Efficient use of ferrate(VI) in the oxidative removal of potassium hydrogen phthalate from aqueous solutions
Article information
Abstract
The aim of this study is to assess the applicability of ferrate(VI) in the efficient treatment of aqueous waste contaminated with potassium hydrogen phthalate (KHP) which is known to be a potent endocrine disrupting chemicals. Simulated batch reactor operations were conducted at a wide range of pH (7.0 to 12.0) and molar ratios of KHP to ferrate(VI). Kinetic studies were performed in the degradation process and overall rate constant was found to be 83.40 L/mol/min at pH 8.0. The stoichiometry of ferrate(VI) and KHP was found to be 1:1. Further, lower pH values and higher KHP concentrations were favoured greatly the degradation of KHP by ferrate(VI). Total organic carbon analysis showed that partial mineralization of KHP was achieved. The presence of several background electrolytes were studied in the degradation of KHP by ferrate(VI).
1. Introduction
Water resources are continually and increasingly contaminated with domestic as well as industrial effluents. Introduction of emerging water pollutants, particularly a variety of micro-pollutants including endocrine disrupting chemicals (EDCs), pharmaceuticals and personal care products etc. are becoming more and more problematic due to its persistency/toxicity in aquatic environment. Potassium hydrogen phthalate (KHP) is an acidic salt compound and forms white powder, colourless crystals, a colourless solution, and an ionic solid. KHP is the simplest form of phthalate esters and having a benzene ring with two attached adjacent carboxylic groups (cf. Fig. 1). Phthalates are known as EDC because of their complex effects on several hormonal systems including the estrogen and androgen hormone systems. It is often used to make plastics soft and flexible. It is primarily employed in the production of polyvinyl chloride resins [1]. They are found in a wide variety of common products including plastics, cosmetics, pharmaceuticals, baby care products, building materials, modelling clay, automobiles, cleaning materials, insecticides, etc. Due to its wide applications in industry and large quantities of use, phthalates are recognized as a significant environmental contaminant and is commonly detected in aquatic environment.
Phthalates easily migrates from plastic materials to the environment and is readily distributed in water, soil, air, food and human organisms [2]. Phthalates are also found in ground, natural and drinking waters [3–5]. Surface water is polluted with phthalates mainly due to the effluents of plastics plants, or washed out from the plastics (bottles, packages, etc.) gathered on waste dumps which enters into the environment. Many countries have listed the permissible/allowable concentrations of some of the phthalates in the drinking water [2].
It was reported that some micro-pollutants are seemingly caused to show direct toxicity to certain aquatic organisms [6–9] and it accumulates slowly, and finally leads to irreversible changes on wildlife and human beings [10]. The micro-pollutants may cause several adverse environmental and human health effects even at very low concentration range i.e., from μg/L to ng/L level [11–12]. Therefore, these water pollutants are received a special interest for its effective and efficient removal, even at very low level, from aquatic environment.
Phthalates may induce proliferation, malignant invasion, and tumor formation in breast cancer cell lines that are low or lack hormone receptors. This indicates that at least some effects of these compounds are independent of their direct estrogenic or androgenic effects [13]. The endocrine-disrupting properties of this class of chemicals are well established in the off-spring of mother rats that are treated with phthalates during pregnancy. Phthalates greatly disrupt the development and functioning of male and female reproductive systems as interfered the production of testosterone and estradiol, respectively [14–15]. Exposure of human mothers to phthalates, analysed their urine samples, is associated with shortened anogenital distance in their new born sons-a measure of feminization of external genitalia [16]. Similarly, very young rats show an increased cellular proliferation in the terminal end buds of mammary tissue once exposed to the phthalates. Phthalate causes the activity of mammary-cell genes that are involved in the regulation of cell differentiation and proliferation, as well as cell-to-cell communication [17].
