Enhancement of Efficiency of Activated Carbon Impregnated Chitosan for Carbon Dioxide Adsorption
Article information
Abstract
The effect of carbon dioxide (CO2) on global warming is serious problem. The adsorption with solid sorbents is one of the most appropriate options. In this study, the most interesting adsorbent is granular activated carbon (GAC). It is suitable material for CO2 adsorption because of its simple availability, many specific surface area, and low-cost material. Afterwards, GAC was impregnated with chitosan solution as impregnated granular activated carbon (CGAC) in order to improve the adsorption capacity of GAC. This research aims to compare the physical and chemical characteristics of GAC and CGAC. The experiment was carried out to evaluate the efficiency of CO2 adsorption between GAC and CGAC. The results indicated that the iodine number of GAC and CGAC was 137.17 and 120.30 mg/g, respectively. The Brunauer-Emmett-Teller results (BET) of both GAC and CGAC show that specific surface area was 301.9 and 531.3 m2/g, respectively; total pore volume was 0.16 and 0.29 cm3/g, respectively; and mean diameter of pore was 2.18 and 2.15 nm, respectively. Finally, the CO2 adsorption results of both GAC and CGAC in single column how the maximum adsorption capacity was 0.17 and 0.25 mol/kg, respectively; how degeneration time was 49.6 and 80.0 min, respectively; and how the highest efficiency of CO2 adsorption was 91.92% and 91.19%, respectively.
1. Introduction
The greenhouse effect is attributed to increase in the emission of the greenhouse gases, such as carbon dioxide (CO2), methane (CH4), nitrous oxide (N2N2O), chlorofluorocarbons (CFC), and sulfur hexafluoride (SF6). Among them, CO2 is the main greenhouse gas that causes global warming [1] because CO2 mostly increases among other greenhouse gases which was increased by 45% between 1990 and 2010 [2].
The most important sources of CO2 emission is the human activities. Including, burning fossil fuels (coal, oil, natural gas) and various industrial processes, such as distillation of petroleum, manufacturing cement, and metal smelting. There were several post-combustion gas separation and capture technologies being investigated, such as amine scrubber, thermal separation, membrane separation, biofixation, and pressure swing adsorption (PSA) [3].
At present, the preferred technology which carries out the separation of CO2 in post-combustion applications is amine scrubbing. However, this technology presents several disadvantages because amine scrubbing requires high energy to regenerate solvent and special management from especial officer. Thus, other technologies for development seek to reduce the cost in the captured steps as adsorption with activated carbon which has high adsorption capacity at ambient pressure, easy regeneration, low-cost, and insensitiveness to moisture.
The adsorption capacity of activated carbon is mainly governed by its texture, but it is also strongly influenced by the surface chemistry. Commercial activated carbon is interesting due to low-cost and simple availability. The commercial activated carbon will be modified with impregnation in chitosan to increase the efficiency for CO2 adsorption because chitosan solution will be ligand for adhesion of adsorbate [4]. Thus, activated carbon will have a more adsorption capacity. Next, activated carbon impregnated chitosan was carried out to adsorb CO2. Finally, in order to determinate CO2 adsorption capacity was carried out in single column of the PSA.
2. Materials and Methods
2.1. Preparation of Activated Carbon
Commercial granular activated carbon (CGAC) was purchased by METRA Co. Ltd. which was selected and separated with the particle size approximately 1.8–2.0 mm. Then, both samples were dried in the hot air oven at 105°C for 24 hr. Keep a dry sample in the desiccator before chitosan impregnation method.
The commercial chitosan (CS) was purchased from SIGMA Co. Ltd. which was used to prepare stock chitosan solution at concentration by 0.1% in 100 mL acetic acid. Next, The GAC would be immersed in chitosan solution for 24 hr at room temperature.
Afterwards, filtering and washing with distilled water to remove the excess chemicals on the surface of adsorbent. After previous steps, this sample was called that CGAC which would be dried in the hot air oven at 105°C for 24 hr.
