Efficient use of ferrate(VI) for the remediation of wastewater contaminated with metal complexes
Article information
Abstract
Remediation of wastewater contaminated with metal(II)-complexed species (Cu(II)-NTA (NTA: nitrilotriacetic acid), Cu(II)-EDTA (EDTA: ethylenediamine tetraacetic acid) and Cd(II)-EDTA is attempted using the potential applicability of ferrate(VI). Kinetics of pollutant degradation is obtained with the removal of ferrate(VI) studied at wide range of pH (8.0–10.0) and the concentration of metal(II)-complexed species (0.3 to 15.0 mmol/L) employing a constant dose of ferrate(VI) i.e., 1.0 mmol/L. Pseudo-first-order and pseudo-second-order rate constants were obtained in the reduction of ferrate(VI) which was then employed to obtain the overall rate constants of the pollutant degradation. The mineralization of NTA and EDTA was obtained with the change in TOC (total organic carbon) values collected by the ferrate(VI) treated pollutant samples. Decrease in pH and molar pollutant concentrations was greatly favored the percent mineralization of NTA or EDTA by the ferrate(VI) treatment. The treated pollutant samples were filtered and subjected for AAS (atomic absorption spectrophotometric) analysis to assess the simultaneous removal of copper and cadmium from aqueous solutions at the studied pH as well at the elevated pH 12.0. Results show that an enhanced removal of cadmium or copper was achieved at pH 12.0. Overall, ferrate(VI) possesses multifunctional application in wastewater treatment as it oxidizes the degradable impurities and removes metallic impurities by coagulation process.
1. Introduction
Metal-complexed species are widely employed for various industrial applications including metal finishing, painting, dying, photography, surface treatment, printed circuit board manufacturing etc. [1]. Mobility, bioavailability and toxicity of these pollutants are largely dependent to the speciation of these species present. It is further indicated that metal complexed species are highly mobile in aqueous solutions [2]. The presence of these metal complex species in aquatic environment creates a serious environmental concern since it get escapes from the wastewater treatment plants (WTP); hence poses serious issues relating to the biochemical hazards [3–5]. Several methods are demonstrated to remove these pollutants from aqueous solutions such as adsorption [6], Fe2+ replacement and precipitation [7, 8], ion exchange [9, 10], membrane filtration [11], electro-coagulation and deposition [12] etc. but are seemingly less or partial effective with these treatment processes.
Ethylenediamine tetraacetic acid (EDTA) is one of strong chelating agent forming relatively stable chelates with several metal cations including copper and cadmium. EDTA is widely employed in electrode-less copper-plating in the printed circuit boards (PCBs) [13–15], electroplating, paper manufacturing [16] and agricultural industries as a potential metal sequester [17, 18]. Similarly, nitrilotriacetic acid (NTA) is a class of synthetic aminopolycarboxylic acid (APCA) forming stable chelates with several metal cations which enables it to utilize in the industries like detergent builder where it chelates with magnesium and calcium ions and preventing the formation of scales. It is widely employed in the food industries, pharmaceuticals, cosmetic, metal finishing, photographic, textile, paper industries, nuclear decontamination etc. [19–22]. These industrial operations, therefore, poses a serious environmental threat due to the discharge of untreated or partially treated industrial wastes into the water bodies which ultimately contaminating the aquatic environment.
Higher oxidation state of iron i.e., ferrate(VI) is found to be a potential and useful oxidant. Ferrate(VI) possesses relatively higher redox potential as compared to commonly used oxidants hence; shows wide applications in the treatment of variety of water pollutants at low levels [23–29]. The use of ferrate(VI) in the wastewater treatment technologies shows multifunctional applicability since during initial stage of treatment, ferrate(VI) serves to oxidize the degradable impurities and in the latter stages the reduced ferric(III) coagulates or even adsorbs the non-degradable metallic impurities. Additionally, ferrate(VI) is also reported to be an efficient disinfectant [23, 26, 28, 29].
