Effects of titanium oxide nanoparticles on Oryzias latipes embryos and sac-fry under different irradiation conditions
Article information
Abstract
Some phototoxicity of titanium dioxide nanoparticles (TiO2 NPs) has been reported in recent years in studies with fish embryos or larvae. However, it is necessary to focus on the potential effects of embryonic exposure due to irreversible abnormalities and mortalities observed in sac-fry, and to expand various fish embryos to generate multiple test species. The aim of this study was to evaluate the effects of TiO2 NPs under different irradiation conditions in exposed Oryzias latipes (O. latipes) at the embryonic and sac-fry stages. The effects of different irradiation conditions were observed using ultra-violet (UV) and visible light, and the corresponding effects were monitored by determining cumulative mortality and abnormality. O. latipes were exposed for 8 d to 0, 1, 5, 10, or 50 mg/L TiO2 NPs under UV (4,818.86 mW/m2 at the bottom of clear vials) or visible light, after which the embryos were transferred to NP-free embryo-rearing solution until 16 days post fertilization (dpf). Abnormalities of embryos and sac-fry increased at high TiO2 NP concentrations under UV irradiation, compared to control samples treated with visible light or UV irradiation alone. This work provides information regarding the phototoxicity of TiO2 NPs using O. latipes at the embryonic and sac-fry stages.
1. Introduction
Titanium dioxide nanoparticles (TiO2 NPs) have been used in beauty products, hygienic goods, and textiles [1–3]. Boxall et al. [2] presented modeling data used to estimate the TiO2 NP concentration in water as 0.245 mg/L, based on a 100% market-penetration scenario of paints and sunscreen, particularly paints containing 5 mg/g TiO2 NPs and sunscreen containing 50 mg/g TiO2 NPs. TiO2 NPs tend to become rapidly distributed throughout the environment because of their small sizes and non-biodegradability [3], and the potential exposure to bio-receptors increases as a consequence.
Several studies have been conducted in recent years to assess the toxicity of TiO2 NPs. Because the photoreactivity of TiO2 NPs can cause differing toxicities based on the bio-receptors that are activated [1, 4–5], the phototoxicities of TiO2 NPs have been tested in various organisms, including fish [1, 4, 6–11], waterfleas [7, 9], algae [12], and bacteria [13]. The phototoxicity of TiO2 NPs induced increased mortality of Daphnia magna [7, 9] and decreased colony formation of Escherichia coli and Bacillus subtilis [13]. Evidence suggests that TiO2 NPs in surface water can readily absorb ultra-violet (UV) irradiation and that the absorbed energy can generate an excited state by electron-hole pair production on the surface of particles [4, 7, 8, 12].
Some authors have reported the phototoxicity of TiO2 NPs using fish embryos or larvae [1, 4, 6–7, 11]. These studies were focused on the effects of Danio rerio (D. rerio) embryos [1, 4, 11, 14] and larvae [11, 14], Oryzias latipes (O. latipes) larvae [7, 9], and Piaractus mesopotamicus juveniles [6]. Although Ma et al. [14] presented evidence to suggest that yolk-sac larvae were most sensitive to the phototoxicity of TiO2 NPs, it is still necessary to study their effects on potential embryonic exposure due to irreversible abnormalities and mortalities observed in sac-fry [15–16]. However, limited phototoxicity data exist regarding the effects of TiO2 NPs on fish embryos, and such studies have mainly focused on D. rerio. Therefore, it is necessary to expand various fish embryos for use as multiple test species in order to assess the phototoxicity of TiO2 NPs in water. O. latipes is recommended as a test fish species by Organization for Economic Co-operation and Development (OECD) No. 212 [17].
The aim of this study was to evaluate the effects of TiO2 NPs under different irradiation conditions in exposed O. latipes at the embryonic and sac-fry stages. The effects of different irradiation levels were observed using UV and visible light, and the corresponding effects were monitored by measuring cumulative mortality and abnormality. O. latipes was chosen as the test species due to the lack of available phototoxicity data. To the best of our knowledge, this is the first study to evaluate phototoxicity of TiO2 NPs using O. latipes embryos.
