syringe filter (Satorius Stedim Biotech, Germany). The fatty acid methyl ester (FAME) composition was analyzed by gas chromatography (GC; HP6890, Agilent, USA) with a flame-ionized detector (FID) and an HP-INNOWAX polyethylene glycol column (HP 19091 N-213, Agilent, USA). Each FAME peak was identified based on the retention time of the internal standard (F.A.M.E. MIX C8–C24, Supelco, USA), and quantified by comparing the peak area with the internal standard. FAME yield was calculated as total weight of FAMEs/weight of biomass input × 100%. Based on the weight percentage of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), the degree of unsaturation (DU) was calculated as [29]:
where MUFA% is the percent weight of MUFAs and PUFA% is the percent weight of PUFAs.
3. Results and Discussion
3.1. Effect of Filtered Illumination on Cell Growth of N. gaditana
The purpose of this study was to test the effect of filtered illumination on the growth and lipid production of Nannochloropsis, as previously reported for monochromatic illumination by blue, red, and green LEDs. Nannochloropsis contains chlorophyll a, β-carotene, and violaxanthin as major pigments [30], and absorbs blue light (400–500 nm) and red light (600–700 nm). Thus, this species may be expected to grow better under blue or red light. In fact, several studies reported greater growth of Nannochloropsis under blue light and red light [17, 18, 31]. In the case of N. gaditana, red light stimulated a greater accumulation of biomass and lipids than blue light, and also led to better biodiesel quality, based on the DU [16]. The present study used a mixture of red and yellow pigments, as a simple and inexpensive light filter, to examine the effect of different light spectra on the cellular growth and fatty acid production by N. gaditana. Because the absorption spectrum of the sole red additive had a main peak around 506 nm (Fig. 1(a)) and did not effectively absorb light below 450 nm, a yellow additive was mixed at a ratio of 4:1 (red:yellow) to achieve a more uniform absorbance below 506 nm (Fig. 1(b)). The half-maximal absorbance was at about 550 nm (Fig. 1(b)). The 0.02% concentration of this mixture was set to pass 50% of incident light intensity through the color filter.
N. gaditana cultures were grown under photoautotrophic conditions under white light or filtered light (Fig. S1). Compared to cells grown under white light, cells grown under filtered light had significantly greater specific growth rate (0.55 h−1 vs. 0.38 h−1) (Fig. 2) and 46.7% more biomass productivity after 9 d (0.107 g L−1 d−1 vs. 0.073 g L−1 d−1) (Table 1). Also, the maximum biomass productivity was greater under filtered light than white light (0.156 g L−1 d−1 on day 4 vs. 0.082 g L−1 d−1 on day 8).
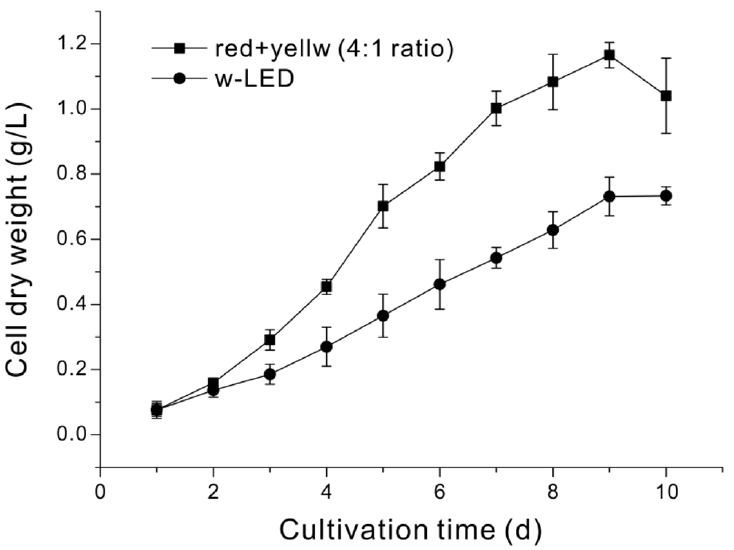
Growth of N. gaditana under white light (circles) and filtered light (squares). Each value is the mean of three biological replicates with standard deviation.

Biomass and FAME Productivity of N. gaditana Grown under White Light and Filtered Light. Maximum Biomass Productivity Was Defined as the Day with the Greatest Cumulative Biomass Production. All Other Values Were Measured from Samples at the Final Day of Cultivation (Day 9). Each Value Is the Mean of Three Biological Replicates with Standard Deviation. Here and Below, Significant Differences Were Determined by t-test and Indicated by Asterisks (*p < 0.05).
Previous research indicated that biomass accumulation of N. gaditana was greater under red light than white light, but the specific growth rates were similar under these different conditions [16]. This implies that red light had a greater effect on accumulation of biomass than cell division. These differences may also occur when light was filtered through the solution described here. However, the light filter used in the present study supplies a broader range of wavelengths to the culture, and some light below 600 nm penetrates through. Petroutsos et al. reported that photo-tropin, a blue light photoreceptor, regulates photosynthesis in microalgae [32]. Thus, the partial penetration of blue light in the experiments reported here might regulate photosynthesis in N. gaditana, and be responsible for the improved specific growth rate under filtered light.