These promising properties of ferrate(VI) enables the chemical to degrade/or decomplex the metal complexed species with simultaneous removal of free metals from aqueous solutions [30–34]. It is successfully employed in the treatment of electroplating wastes contained with Cu(II) and Ni(II) complexed cyanides [34]. The polycarboxylic acid complexes viz., Zn(II)-NTA, Cd(II)-NTA are treated with ferrate(VI) and shows that NTA is degraded significantly with simultaneous removal of Zn(II) or Cd(II) from aqueous solutions. Further, the presence of several background electrolyte concentrations is studied in the treatment [35, 36]. The study is extended to assess the suitability of ferrate(VI) in the treatment of Cu(II)- iminodiacetic acid (IDA) or NTA or EDTA and ethylenediaminediacetic acid (EDDA) and the order of the oxidation is as: Cu(II)-EDDA > Cu(II)-IDA >> Cu(II)-NTA~Cu(II)-EDTA which is in accordance to their respective stability constant values [37]. The sulphide mine tailings having metal complexed sulphides are treated with ferrate(VI) and results show that the sulphide is oxidized rapidly to sulphate [38]. A similar simulated study is performed in the metal-sulphide treatment [39] and shows that a fast oxidation is affected greatly in presence of NaNO2 and Na2SO3 background electrolytes. Recent studies enable to simulate the treatment of copper(II)-IDA and zinc(II)-IDA species as a function of pH, concentration of pollutants as studied under batch reactor operations [40]. Kinetics of ferrate(VI) reduction in the treatment of cadmium(II)-IDA and nickel(II)-IDA complexed species from aqueous solutions is demonstrated elsewhere [41]. The present investigation deals with the treatment of wastewaters contaminated with copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA metal complexed species using ferrate(VI) as an alternative safe and relatively green degradation process. The study is extended to discuss various physico-chemical parametric studies viz., the effect of pH and pollutant concentrations.
2. Materials and Methods
2.1. Materials
Nitrilotriacetic acid (C6H9NO6), iron(III) nitrate nanohydrate (Fe(NO3)3. 9H2O), diethyl ether (C4H10O) and hexane (C6H14) are procured from Sigma Aldrich. Co., USA. Disodium ethylenediamine tetraacetic acid (C10H14N2Na2O8·2H2O) and cadmium nitrate (Cd(NO3)2·4H2O) are obtained from Loba Chemie, Pvt. Ltd., India. Potassium hydroxide (KOH), copper sulphate pentahydrate (CuSO4 ·5H2O), hydrochloric acid (HCl), phosphoric acid (H3PO4) is procured from Merck India Ltd., India. Moreover, the disodium tetraborate decahydrate (Na2B4O7·10H2O), disodium hydrogen phosphate (Na2HPO4) is obtained from Himedia India Ltd., India. Purified sodium hypochlorite (NaClO) is obtained from Palanad Enterprises, Nagpur, India. Purified water (10–15 MΩ cm) is obtained from Millipore Water Purification system (Model: Elix 3).
Glass filtration system having fritted funnel (10–15μm) is obtained from Merck India Ltd., India. Whatman Filter Paper (GF/C grade, 47mm; USA) is used for filtration during ferrate(VI) preparation. Syringe filter 25 mm diameter having porosity of 0.47 μm is obtained from Whatman, USA. This is used for ferrate(VI) treated samples filtration. Electronic balance (BSA 224S-CW; Sartorius) is employed for weighing purpose. A pH-meter having glass and calomel electrode assembly (Cyberscan pH 310; EUTECH Instruments, pH/MV/°C/°F Data meter) is used for entire pH measurements in aqueous solutions.
Ferrate(VI) is synthesized in laboratory by wet chemical oxidation method as described elsewhere [34, 35, 42]. The ferrate(VI) was obtained to its potassium salt form (K2FeO4).The purity of the synthesized potassium ferrate(VI) was found to be Ca 95%. The product is carefully kept in a vacuum desiccator containing excess pellets of sodium hydroxide.
2.2. Batch Reactor Operations
UV-visible measurements, in particular the change in ferrate(VI) concentrations, along with the TOC (total organic carbon) analysis clearly demonstrate to observe the decomplexation or degradation of Cu(II)/Cd(II)-complexed species present in simulated waste-waters by ferrate(VI). Batch reactor operations are performed by treating different concentrations of the metal-complex species (0.5 to 15.0 mmol/L of copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA with a constant dose of ferrate(VI), i.e., 1.0 mmol/L and at varied pH conditions (i.e., pH 8.0, 9.0 and 10.0). The decomposition of ferrate(VI) is obtained in correlation with the degradation of NTA/or EDTA complex species. A known quantity of ferrate(VI) is introduced with the pollutant solution and immediately the solution is subjected to the UV-visible spectrometer as to record the change in absorbance value. The absorbance is recorded at the wavelength of 510 nm and at a regular interval of times for a total period of 20 min. Similarly, the absorbance of ferrate(VI) blank solution is also recorded in parallel for making necessary blank corrections which occurs due to the self-decomposition of ferrate(VI). Following with the UV-Vis analysis, the ferrate(VI) treated samples are stirred for another 2 hrs at room temperature and then filtered with 0.47 μm syringe filter. The filtrates are subjected for its TOC analysis (TOC-VCPH/CPN; Shimadzu, Japan) so as to obtain the TOC values of the treated samples. Thereafter, the percent mineralization of NTA/or EDTA is calculated by using the initial TOC values of the untreated samples.
Moreover, the UV-visible data obtained as a function of time is further utilized to study the kinetics of the oxidation of metal(II)-complex species indirectly with the ferrate(VI) reduction to ferric(III) hydroxide.