The existing water treatment technology is not sufficient to treat many of the EDCs from aqueous solutions. In view of this, it is necessary to identify an efficient treatment process for the effective degradation of pollutants from contaminated waters and wastewaters. Therefore, the low level removal of EDCs from the wastewater is a greater need to reduce the potential risk caused due to the presence of EDCs. Ferrate(VI) is a promising oxidant over other commonly employed oxidants having the redox potential of 2.20 V and 0.72 V at pH 1.0 and 14.0, respectively. Relatively high redox potential makes this chemical a useful oxidizing agent which show potential applicability in the treatment of wastewaters [18–21]. Several studies stated the applicability of ferrate(VI) in the treatment of several emerging contaminants including the EDCs or pharmaceuticals from aqueous solutions [22–27]. These studies show the potential use of ferrate(VI) in the possible degradation of water pollutants including the EDCs or pharmaceuticals. Moreover, our previous studies also showed potential application of ferrate(VI) in the treatment of several metal(II)-complexed species from aqueous solutions. Multifunctional use of ferrate(VI) enabled to decomplex and degrade the organic pollutant and simultaneously may remove the free metals by coagulation efficiently [28–34]. The present communication deals further the application of ferrate(VI) in the degradation of an important micro-pollutant viz., potassium hydrogen phthalate from aqueous solutions. The study is conducted for various physico-chemical parameters as to obtain the mechanism and kinetics of phthalates degradation by ferrate( VI). Also the mineralization of phthalate was assessed by the total organic carbon measurements.
2. Material and Methods
2.1. Materials
Potassium hydrogen phthalate monobasic salt (C8H5KO4), iron(III) nitrate nonahydrate (Fe(NO3)3.9H2O), diethyl ether (C4H10O), hexane (C6H14) are obtained from Sigma Aldrich Co., USA. Potassium hydroxide (KOH), hydrochloric acid (HCl), phosphoric acid (H3PO4) is obtained from Merck India Ltd., India. Disodium tetraborate decahydrate (Na2B4O7.10H2O), disodium hydrogen phosphate (Na2HPO4) is obtained from Himedia India Ltd., India. Purified sodium hypochlorite (NaClO) is procured from Palanad Enterprises, Nagpur, India. Purified water (10–15 MΩ cm) is obtained from Millipore Water Purification system (Model: Elix 3).
Glass filtration system and fritted funnel (10–15 μm) is obtained from Merck, India Ltd., India. Whatman Filter Papers (GF/C grade, 47 mm) are used for filtration during ferrate(VI) preparation. Syringe filter of 25 mm diameter in size and porosity of 0.45 μm is obtained from Whatman, USA.
2.2. Methods
2.2.1. Preparation of potassium ferrate(VI)
Ferrate(VI) is synthesized in its potassium salt by the wet chemical oxidation process followed the previously described method [31–34]. The purity of the synthesized potassium ferrate was found to be >95% as measured by the known spectrophotometric method [21]. The product was carefully kept in a vacuum desiccator contained with excess of potassium hydroxide pellets.
2.2.2. Batch reactor method
1.0 mmol/L of KHP stock solution is prepared by dissolving an appropriate amount of KHP in the phosphate/borate (0.01 mol/L) buffer solutions. The pH is adjusted by the drop-wise addition of phosphoric acid/sodium hydroxide (0.1 mol/L) solutions. The required concentration of KHP is obtained by the successive dilution of stock solutions. Batch experiments are conducted by taking varied concentrations of KHP solutions (0.03 to 0.5 mmol/L; 100 mL each) at varied but constant pH values (pH 7.0 to 12.0) in a small reactor vessel and then a constant amount of solid potassium ferrate (0.1 mmol/L) is introduced in the reactor vessel under stirred condition. The decomposition of ferrate(VI) is recorded which indicates the degradation of the KHP. Once after introducing the ferrate(VI), immediately the solution is subjected to measure the absorbance of the solution at 510 nm at regular interval using the UV-Vis Spectrophotometer (UV-Visible Spectrophotometer: Thermo Electron Corporation, England; Model: Thermo Spectronic UVI). For blank experiments, having the same pH and having the same buffer solutions a blank of ferrate(VI) decomposition (in absence of KHP) is recorded by observing the change in absorbance recorded at 510 nm for an identical time intervals. A necessary absorbance correction is conducted to nullify the self-degradation of ferrate(VI) using the blank absorbance data. The samples are kept in the reactor for another 2 h under the stirred conditions. It is then filtered using the syringe filter (0.45 μm) and the solution was divided into two parts. One part is subjected for the HPLC (high performance liquid chromatography) measurements and the other part for the TOC (total organic carbon analyzer) (Shimadzu, Japan; Model: TOC-VCPH/CPN) analysis.