2.2. Characterization of Samples
The chemical and physical characteristics of GAC and CGAC were analyzed as shown.
The measurement of iodine number corresponding to procedure established by the standard method ASTM D4607-94 (2011) [5]. The measurement of specific surface area, pore volume, and pore size were determined by nitrogen adsorption-desorption isotherms at 77 K with discontinuous volumetric apparatus (Quantachrome AUTOSORB 1) [6].
Analysis of the surface functional group by using Fourier transforms infrared spectroscopy (FTIR) of the GAC before and after chitosan treating which is evaluated using Nicolet Magna 560 [7].
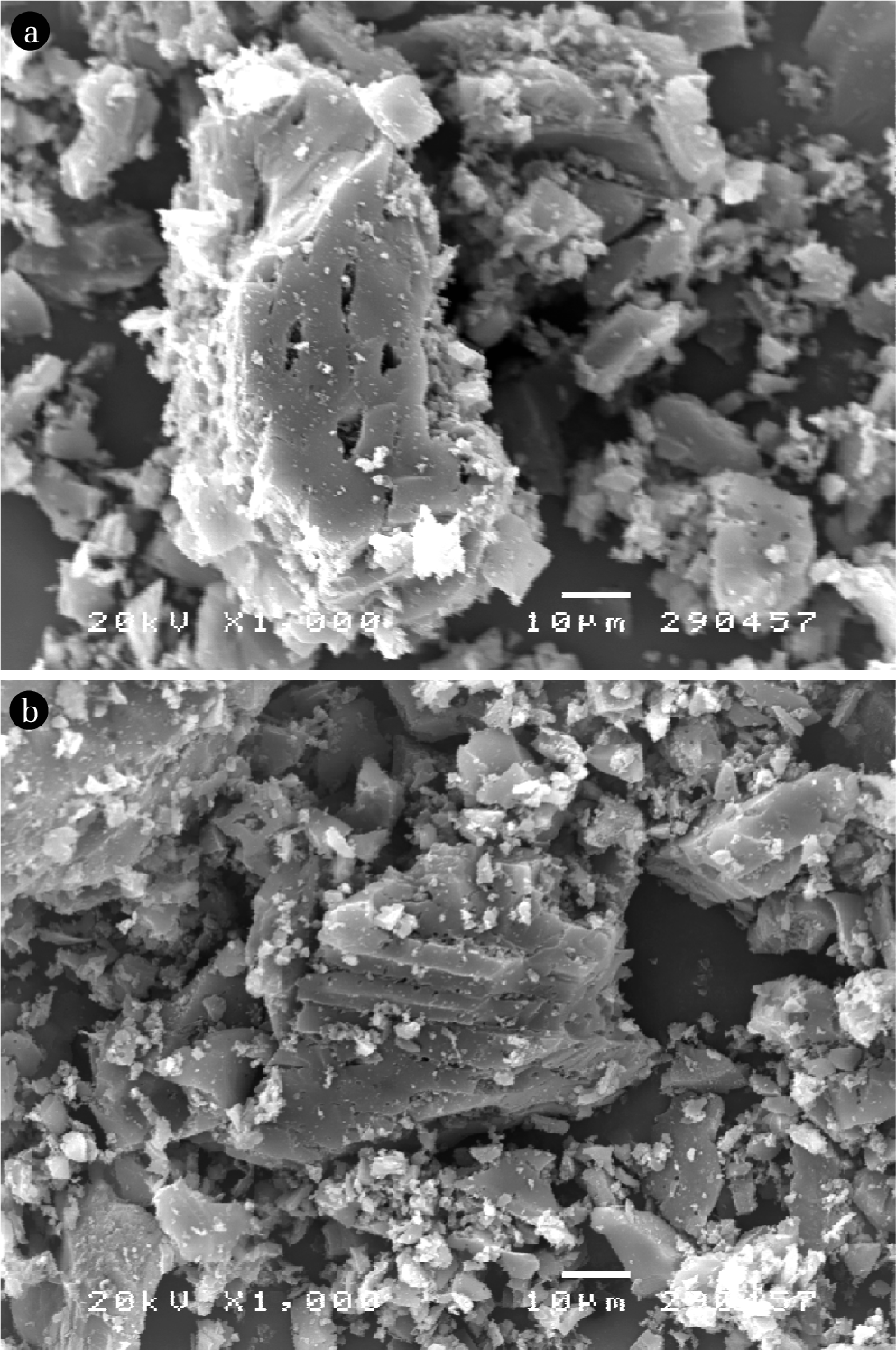
Analysis of morphology of samples. In order to know the surface structure of GAC and CGAC. Morphological analysis was carried out by scanning electron microscope (SEM) by using JSM-6400 scanning microscope model JEOL.