The removal of free metal ions in the treated sample solutions by ferrate(VI) is further analyzed. The filtrates obtained by ferrate(VI) treated samples are divided into two portions. One portion of the filtrate samples are subjected to atomic absorption spectrophotometric (AAS) analysis (Flame Atomic Absorption Spectrophotometer, Perkin-Elmer, Analyst 200) for the quantitative estimation of total metals, i.e., copper(II) or cadmium(II). The pH of other portion of the filtrate is raised to 12.0 by the addition of conc. NaOH solution so as to investigate the effect of enhanced coagulation at higher pH values in the removal of metals from aqueous solutions. The samples at pH 12 are again filtered using 0.47 μm syringe filter and subjected to the AAS analysis as to obtain the total metal concentrations. The percent removal of metals is finally estimated.
3. Results and Discussion
3.1. Decomplexation/degradation of Metal(II)-complex Species by Ferrate(VI)
3.1.1. Effect of pH and concentration of metal(II)-complex species
Varied molar concentrations of metal(II)-complex solutions i.e., from 0.5 to 15.0 mmol/L at different pH values (pH 8.0 to 10.0) are taken in a reaction reactor. The known amount of the solid K2FeO4 is added in reactor as to obtain a constant dose of ferrate(VI), i.e., 1.0 mmol/L. The reduction of ferrate(VI) concentration indirectly correlates the decomplexation/degradation of metal(II)-complexed species. Therefore, the change in ferrate(VI) concentration as a function of time for different metal(II)-complex species concentrations are calculated from the absorbance data recorded at 510 nm wavelength and at different pH values i.e., pH 8.0, 9.0 and 10.0. The results obtained e.g., at pH 8.0 are presented in Figs. 1, 2 and 3, respectively for copper(II)-NTA and copper(II)-EDTA and cadmium(II)-EDTA systems. The results indicate that, in presence of metal(II)-complex species, with the lapse of time the ferrate(VI) is decomposed gradually and attains almost a constant value within Ca 14 min of contact. Moreover, this decrease in ferrate(VI) concentration is highly dependent to the solution pH, reaction time and concentrations of complex species in solution. This is demonstrated with the fact that ferrate(VI) is found to be degraded rapidly in presence of metal(II)-complex species during the initial period of contact. Quantitatively, within initial 10 min, the ferrate(VI) removal is 20% to 40% for various molar concentrations of metal(II)-complex species at pH 8.0. This fast initial reduction of ferrate(VI) is in general increased with decreasing the solution pH from 10.0 to 8.0. This is explicable with the fact that the redox potential of ferrate(VI) is significantly high at lower pH values (2.20 V and 0.72 V at pH 1 and pH 14, respectively) which enables ferrate(VI) to decomplex/or degrade the complex species from aqueous solutions [25, 26]. It is further observed that increasing the molar concentration of Cu(II)-NTA, Cu(II)-EDTA or Cd(II)-EDTA, in general, was caused to enhance the decomplexation/degradation of these complexed species in solutions since more and more ferrate(VI) is reduced with an increased rate. Increasing the concentration of copper(II)-NTA from 0.5 mmol/L to 15.0 mmol/L, the corresponding percent reduction of ferrate(VI) is increased from 11.0% to 43.5% within 20 min of contact at pH 8.0. Similarly, the copper(II)-EDTA and cadmium(II)-EDTA systems, the similar increase was recorded from 5.0% to 25.6% and 14.6% to 41.6%, respectively at pH 8.0. The increase in reduction of ferrate(VI) with increase in pollutant concentrations is again pronounced at lower pH values. This again indicates that the acidic conditions favors greatly the decomplexation of metal(II)-complex species in presence of ferrate(VI). Several reports for other metal organo-complex systems indicate somewhat similar findings [35, 40].
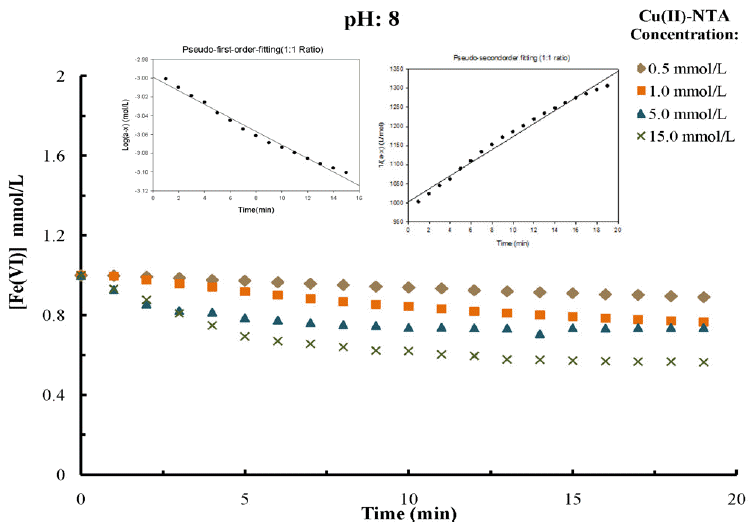
Degradation of NTA by ferrate(VI) as a function of time for different concentrations of Cu(II)-NTA at constant pH: 8.0 [Initial [Ferrate(VI)]: 1.0 mmol/L].