The HPLC measurements are performed using the Waters HPLC instrument (Waters 515 HPLC Pump) equipped with the Waters 2489 UV/Visible detector and having C18, 5 μm (4.6 × 250 mm) column. 20 μL sample is injected and the flow rate is maintained at 0.5 mL/min. The acetonitrile:water (70:30) is used as mobile phase and the absorbance is recorded at 230 nm. The peak area calculation is adopted for concentration of KHP determination. The standard solutions of KHP are prepared dissolving appropriate amount of KHP in the HPLC grade water (Merck, India). Further, the area of peak calculation is used to obtain the percentage removal of KHP. The results are then presented as percentage of KHP removed as a function of KHP concentration and pH.
The total organic carbon (TOC) measurement is converted into the percentage removal using the initial TOC value of KHP. The total organic carbon value is an indicative of the mineralization of the KHP from aqueous solution. Therefore, the percentage mineralization of KHP is obtained and presented as a function of KHP concentration and pH. Moreover, the difference in TOC values i.e., the initial and ferrate(VI) treated samples is utilized in the calculation of amount of KHP removed and shown as a function of pH and pollutant initial concentration.
The degradation of KHP by ferrate(VI) is also assessed in presence of several background electrolyte concentrations viz., Na2HPO4, Na2SO4, NaNO3, NaCl, NaNO2 and Na2SO3. The concentration of background electrolytes, KHP and ferrate(VI) are taken as 0.1 mmol/L and the samples are treated at pH 8.0. The solutions are stirred for 2 h and then filtered using the syringe filter (0.45 μm) and subjected for the KHP concentration measurements using the HPLC.
3. Results and Discussion
3.1. Effect of KHP Concentration
The KHP solution having the concentrations of 0.03 mmol/L to 0.5 mmol/L was treated with ferrate (0.1 mmol/L) at a constant pH 8.0. The concentrations of KHP was varied in such a way so that a wide range of ferrate(VI) to KHP molar ratio was obtained (i.e., from 1:0.3 to 1:5). The time dependence degradation of ferrate(VI) in presence of KHP was observed by monitoring the change in concentration of ferrate(VI) using the UV-Visible spectrophotometer. The results were presented in Fig. 2. It is evident from the figure that increasing the concentration of KHP the reduction of ferrate(VI) was increased sharply. Therefore, the degradation of the pollutant was increased significantly with the increase of pollutant concentration. Further, it was observed that during the initial period the degradation of ferrate(VI) was relatively fast which attained almost a constant value in the later stage of time i.e., beyond 10 min of contact. These results showed that a maximum degradation of KHP was achieved during the initial period of contact. Therefore, ferrate(VI) was found to be efficient oxidant at least in the degradation of KHP from aqueous solutions. Further, quantitatively, increasing the contact time from 0 to 19 min the amount of ferrate(VI) was decreased from 0.10 to 0.087 mmol/L for the 1:1 molar ratios of ferrate(VI) to KHP.