2.3. Adsorption Experiments
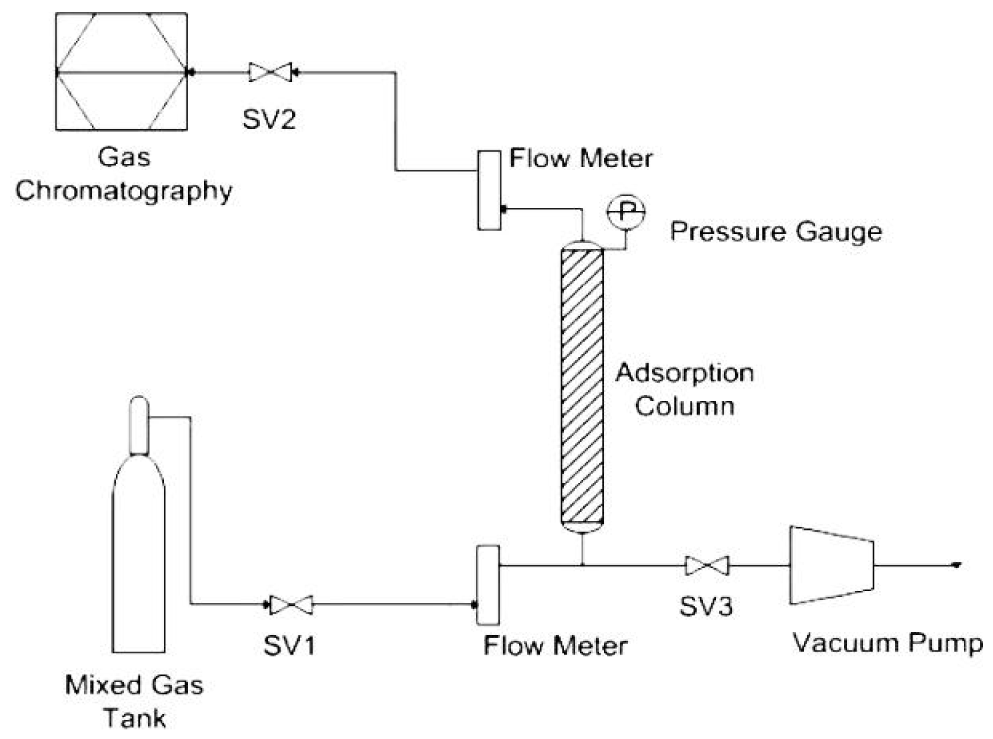
Both samples were analyzed CO2 adsorption in single column of PSA (Fig. 1).
The chemical compositions of mixed gas were 40% CO2 and 60% N2. Next, feeding mixed gas at controlled pressure of 3 bars with the ratio of flow rate (inlet/outlet) of 1.
Finally, the outlet gas will be collected to analyze the composition of gas by gas chromatograph (Shimadzu model GC2014).