2. Materials and Methods
2.1. Characterization and Preparation of Nanoparticles
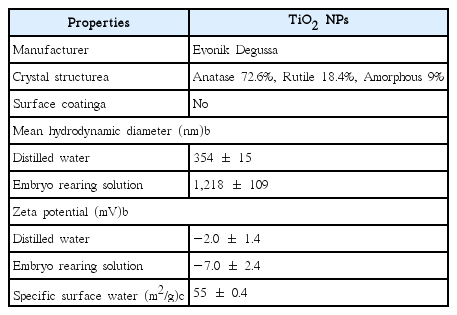
TiO2 NPs (particle size 21 nm, P25, white powder form) were purchased from Evonik Degussa (Germany). A field emission transmission electron microscope (FE-TEM, JEM2200FS, JEOL, Japan) was used to observe the morphology of the NPs. Specific surface area of the TiO2 NPs was analyzed by particle size analyzer (UPA-150, Microtrac, USA). An electrophoretic light scattering spectrophotometer (ELS-8000, Otuska Electronics Co., Japan) was used to measure particle size distributions of hydrodynamic diameters and zeta potential of 10 mg/L TiO2 NPs dispersed in deionized water (DW) and embryonic rearing solution (ERS). UV absorption spectra of 10 mg/L TiO2 NPs suspended in ERS was measured by a UV/Vis spectrophotometer (Libra S32 PC, Biochrom Ltd., Cambridge, UK) at 10-min intervals for 1 h, to verify aggregation of TiO2 NPs. ERS included NaCl (1 g/L), KCl (0.03 g/L), CaCl2·2H2O (0.04 g/L), MgSO4·7H2O (0.163 g/L), and methylene blue (1.0 mg/L) to prevent fungal growth [18]. When UV spectra analysis, Methylene blue was eliminated from the ERS due to photo-degradation [18]. A stock solution of 100 mg/L TiO2 NPs was prepared by dispersing the NPs in ERS with sonication for 10 min in a water bath sonicator (40 kHz frequency, Powersonic 420, Hwashin Technology, Korea). A series of exposure concentrations of TiO2 NPs (0, 1, 5, 10, and 50 mg/L) were diluted with ERS.
2.2. Test Species and Pre-culture Conditions
O. latipes (Japanese medaka) was received from the National Academy of Agricultural Science (NAAS, Suwon, Korea). Adult orange-red type O. latipes for broodstock were kept in dechlorinated tap water (pH 7.0 ± 0.2; temp. 25 ± 2°C; light:dark = 16:8 h), with a diet of commercial Tetra Min (Tetra Werke, Germany) and brine shrimp (OSI PRO80TM, USA) twice per day. On the day of the experiment, newly fertilized and healthy eggs of O. latipes were collected from the broodstock.
2.3. Phototoxicity Assay Using Fish Embryo and Sac-fry
The fish embryo and sac-fry assay was performed based on the OECD guidelines (No. 212) for chemical testing [10, 14, 15, 19]. Embryos (9–10 stages) were transferred into 2 mL clear vials with screw caps (Wheaton), one embryo per vial and one mL exposure solution per vial, and there were 10–15 replicates prepared. All experiments were repeated 1 to 4 times for treatments and 3 to 4 times for controls. The capped vials were placed upside down in a photoreactor (model LZC-4, Luzchem Research Inc., Ottawa, ON, Canada) under a UV lamp for UV irradiation and in the laboratory under standard fluorescent lamps for visible light irradiation, before hatching. The photoreactor has one UV lamp (spectral distribution of 316 nm to 400 nm), positioned 18 cm above the test units. The light intensity was measured by a spectroradiometer (SPR 4001, Luzchem Research Inc., Ottawa, ON, Canada) and its values were 6,198.03 mW/m2 at the top of the clear vials and 4,818.86 mW/m2 at the bottom of the clear vials. At 8 days post fertilization (8 dpf, when the control embryos hatched), exposure solutions were changed with NP-free embryo-rearing solution. UV irradiated- or visible light irradiated-vials were maintained in the incubator under pre-culture conditions (without oxygen and food supply in both the control and exposure groups). Fish embryos and sac-fry were observed daily under a dissection microscope based on cumulative mortality and various abnormalities; abnormal embryos and sac-fry: Heart damage (e.g., tube heart, blood circulation disorder, pericardial necrosis, pericardial edema, and hemostasis), whole body necrosis, malformation of the pericardium and peritoneum, gallbladder edema, small eyes, tail deformity, swim bladder deformity (e.g., undeveloped, over-inflated, and deflated swim bladder), and erratic swimming activity. The dead embryos or sac-fry were immediately eliminated from the test units. The detailed experimental design of fish embryo and sac-fry assay is shown in Fig. 1. The effect of Ti ions was not measured because TiO2 NPs are insoluble in water [3].