3.2. Kinetics of KHP Degradation
The kinetics of KHP degradation by ferrate(VI) was performed using the time dependence data obtained for the change in ferrate( VI) concentration at pH 8.0 having varied concentration of KHP. The rate of ferrate(VI) reduction in presence of KHP is presented as Eq. (1):
where kapp is eventually an apparent rate constant in the degradation of ferrate(VI) in presence of KHP. Further, the Eq. (1) could be reduced to Eq. (2) at varied concentrations of KHP and at constant pH:
where,
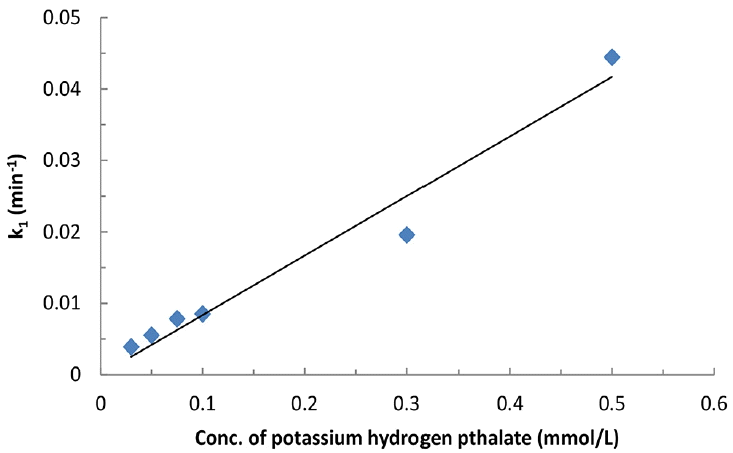
The time dependence change in ferrate(VI) concentration (i.e., from Fig. 3) is utilized to fit into the pseudo-first-order rate and pseudo-second-order rate equations to estimate the value of ‘m’ either one or two at pH 8.0. The kinetic data is suitably fitted well to the pseudo-first order rate kinetics (cf. Fig. 3). This clearly inferred that the value of ‘m’ is 1 with respect to each concentration of KHP. The pseudo-first order rate constant values at pH 8.0 was obtained and included in Table 1 with the regression coefficient (R2). Table 1 further indicated that increasing the concentration of KHP (from 0.03 to 0.5 mmol/L) apparently caused to an increase in pseudo-first order rate constant values. This was possibly due to the fairly high oxidizing capacity of ferrate(VI) towards the pollutants that enabled it faster oxidation of the pollutant species in aqueous solutions [34–35].
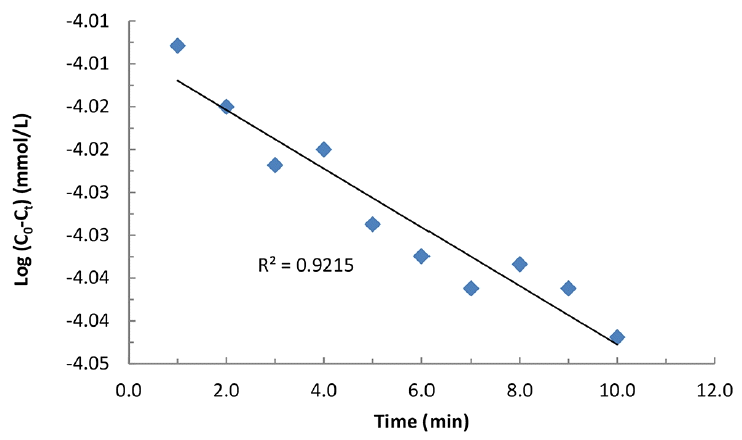
Plot of Log(C0-Ct) against time ‘t’ in the degradation of KHP by ferrate(VI) [pH: 8.0; [KHP]: 0.10 mmol/L; [Ferrate(VI)]: 0.10 mmol/L and C0 and Ct are the concentration of ferrate(VI) at time ‘0’ and ‘t’ mins, respectively]
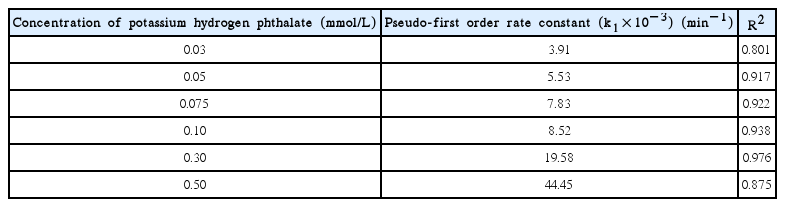
Pseudo-first Order Rate Constant Values Obtained for the Degradation of Ferrate(VI) in Presence of Potassium Hydrogen Phthalate at pH 8.0
The pseudo-first order rate constant values obtained at varied concentrations of KHP at pH 8.0 (cf. Table 1) is then utilized to obtain the value of ‘n’ hence, the stoichiometry of ferrate(VI) and KHP along with the apparent rate constant value kapp. A plot is drawn between the KHP concentrations against the ‘k1’ values and is illustrated in Fig. 4. The results clearly demonstrated that reasonably a good linearly was obtained between the concentration of KHP against the k1 values at pH 8.0 (cf. Fig. 3). Hence, this indicated that the value of ‘n’ is ‘1’. These results therefore, concludes that the stoichiometric ratio of the ferrate(VI) and KHP is 1:1. Moreover, the apparent or overall rate constant kapp is found to be 83.40 L/mol/min (R2 = 0.964). Relatively high value of kapp indicates that the ferrate(VI) is efficient in the degradation of KHP from aqueous solutions [36–37].