3.2. Effect of Filtered Light on Lipid Accumulation
Lipids in N. gaditana were analyzed by GC, after extraction and conversion to FAMEs. The FAME yield under filtered light was greater than under white light (19.18% vs. 23.52%, p < 0.05) (Table 1). The FAME productivity was also greater under filtered light, but the difference was not statistically significant (25.20 mg L−1 d−1 vs. 14.00 mg L−1 d−1, p > 0.05)
Growth of cells under filtered light also led to several significant changes in FAME composition (Table 2). In particular, growth under filtered light significantly increased the amount of palmitic acid (C16:0), and significantly decreased the amounts of eicosa pentaenoic acid (EPA, C20:5n3) and tetradecanoic acid (C14:0). As a result, the proportion of saturated fatty acids (SFAs) increased and the proportion of PUFAs decreased, although there was no significant change in the proportion of MUFAs (Fig. 3). Similar results were reported in previous studies of N. gaditana [16] and other Nannochloropsis sp. exposed to red light [31] under photoautotrophic conditions.

FAME Composition and Degree of Unsaturation (DU) after Growth of N. gaditana Grown under White Light and Filtered Light for 9 Days. Each Value Is the Mean of 9 Replicates (Three Biological Replicates × Three Technical Replicates) with Standard Deviation. DU Was Calculated As Described in the Methods (Eq. (1)) (*p < 0.05, **p < 0.01, ***p < 0.001).
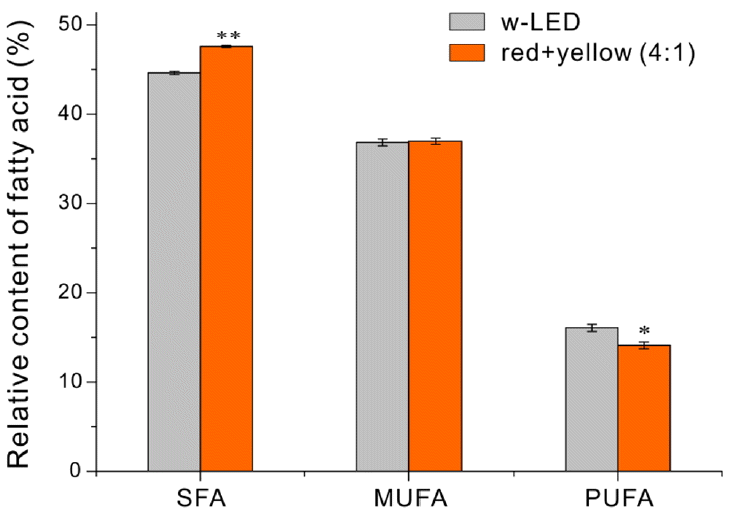
Percentages of saturated (SFAs), monounsaturated (MUFAs), and polyunsaturated (PUFAs) fatty acids. Each value is the mean of six replicates (three biological replicates × three technical replicates) with standard deviation. Significant differences were determined by t-test and indicated by asterisks (*p < 0.05, **p < 0.01).
The changes in FAME composition directly alter the DU, which is a function of the proportions of SFAs, MUFAs, and PUFAs. The DU correlates negatively with cetane number (CN) and positively with iodine value (IV) [29, 33]. CN affects various engine performance parameters like combustion quality and ignition delay [34], even emission of NOx [35]. IV reflects total unsaturation in biodiesel mixture and generally affects oxidation stability of biodiesel [33]. Thus, a higher CN value and a lower IV value imply better quality of biodiesel, which shows shortened ignition delay, lower emission of NOx and higher oxidation stability. N. gaditana cultivated under filtered light had a lower DU (Table 2). The present study using tar color additives did not show as much significant decrease of DU value as previous study that N. gaditana was cultured under the red LED illumination [16]. However, an optimal use such as different composition and/or concentration of color additives could improve a better microalgae-based biodiesel property.
In conclusion, the results presented here indicate that use of an inexpensive pigment solution can mimic the effect of different colored LEDs in increasing lipid content, lipid quality, and biomass productivity of N. gaditana. This strategy of light filtering using different pigments may also improve biodiesel productivity and quality of other microalgal species.
4. Conclusions
This study investigated the effect of using a simple and inexpensive light filter, based on tar color additives, on the photoautotrophic growth of N. gaditana. Relative to cells grown under white light, filtered light, obtained by a 4:1 mixture red and yellow pigments, improved biomass productivity by 44.6%, improved FAME yield by 22.6%, and improved biodiesel quality. Filtered light also led to 80.0% higher FAME productivity. These results imply that use of a simple light filter can enhance lipid productivity in N. gaditana. Further studies are needed to determine the optimal spectral composition for FAME production in N. gaditana and other microalgal species, and the applicability of this method in outdoor mass cultivation systems as a cost-effective replacement for artificial light sources.
Supplementary Material
Acknowledgments
This work was supported by Development Fund Foundation, Gyeongsang National University, 2015. This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (2015R1C1A 1A01054303) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2017R1A4A1015628).