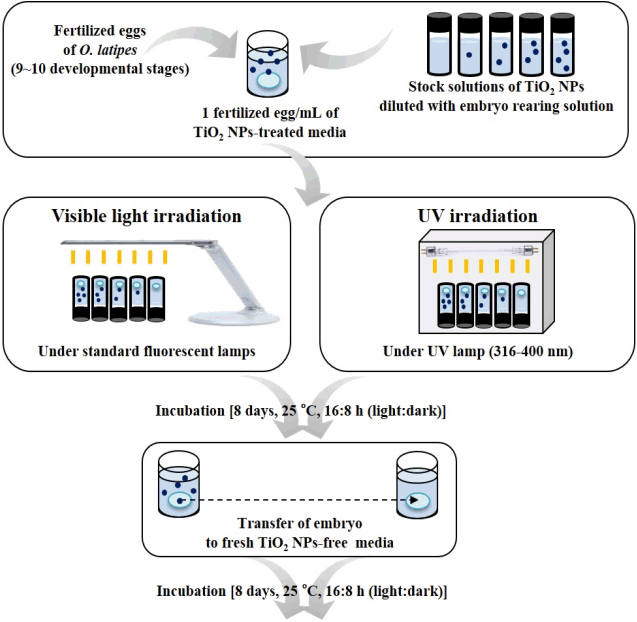
Experimental study design. Fertilized eggs of Oryzias latipes were exposed to TiO2 NPs in embryo rearing solution until 8 days post fertilization (dpf), under visible light or UV irradiation. At 8 dpf, the exposure solution was replaced with TiO2 NP-free embryo rearing solution. All tests were assessed with respect to cumulative mortality and abnormalities of embryos and sac-fry until 16 dpf (modified from Shin et al. [10, 15]).
2.4. Statistical Analysis
The results were reported as the sum of cumulative mortalities and abnormalities of embryos and sac-fry. If abnormal embryo was counted, sac-fry developed from an abnormal embryo was excluded from the calculation (even if the abnormal sac-fry manifested). The data were presented as mean ± standard error. Means were compared using one-way analysis of variance (ANOVA) (Origin 8, OriginLab Corp.). Multiple comparisons were conducted using Fisher test and differences were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Physicochemical Characterization and Dissolution of TiO2 NPs
Fig. 2 shows physicochemical characterization of TiO2 NPs. Morphological TEM image of TiO2 NPs is shown in Fig. 2(a). Fig. 2(b) shows distributions of the hydrodynamic diameter of TiO2 NPs suspended in DW and ERS. According to Table 1, mean hydrodynamic diameter of TiO2 NPs dispersed in ERS was estimated to be 1,218 ± 109 nm, which is 3.4-fold greater than the TiO2 NPs dispersed in DW (354 ± 15 nm). The zeta potential of TiO2 NPs was estimated to be -7.0 ± 2.4 mV (in ERS) and -2.0 ± 1.4 mV (in DW). This indicates that TiO2 NPs are rapidly coagulated or flocculated in solution. The specific surface area of TiO2 NPs analyzed using the Brunauer-Emmett-Teller (BET) method was estimated to be 55 ± 0.4 m2/g. The UV absorption spectra of 10 mg/L TiO2 NPs suspended in ERS were determined at 10-min intervals for 1 h (Fig. 2(c)). TiO2 NPs absorbed UVA light (320–400 nm) and peaked at 330–340 nm for TiO2 NPs. The absorbance of TiO2 NPs decreased as a function of increased irradiation time. This is possibly due to aggregation of NPs. When TiO2 NPs were re-sonicated for 10 min, the absorbance of TiO2 NPs increased (data not shown). In a previous study, Lee and An [12] reported the absorbance of TiO2 NPs as a function of time until 1 h. They reported the absorbance of TiO2 dispersed in OECD algal medium decreased as a function of increased irradiation time, similar to the results obtained in this study.