3.3. Effect of pH and Concentration of KHP in the Degradation of KHP with Ferrate(VI)
The pH dependence study is a useful parameter in order to optimize the selectivity of solution pH to degrade the pollutant from aqueous solutions. Additionally, it provides the mechanism involved in the degradation of pollutant by ferrate(VI). The degradation of KHP was further studied using the HPLC measurements and the results are illustrated in Fig. 5 as percentage removal of KHP against the pH and concentration of KHP. It is observed, in general, that increasing the concentration of KHP i.e., 0.03 to 0.5 mmol/L has caused to decrease in percentage removal of KHP at a constant concentration of ferrate(VI). More quantitatively, increasing the concentration of KHP from 0.03 to 0.5 mmol/L, the corresponding decrease in percentage removal of KHP was found to be from 76.13 to 16.03%, respectively at pH 7.0 and at ferrate(VI) concentration of 0.1 mmol/L. This decrease in percentage removal is due to the fact that at lower concentration of KHP relatively more number of ferrate(VI) molecules are present to degrade the lesser number of KHP molecules. However, increasing the concentration of KHP an apparent increase in content of KHP removal is obtained. This indicated that although at lower concentration the percentage removal of KHP is higher however, the increase in KHP concentration favoured the content removal of KHP at a constant concentration of ferrate(VI).
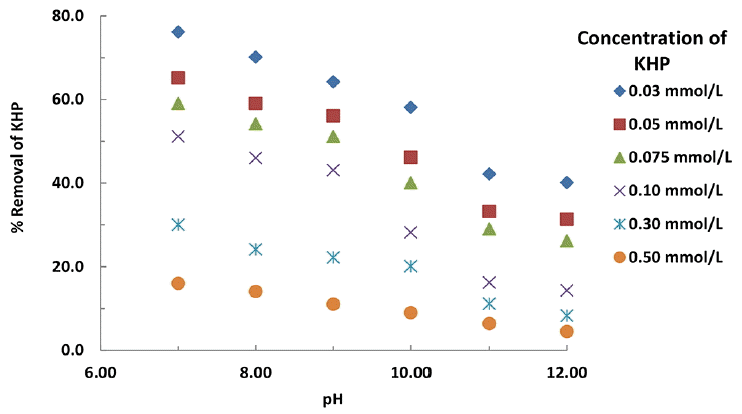
Percentage removal of potassium hydrogen phthalate as a function of solution pH and pollutant concentrations using a constant dose of ferrate(VI): 0.1 mmol/L.