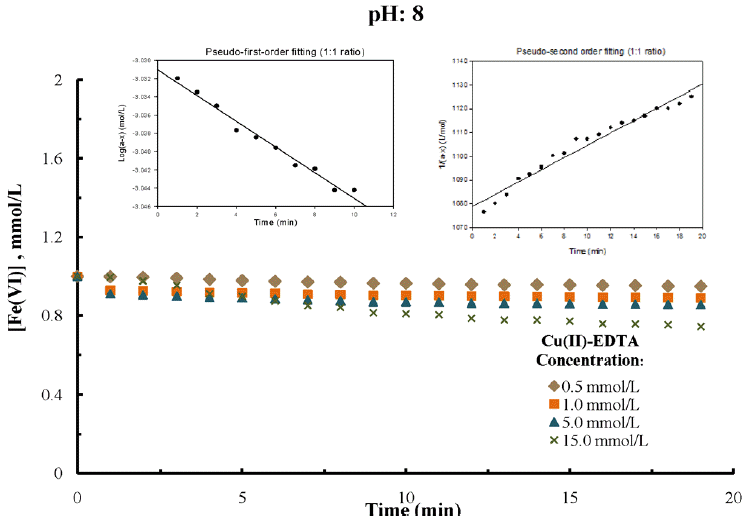
Degradation of EDTA by ferrate(VI) as a function of time for different concentrations of Cu(II)-EDTA at constant pH: 8.0 [Initial [Ferrate(VI)]: 1.0 mmol/L].
3.1.2. Kinetics of metal(II)-complex decomplexation/degradation
The reduction kinetics of ferrate(VI) in presence of various concentrations of copper(II)-NTA, copper(II)-EDTA or cadmium(II)-EDTA at different pH values and at varied molar ratios (i.e., pollutant to Fe(VI) ratios) were carried out utilizing the time dependence absorbance/or ferrate(VI) concentration data collected at different pH values and molar ratios of ferrate(VI) to metal(II)-complex species. It was demonstrated previously that the reduction of potassium ferrate(VI) into iron(III) as in the form of ferric(III) hydroxide was occurred with the below mentioned reductive pathways [40, 43]:
The hydroxyl free radical generated is one of strong oxidizing agent [44] which is readily decomplex/degrade the pollutants from aqueous solutions. The simple kinetics of the degradation of copper(II)-NTA, copper(II)-EDTA or cadmium(II)-EDTA is then discussed. The basic equation for reduction of ferrate(VI) could be represented as:
It was assumed that ferrate(VI) caused, partly/or fully, to mineralize the NTA/or EDTA to its end products, which was analyzed by the TOC values. The rate of decomposition of ferrate(VI) is simply expressed as:
where,
The time dependence change in ferrate(VI) concentration is further utilized to simulate in the Eq. (7) to optimize the value of m=1 or 2 i.e., to employ the pseudo-first and pseudo-second order rate equations. Results are presented graphically in figs. 1, 2 and 3 (as in insets), respectively for copper(II)-NTA, cop-per(II)-EDTA and cadmium(II)-EDTA systems (e.g., at pH 8.0 having 1:1 molar ratio of metal(II)-complex and ferrate(VI)). The estimated rate constant values along with the R2 values both for the pseudo-first and pseudo-second-order rate equations were returned in Table 1, 2 and 3, respectively for the copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA systems studied at varied molar ratios and at different pH values. Results showed that the rate constant was decreased significantly with the increase in pH from 8.0 to 10.0; clearly indicated the rate of reduction of ferrate(VI) or the degradation of M(II)-complex species was more pronounced at lower pH values.