Transmission electron microscope image (a), particle size distributions of hydrodynamic diameters (b), and absorption spectra (c) of TiO2 NPs.
3.2. Effects of TiO2 NPs on the Embryos and Sac-fry of O. latipes
Fig. 3–5 show embryos and sac-fry at 16 dpf of exposure to TiO2 NPs, under either visible light or UV irradiation. The abnormalities of embryos and sac-fry were increased at 10 and 50 mg/L NPs under UV irradiation, compared to the control under visible light irradiation or UV irradiation (p < 0.05) (Fig. 3). Under UV irradiation, compared to the control, at 50 mg/L TiO2 NPs, some TiO2 NP-treated embryos developed but exhibited abnormalities (p < 0.05). Tube heart, pericardial edema, and undeveloped or over-inflated swim bladder deformity were primarily induced as symptom of embryo and some exhibited hemostasis, malformation of pericardium, whole-body necrosis, pericardial edema, pericardial necrosis, and tail deformity (Fig. 4). In particular, the effect of TiO2 NPs during the embryonic stages of some fish (10% for 1 mg/L and 10% for 50 mg/L) died as sac-fry, and embryonic exposure generated irreversible developmental impairment. Under visible light irradiation, some TiO2 NP-treated embryos developed but exhibited abnormalities [e.g., deflated yolk, undeveloped or over-inflated swim bladder deformity, blood circulation disorder] at 50 mg/L TiO2 NPs. The abnormalities shown by sac-fry included undeveloped or over-inflated swim bladder deformity and hemostasis. However, there was no significant increase of abnormality under visible light irradiation, compared to control (p < 0.05). There was no significant difference of abnormality between UV and visible light at the same exposure concentrations in the range of tested exposure concentrations (p < 0.05). No significant mortality of TiO2 NP-treated sac-fry was observed under UV irradiation and visible light irradiation (p < 0.05) (Fig. 5).
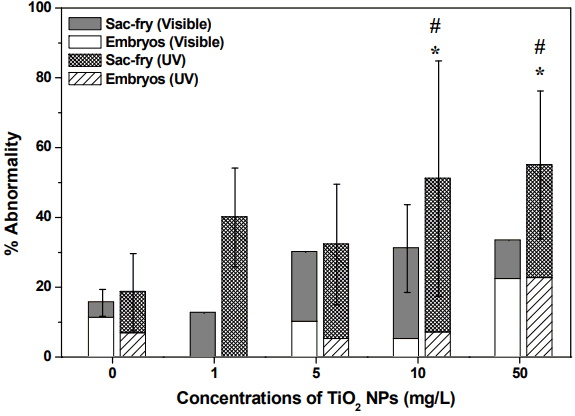
Cumulative abnormality of TiO2 NPs under visible light or UV irradiation on Oryzias latipes. Asterisks (*) indicate significant differences from controls under visible light irradiation (p < 0.05). Crosshatches (#) indicate significant differences from controls under UV light irradiation (p < 0.05).