3. Results and Discussion
3.1. Iodine Number Result
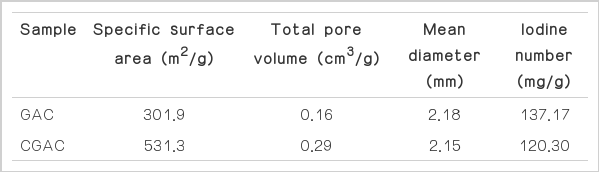
The iodine number of GAC and CGAC were shown in table 1 which was 137.17 and 120.30 mg/g, respectively. Reduction of iodine number in CGAC after impregnation which was caused blocking of iodine in microporous CGAC, but CGAC that will get smoother surface [4].
3.2. BET Surface Area Analysis Result
Analytical results by adsorption and desorption technique with N2 gas in both GAC and CGAC in Table 1 show that specific surface area were 301.9 and 531.3 m2/g, respectively; total pore volume were 0.16 and 0.29 cm3/g, respectively; and mean diameter of pore were 2.18 and 2.15 nm, respectively. Conclusion, the results show that impregnation with chitosan that increases the specific surface area and pore volume because film layer of chitosan which coat surface area on GAC [8].
3.3. Fourier Transform Infrared Spectroscopy Result
Fourier transform infrared spectroscopy (FTIR) of chitosan and both samples that the peaks with about the C=O stretching, amide and amine group in chitosan have appeared at wave numbers 1,704 and 1,650 cm−1. Thus conferred peaks of wave number were distinguished in CGAC (Fig. 2), but those wave numbers were not clearly appear in GAC.
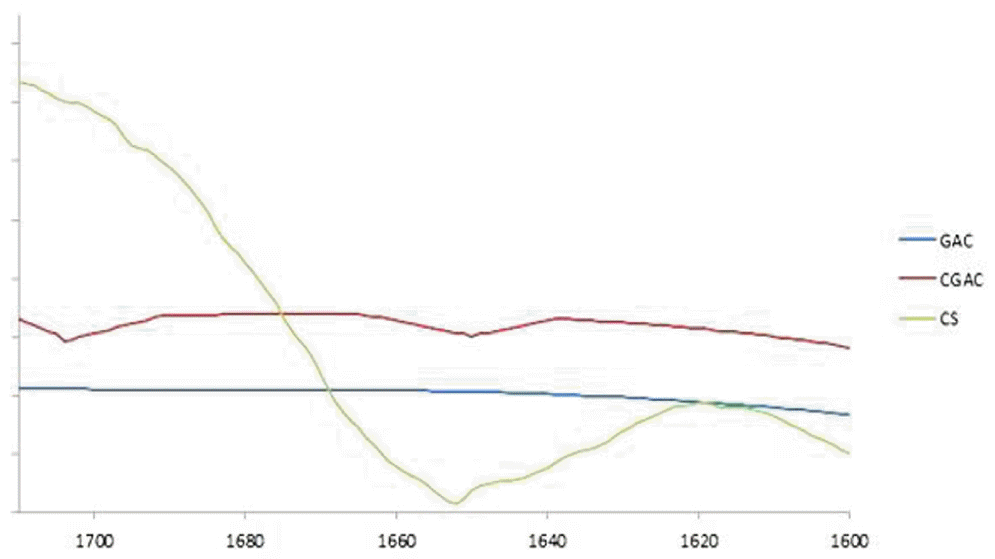
Fourier transforms infrared spectroscopy spectrums of granular activated carbon (GAC), commercial granular activated carbon (CGAC), and commercial chitosan (CS).
Conclusion, impregnation with Chitosan will have more amino ketone and aldehyde functional group while other functional group is not clearly different.
3.4. Scanning Electron Microscope Studies
SEM technique was applied in order to study regarding surface morphology of adsorbent before and after impregnation with chitosan solution. The pictures were shown in Fig. 3. It can be seen that the surface of the GAC and CGAC are not clearly different.