On the other hand the decrease in pH from pH 12.0 to 7.0 enabled to increase significantly the percentage removal of KHP from aqueous solutions (Fig. 5). Quantitatively, decreasing the pH from 12.0 to 7.0 the corresponding percentage degradation of KHP is increased from 14.35 to 51.16% at the ferrate(VI) to KHP molar ratio 1:1 (Fig. 5). The speciation studies showed that at around pH 7.0 the protonated species of the ferrate(VI) i.e., HFeO4− dominated since the pka value for the acid dissociation of HFeO4− is reported to be 7.3 [38]:
The reactivity of protonated species is significantly high since the protonated species possesses larger spin density [34, 39, 40]. Moreover, the alkyl groups are found to be electron releasing groups, hence enhances the reactivity of protonated species HFeO4− in aqueous solutions [19]. Similarly, the redox potential of ferrate(VI) gradually increases with the decrease in pH which again enhances the reactivity of the ferrate(VI) at lower pH conditions. Previously, it was reported that 4-chlorophenol was degraded maximum at pH 9.0 whereas relatively less degradation was achieved at pH 11 and 7.0. This was explained with the fact that at this pH phenol is predominantly present in the deprotonated species which is readily attacked by the ferrate(VI) and led to the formation of phenoxy radicals [41]. Similarly, the higher degradation was reported with the protonated species of ferrate (HFeO4−) than the deprotonated species (FeO42−) in the degradation of various steroid estrogen from aqueous solutions [33]. In a line, it was reported that the dissociated species of ciprofloxacin was relatively stable whereas the un-dissociated species of ciprofloxacin was unstable. Therefore, the degradation of dissociated species by ferrate(VI) was found lesser than the un-dissociated ciprofloxacin [42].
3.4. Mineralization of KHP
Further, it is interesting to obtain the mineralization of KHP after the ferrate(VI) treatment. The samples of KHP having varied concentrations (0.03 to 0.5 mmol/L) were treated with a constant dose of ferrate(VI) (0.1 mmol/L) for a period of 2 h at constant but varied pH conditions (pH 7.0 to 12.0). The samples were then subjected for the estimation of TOC values. The untreated samples of KHP were also employed to measure the initial TOC values. Based on the TOC measurements the percentage TOC removal of the KHP was obtained and presented as a function of pollutant concentration and solution pH in Fig. 6(a). It is evident from the Fig. 6(a) that the decrease in KHP concentration and pH greatly favoured the percentage removal of TOC. Quantitatively, decreasing the KHP concentration from 0.5 to 0.03 mmol/L was caused to increase the TOC removal from 26.80 to 51.96% as observed at pH 7.0. Similarly, decreasing the solution pH from 12.0 to 7.0 the corresponding increase in percentage TOC removal was found to be 12.38 to 30.53% at the 1:1 molar ratio of the ferrate(VI) to KHP. The trend of this result was in a line to the HPLC results obtained previously. It was also evident from the TOC data that although a partial mineralization of this micro-pollutant was obtained but a significant percentage of the KHP was mineralized at a single dose of ferrate(VI) 0.1 mmol/L.

(a) Percentage removal; and (b) extent removal of potassium hydrogen phthalate (KHP) as a function of pH and KHP concentrations at a constant concentration of ferrate(VI): 0.1 mmol/L.
On the other hand, the data was further presented in terms of total amount of the KHP mineralized by the ferrate(VI) treatment and were presented as a function of KHP initial concentration and solution pH (cf. Fig. 6(b)). It is interesting to observe that increasing the initial concentration of KHP apparently caused to increase the content removal of KHP from aqueous solutions. Similarly, decrease in pH (i.e., from pH 12.0 to 7.0) also favoured the content removal of KHP.