Pseudo-first and Pseudo-second Order Rate Constants Obtained for the Reduction of Ferrate(VI) Using Various Concentrations of Cu(II)-NTA at Different pH Conditions

Pseudo-first and Pseudo-second Order Rate Constants Obtained for the Reduction of Ferrate(VI) Using Various Concentrations of Cu(II)-EDTA at Different pH Conditions

Pseudo-first and Pseudo-second Order Rate Constants Obtained for the Reduction of Ferrate(VI) Using Various Concentrations of Cd(II)-EDTA at Different pH Conditions
Quantitatively, it is observed that increasing the pH from 8.0 to 10.0, the rate constant value is decreased, respectively from 1.66×10−2 to 0.55×10−2 min−1 (for pseudo-first-order) and from 17.10 to 6.10 L/(mol·min) (for pseudo-second-order) at 1:1 molar ratios of ferrate(VI) and copper(II)-NTA system. Similarly, for copper(II)-EDTA system increasing the pH from 8.0 to 10.0 the rate constant is decreased, respectively from 0.32×10−2 to 0.16×10−2 min−1 (for pseudo-first-order) and from 3.73 to 1.62 L/(mol·min) (for pseudo-second-order) at the 1:1 molar ratio of ferrate(VI) to copper(II)-EDTA. Moreover, in case of cadmium(II)-EDTA system, the rate constant is decreased from 0.92×10−2 to 0.23×10−2 min−1 (for pseudo-first-order) and from 12.62 to 2.68 L/mol/min (for pseudo-second-order) for the similar increase in pH, respectively from pH 8.0 to 10.0 at the 1:1 molar ratio of ferrate(VI) and cadmium(II)-EDTA. Interesting to note that the reduction of ferrate(VI) is quite insignificant or not much pronounced for copper(II)-EDTA system which indicates the relative stability of copper(II)-EDTA chelate (stability constant value = 1018.80) [45]. This apparently hinders the rate of reduction of ferrate(VI). It was reported previously that the degradation of EDTA and NTA in natural conditions proceeded with the growth of specific bacteria from the subclass of Procteobacteria. Further, reported that the metal(II)-EDTA complexes with the stability constants below 1012 such as Ba(II), Mg(II), Ca(II) and Mn(II) were degraded efficiently whereas chelates with higher stability constants such as Fe(III), Co(II), Cd(II), Pb(II), Ni(II) or Cu(II) were not metabolized [46]. It was also observed that the rate of reduction of ferrate(VI) was significantly higher at lower pH values, this is because of an enhanced reactivity of ferrate(VI) at lower pH values [26, 27]. These results are in a line with the other studies conducted for the decomposition of other complex species by ferrate(VI) [40].
Further, the overall rate constant ‘k’ (Eq. (6)) is estimated using Eq. (8). The k1 values obtained at different concentrations of [Metal(II)-complex] is plotted against the concentration of metal(II)-complex both for pseudo-first-order and pseudo-second order rate constant values. Additionally, the value of ‘n’ is also optimized with its probable values of 1 and 2; but the data is best fitted for the m=1 and for n=1 as relatively higher value of R2 is obtained for these systems at various pH conditions (cf Figs. 4, 5 and 6, respectively for the copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA systems at pH 9.0). Employing these plots obtained separately at different pH i.e., 8.0, 9.0 and 10.0 are utilized to deduce the overall rate constant values (k) using the slope of these lines. The values of k along with R2 values are then returned in Table 4. In general, increasing the pH from 8.0 to 10.0, the overall rate constant values are decreased. As pH is increased from 8.0 to 10.0, the overall rate constant is found to be decreased from 4.8 to 1.2 L/(mol·min) for copper(II)-NTA system. Whereas the increase in pH from 8.0 to 9.0 the overall rate constant k is decreased from 2.2 to 1.3 for copper(II)-EDTA and from 4.1 to 0.9 L/(mol·min) for cadmium(II)-EDTA systems, respectively. The decrease in rate constant values at higher pH values is ascribed due to the fact that the reactivity of ferrate(VI) is increased at lower pH values. The speciation studies conducted elsewhere showed that at around pH 8.0 the protonated species of the ferrate(VI) i.e., HFeO4− was gradually increased (i.e., Ca. 50% at pH 8.0) since the pka value for the acid dissociation of HFeO4− was reported to be 7.3 [34]:
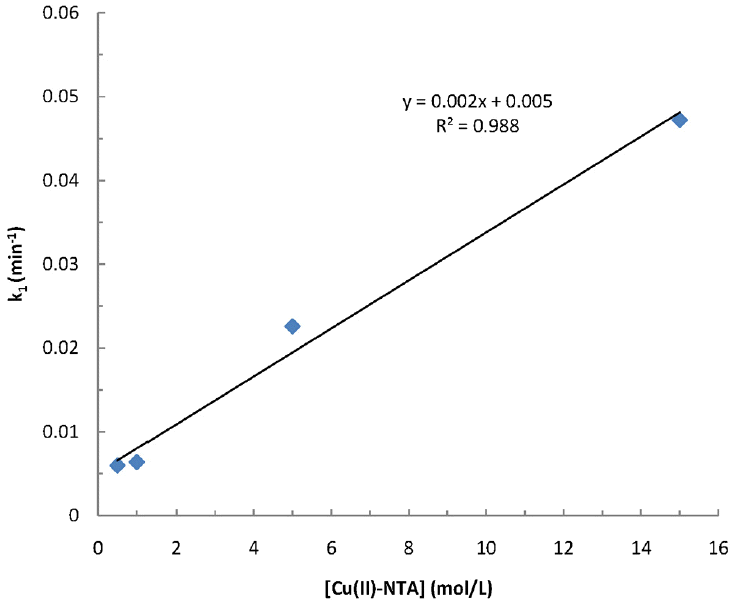
Fitting of pseudo-first-order rate constant values for the degradation of ferrate(VI) as a function of Cu(II)-NTA concentrations at pH 9.0.