3.5. Carbon Dioxide Adsorption Result
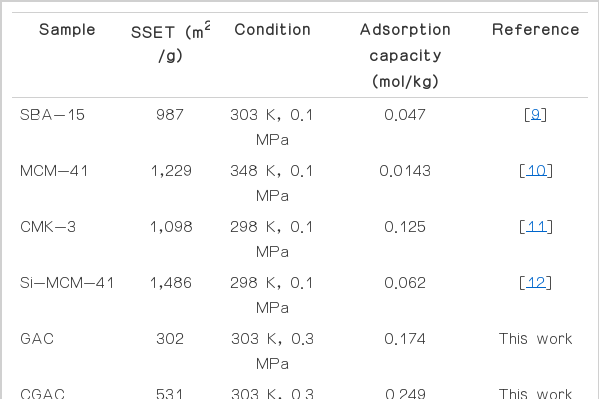
Analytical results for purity of outlet CO2 gas were set as the declining point by 50% of inlet CO2 concentration. GAC and CGAC were degenerated at 49.6 and 80.0 min, respectively (Fig. 4). The maximum adsorption capacity (qm) of CO2 in GAC and CGAC adsorbents were shown in Fig. 5. The maximum adsorption capacity for GAC and CGAC which obtained from the following balance Eq. (1) were 0.174 and 0.249 mol/kg, respectively. Other available adsorption capacity results were shown in Table 2.
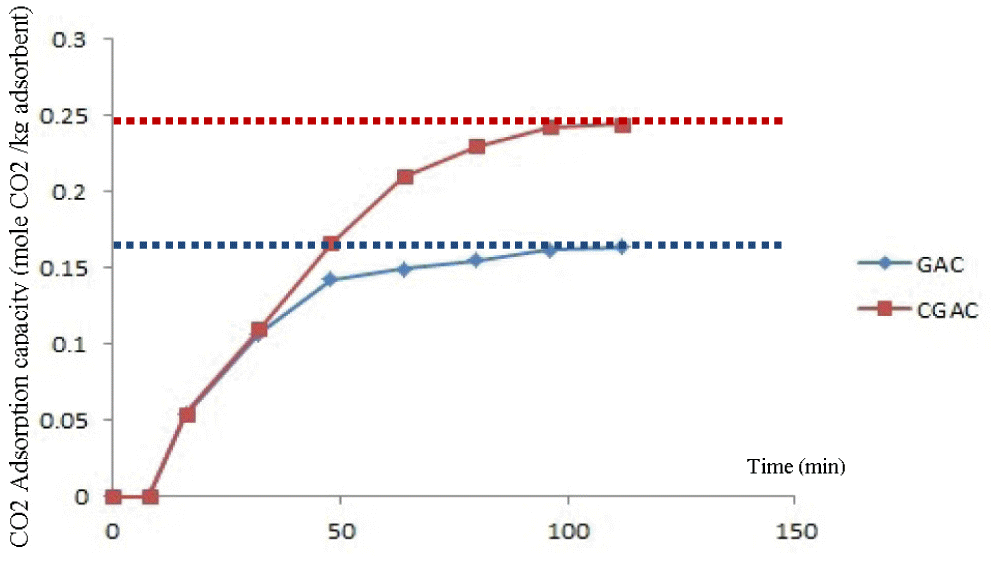
Adsorption capacity of CO2 on granular activated carbon (GAC) and commercial granular activated carbon (CGAC).
Conclusion, the effect of Chitosan on the increasing of degeneration time (the time which adsorbate starts to release from adsorbent because of full capacity) and maximum adsorption capacity of CGAC because CGAC was with more amino and hydroxyl functional groups than GAC after Chitosan impregnation which well adsorb a weak acid as CO2 than GAC before impregnation with Chitosan [7].
4. Conclusions
The impregnation with Chitosan on CGAC has influence to improve the physical characteristics. Including, increasing Specific surface area and total pore volume. Moreover, impregnation with Chitosan of CGAC also increases the degeneration time, maximum adsorption capacity and its adsorption efficiency.
Acknowledgement
This work was carried out with financial support from the corporation between Thailand Institute of Scientific and Technological Research (TISTR) and Academic Institute on Graduate Program Development. In addition, the authors also acknowledge the financial support from the graduate school, Chulalongkorn University, Thailand.