3.5. Effect of Co-existing Ions
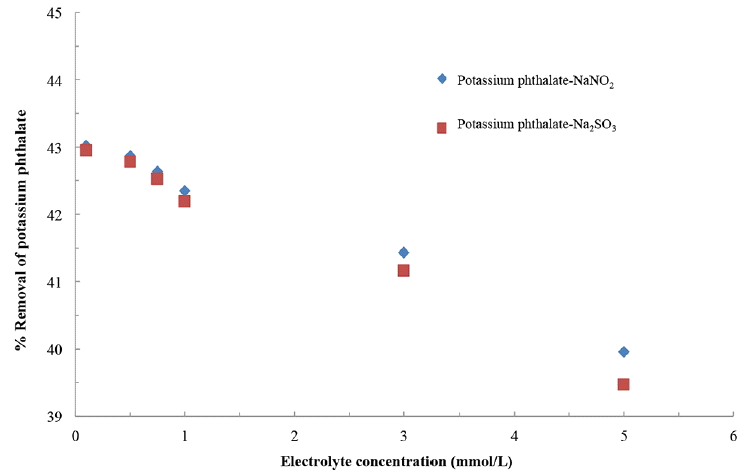
The presence of co-exiting ions in the oxidative removal of KHP (0.1 mmol/L) was studied using the ferrate(VI) dose 0.1 mmol/L at constant initial pH 8.0. The Na2HPO4, Na2SO4, NaNO3, NaCl, NaNO2 and Na2SO3 are used as co-existing ions having the concentration of 0.1 mmol/L of each. Variety of electrolytes was used having partly or fully oxidized ions. The results are illustrated in Fig. 7. Results indicated that the presence of fully oxidized electrolytes viz., NaCl, NaNO3 and Na2HPO4 could not affect significantly the oxidation of KHP by ferrate(VI). However, on the other hand the partly oxidized NaNO2 and Na2SO3 electrolytes are caused significantly less percentage decomposition of KHP from aqueous solutions. The results inferred that the presence of NO2− and SO32− greatly affect the degradation of KHP by the ferrate(VI). Previously, it was reported that the presence of NO2− and SO3− greatly affected the degradation of the Zn(II)-NTA complex by the ferrate(VI) since the ferrate(VI) decomposition was increased significantly in presence of these electrolytes [32]. Further, the extent of oxidation of KHP by the ferrate(VI) was studied increasing the NaNO2 or Na2SO3 concentration from 0.1 to 5.0 mmol/L at a constant KHP and ferrate(VI) concentration of 1.0 mmol/L and at pH 8.0. The results are returned graphically in Fig. 8. It is noted that increasing the background concentration from 0.1 to 5.0 mmol/L NaNO2 the corresponding decrease in percentage removal of KHP was from 43.02 to 39.96% (i.e., 3.06% only). Almost similar decrease was observed for the Na2SO3 i.e., from 42.95 to 39.47% (i.e., 3.48% only). The insignificant decrease in percentage removal of KHP even with increasing the partially oxidized electrolytes viz., NaNO2 and Na2SO3 pointed the affinity and potential application of ferrate(VI), at least, in the degradation of KHP from aqueous solutions.
4. Conclusions
High purity ferrate(VI) was synthesized and employed in the treatment of KHP from aqueous solutions under the simulated batch reactor operations. The treatment of KHP by ferrate was conducted at a wide concentration range of KHP i.e., from 0.03 to 0.5 mmol/L and solution pH from pH 7.0 to 12.0 having a constant concentration of ferrate(VI) 0.10 mmol/L. The increase in KHP concentration and decrease in pH greatly favoured the removal of KHP from aqueous solutions. The HPLC data showed that very high percentage removal of KHP was achieved using ferrate(VI) using a single dose of 0.10 mmol/L. The kinetic studies performed at pH 8.0 indicated that the pseudo-first order rate kinetics was followed to each reactants and showed 1:1 stoichiometry of degradation for KHP and ferrate(VI). Moreover, the overall rate constant was found to be 83.40 L/mol/min. Further, partial but significant percentage TOC removal was obtained by the ferrate(VI) treatment which indicated that ferrate( VI) could mineralize significantly the KHP from aqueous solutions. Further, the TOC removal was increased by decreasing the pollutant concentration and pH. However, the content of pollutant mineralization was increased with the increase in initial KHP concentration. The presence of co-existing electrolytes viz., NaNO3, NaCl, NaSO4 and Na2HPO4 could not affect the degradation of KHP by ferrate(VI). But the presence of NO2− and SO32−affected the degradation of KHP by ferrate(VI). Overall, ferrate(VI) is a promising, safe and viable oxidant could be employed in the treatment of wastewater contaminated with KHP.