Fitting of pseudo-first-order rate constant values for the degradation of ferrate(VI) as a function of Cu(II)-EDTA concentrations at pH 9.0.
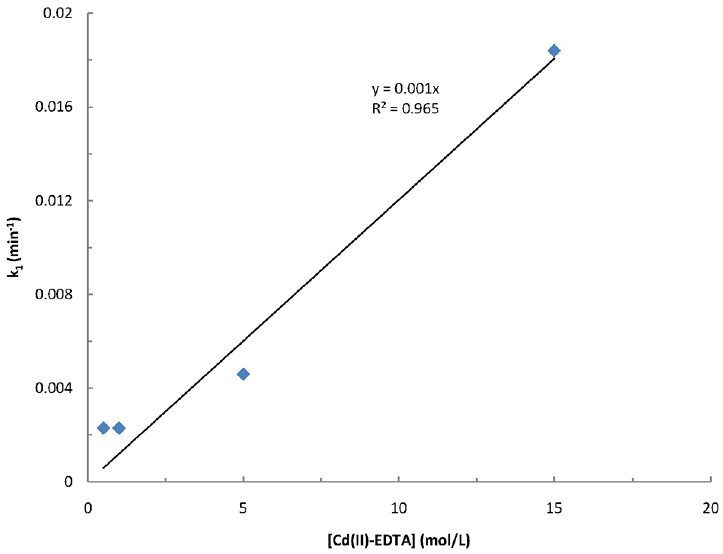
Fitting of pseudo-first-order rate constant values for the degradation of ferrate(VI) as a function of Cd(II)-EDTA concentrations at pH 9.0.

Overall Rate Constant in the Decomplexation/Degradation of Metal(II)-complex by Ferrate(VI) at Different pH Conditions
Since the protonated species HFeO4− were possessed with larger spin density hence, the reactivity of protonated species increased significantly [47, 48]. Moreover, the alkyl groups are found to be electron releasing groups, hence enhances the reactivity of protonated species HFeO4− in aqueous solutions [32]. Similarly, the higher degradation was reported with the protonated species of ferrate (HFeO4−) than the deprotonated species (FeO42−) in the degradation of various steroid estrogen including BPA from aqueous solutions [49].
It is also noted that 1:1 stoichiometry prevailed in decomplexation/degradation of metal(II)-complex with ferrate(VI) at various pH values. Previously, it was indicated that the oxidation of thiocyanate with ferrate(VI) followed pseudo-first-order rate kinetics with respect to the ferrate(VI) concentration. Therefore, the rate constant (k1) at various concentrations of thiocyanate was evaluated [50]. Further, the plot between k1 versus [SCN−] was found to be linear, suggesting that the rate law followed first order with respect to both the reactants i.e., ferrate(VI) and SCN−. The rate of reaction was increased with decreasing the pH. This inferred that protonation of ferrate(VI) caused to enhance the reactivity of ferrate(VI). Similar results were also reported for the decomposition of copper(I)-cyanide complex systems [51]. A recent report also indicated that the copper(II)-IDA and zinc(II)-IDA followed the first-order rate kinetics for both reactants i.e., ferrate(VI) and metal(II)-IDA and the overall rate constant values were greatly decreased with increasing the solution pH from 8.0 to 10.0 [40].