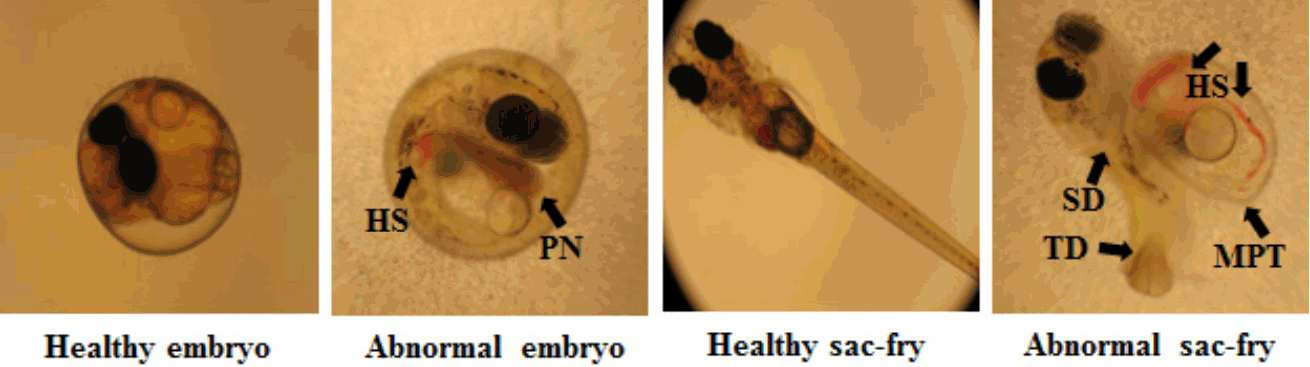
Morphological appearance of Oryzias latipes exposed to TiO2 NPs. HS: hemostasis, PN: pericardial necrosis, SD: swim bladder deformity, TD: tail deformity, and MPT: malformation of the pericardium and peritoneum.
3.3. Comparison of TiO2 NPs on the Embryos and Sac-fry of O. latipes under UV and Visible Light
We studied the phototoxicity of TiO2 NPs under UV and visible light using O. latipes embryos and sac-fry, and found that there are no significant differences at low exposure concentrations (0–50 mg/L). Likewise, Bar-Ilan et al. [4] studied the phototoxicity of anatase/rutile TiO2 NPs using zebrafish embryos. They reported that there are no significant differences in the survival of embryos under UV or visible light at TiO2 NP concentrations of 0, 1, and 10 mg/L. However, their significant differences of survival are observed at 100, 500, and 1,000 mg/L. Clemente et al. [1] studied the phototoxicity of anatase and anatase/rutile TiO2 NPs using zebrafish eggs and larvae. They reported that there are no significant differences in the survival of eggs and larvae under UV or visible light at TiO2 NP concentrations of 0, 1, and 10 mg/L. However, their significant differences of survival are observed at 100 mg/L. Meanwhile, Faria et al. [5] studied the phototoxicity of rutile and anatase/rutile TiO2 NPs using zebrafish embryos. They reported that rutile TiO2 NPs did not cause significant mortality at a concentration of 1 mg/mL under solar radiation. However, anatase/rutile TiO2 NPs caused significant mortality at the same concentration and radiation. Felix et al. [11] studied the phototoxicity of anatase/rutile and anatase poly (acrylic acid)-coated TiO2 NPs using zebrafish embryos and larvae. They reported that 10 mg/L uncoated TiO2 NPs induced hydroxyl radicals, production of lipid peroxidation, and increase of total glutathione level under UV illumination, unlike poly (acrylic acid)-coated TiO2 NPs, due to surface coating. It has been suggested that phototoxicity is due to the production of reaction oxygen species (ROS) that are generated by the exposure of TiO2 NPs to UV light and damage the receptors [4, 7–8, 12]. However, the results showed that lethal phototoxicity occurs only at relatively high exposure concentrations (100–1,000 mg/L).
4. Conclusions
This study showed that TiO2 NPs under UV or visible light cause abnormalities to O. latipes embryos and sac-fry at tested concentrations (10 and 50 mg/L), while the mortality of TiO2 NP-treated sac-fry was not significant under UV or visible light. Additionally, no significant differences were identified under UV or visible light at the same low exposure concentrations (0–50 mg/L) of bare TiO2 NPs. Therefore, further research is needed to assess the effects of higher exposure concentrations of TiO2 NPs than those tested in this study and the effects of different surface coating of nanoparticles. Further research is also needed to evaluate the toxicity of NPs in natural surface waters for investigating their potential photo-activation and risk involved in biotic partitioning of NPs.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2016R1A2B3010445). This work was supported by Korea Environment Industry & Technology Institute (KEITI) through “The Chemical Accident Prevention Technology Development Project”, funded by Korea Ministry of Environment(MOE) (No. 2016001970001). The authors thank the Korean Basic Science Institute (KBSI) for FE-TEM, particle size analyzer, and ELS analyses.