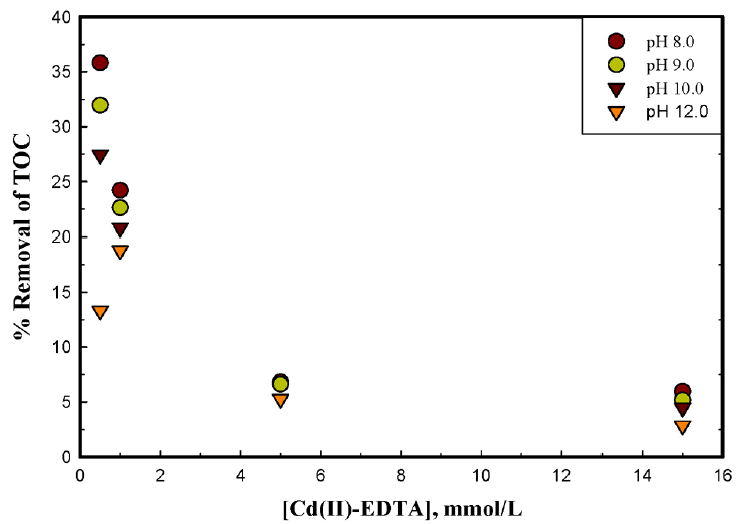
3.2. Mineralization of NTA/or EDTA
Oxidation of decomplexed or free NTA/or EDTA by ferrate(VI) is assessed by the TOC measurements. The total organic carbon data are obtained for various stoichiometric ratios of ferrate(VI) and metal(II)-complex (1.0:0.5 to 1.0:15.0) treated at different pH conditions i.e., pH 8.0 to 12.0. Results are shown graphically in Figs. 7, 8 and 9, respectively for the copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA systems. The results show that NTA/or EDTA is effectively degraded/mineralized by ferrate(VI) at least at lower pH 8.0 and lower metal(II)-complex concentrations. At higher concentrations of metal(II)-complex and at higher pH values i.e., pH 12.0 the percent TOC of NTA or EDTA is significantly decreased. This demonstrates that ferrate(VI) is able to oxidize NTA/or EDTA more effectively at lower concentrations of the metal(II)-complex. More quantitatively, increasing the pH from 8.0 to 12.0, the percent TOC removal was decreased from 25.32% to 17.33% (for copper(II)-NTA), from 64.55% to 32.11% (for copper(II)-EDTA) and from 24.21% to 18.80% (for cadmium(II)-EDTA), respectively for the 1:1 molar ratios of ferrate(VI) and metal(II)-complex species. The results again inferred that the ferrate(VI) is relatively more reactive at lower pH values i.e., at pH 8.0 comparing to higher pH values i.e., at pH 12.0. These results are in a line to the kinetic data obtained by the UV-vis measurements where it was noted that an increase in metal(II)-complex concentration invariably caused to increase the degradation rate of ferrate(VI). Previously, it was reported that an increase in pollutant concentration caused to increase the degradation rate of ferrate(VI) however, the percent removal of TOC was found to be less and an enhanced level of intermediates were, perhaps, formed [42, 52]. Naphthalene and trichloroethylene were mineralized almost completely by the ferrate(VI) [53]. Similarly, increasing the ferrate(VI) dose in the treatment of bisphenol A (BPA) (ferrate(VI):BPA ratio) the value of DOC (dissolved organic carbon) was decreased from 60% to 20%. It was also reported that the degree of degradation and mineralization for ferrate(VI):BPA ratios was obtained to be greater than 1, hence, it indicated that some intermediates of BPA was formed but with the time, the intermediates were also mineralized [42].

Degradation of NTA for different concentrations of Cu(II)-NTA treated with ferrate(VI) at different pH values [Initial [Ferrate(VI)] :1.0 mmol/L].
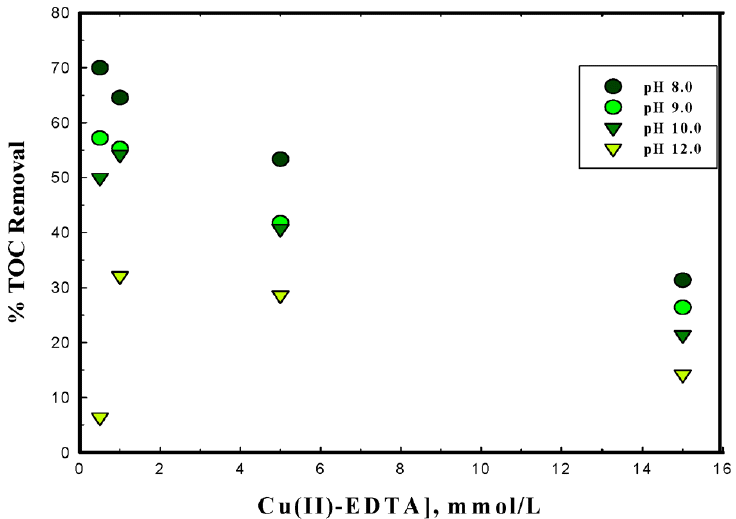
Degradation of EDTA for different concentrations of Cu(II)-EDTA treated with ferrate(VI) at different pH values [Initial [Ferrate(VI)] :1.0 mmol/L].
3.3. Simultaneous Removal of Copper(II)/or Cadmium(II)
The reduced ferrate(VI) into iron(III)hydroxide is an useful coagulant or the presence of Fe(OH)3 is even an excellent adsorbent for heavy metal toxic ions hence, ferrate(VI) may serve as multifunctional use in such wastewater treatment strategies. Therefore, in order to assess the suitability of ferrate(VI) in simultaneous removal of copper(II)/or cadmium(II) from the ferrate(VI) treated samples is further investigated. The ferrate(VI) treated samples are filtered and part of this sample is subjected to increase the solution pH 12.0 by the drop wise addition of concentrated NaOH and again filtered. Thereafter, both the samples are subjected to the total bulk copper(II) or cadmium(II) concentrations using AAS. The percent removal of copper(II) or cadmium(II) by the ferrate(VI) dose is presented graphically in Figs. 10, 11 and 12, respectively for the copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA systems. The data pertained with these figures clearly indicated that relatively less percent removal of copper(II) or cadmium(II) was obtained at lower pH values. However, once the treated samples pH was increased to 12.0, a significant increase in percent removal, i.e., almost 100%, of copper(II) or cadmium(II) was achieved at least for the lower concentrations of metal(II)-complex species. It was observed that the elevated pH favored greatly the removal of copper(II) or cadmium(II) from aqueous solutions. The enhancement in metal(II) percent removal by the ferrate(VI) treatment at higher pH values is attributed due to the enhanced coagulation occurred at higher pH values. Previously it was obtained that a complete removal of copper(II) was obtained in case of Cu(II)-cyanide complex with partial removal of Ni(II) from Ni(II)-cyanide complexes [30]. Similarly, Cu(II) and Zn(II) were removed simultaneously when their IDA complexes were treated with ferrate(VI) [40].
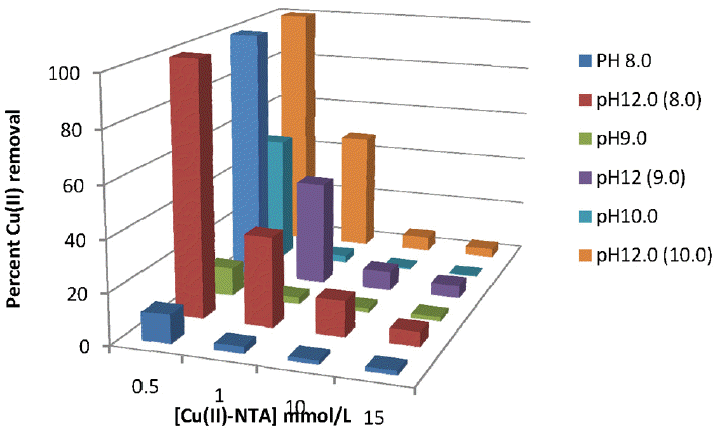
Simultaneous removal of Cu(II) for different concentrations of Cu(II)-NTA treated with Ferrate(VI): 1.0 mmol/L at different pH values.

Simultaneous removal of Cu(II) for different concentrations of Cu(II)-EDTA treated with Ferrate(VI): 1.0 mmol/L at different pH values.
4. Conclusions
The higher oxidation state of iron i.e., ferrate(VI) is utilized in the treatment of wastewaters contaminated with metal(II)-complex species (viz., copper(II)-NTA; copper(II)-EDTA and cadmium(II)-EDTA). The kinetics of degradation is discussed with the reduction of ferrate(VI) studied at wide range of pH (8.0 to 10.0) and at varied metal(II)-complexed species (0.5 to 15.0 mmol/L) using a constant dose of ferrate(VI) 1.0 mmol/L. The pseudo-first-order and pseudo-second order rate constants are estimated in the reduction of ferrate(VI) which is then utilized to optimize the overall rate constants in the degradation process. The overall rate constant values are, in general, increased with decreasing the pH from 10.0 to 8.0 which indicates the enhanced applicability of ferrate(VI) at relatively lower pH values. Further, the mineralization of organic species i.e., NTA and EDTA is obtained by the change in TOC values of ferrate(VI) treated samples. Results show that the percent TOC values are decreased with increasing the pH and molar ratios of ferrate(VI): metal(II)-complex. The simultaneous removal of metallic impurities i.e., copper(II) or cadmium(II) is obtained at the treated pH and also at the elevated pH i.e., 12.0 as to enhance the coagulation to remove the metals. Almost a complete removal of free copper or cadmium is obtained at pH 12.0 at least at lower stoichiometric ratios and at all studied pH values. Overall, the ferrate(VI) is found to be an useful chemical possesses multifunction application in wastewater treatment as in the first step it oxidizes the degradable impurities and in the second step it removes the non-degradable metallic impurities perhaps by the coagulation process. These results show that ferrate(VI) treatment is effective at least for the treatment of wastewaters contaminated with copper(II)-NTA, copper(II)-EDTA and cadmium(II)-EDTA complex species. Moreover, the treatment process is free from toxic by-products hence to be known as ‘Green Treatment’.
Acknowledgement
One of the authors DT wishes to acknowledge the Department of Science & Technology, New Delhi for the financial support; received in the form of Research Project vide No: SR/S1/IC-35/2010.