Effect of Kaolin on Arsenic Accumulation in Rice Plants (Oryza Sativa L.) Grown in Arsenic Contaminated Soils
Article information
Abstract
The As accumulation in part of roots, shoots, husks and grains of rice plants was significantly decreased with the increasing dosage of kaolin addition from 0.5% to 10% w/w. Kaolin addition could reduce As accumulation in rice plants, which mainly could be attributed to the formation of stable crystalline Al oxides bound As that decreased the available As in soil with decreased As accumulation in rice plants. The pH values of the soils did not change significantly when amended with kaolin. The pH values of the soils was neural that proper to adsorb of arsenic with Al2O3. Arsenic tends to adsorb with Al2O3 at acid neutral pH and with desorbing at alkaline pH. The dry weight of rice plant was significantly increased with the increasing dosage of kaolin addition from 2.5% to 10% w/w. The highest dry weight of rice plants was 6.67 g/pot achieved at kaolin addition of 10% w/w with about 13% increasing over the control, which was probably attributed to the highest As concentration formation with kaolin at this dosage. The results of this study indicated that kaolin has the potential to reduce As accumulation in rice plants and enhance the dry weight of rice plants.
1. Introduction
It is well-known that arsenic is a toxic and carcinogenic element to human beings, and a number of environmental problems have been caused by arsenic worldwide. This contamination is mostly originated from mining activity and arsenic leaching produced by the mining activity, which could be discharged to the surrounding area. Furthermore, the leaching could penetrate to the lower parts of soil and endanger the groundwater. Soil is ready to be the recipient of the large amount of arsenic. In Ron Phibun district, Nakhon Si Thammarat province, Thailand, sources of arsenic contamination in the place were thin mining activities around that area. It was reported that people lived in that place suffered from chronic arsenic (As) poisoning with skin cancer, “black fever”, or called arsenic poisoning. They found that arsenic concentration in the soil ranged between 0–3, 931 mg As/kg soil [1]. As polluted soil is considered a major source of contamination in the food chain. However, the remediation of arsenic polluted soil is the great importance for reducing the potential risk of human exposure to arsenic.
Stabilization is regarded as one of the most effective remediation techniques whereby various amendments are applied to reduce arsenic mobility and bioavailability [2, 3]. Kaolin is the common adsorbent used in the treatment of arsenic contaminated soil [4]. Yong Zhou et al., 2010 indicated that kaolin is the effective adsorbent of reducing arsenic(III) in the aqueous phase [4].
Rice (Oryza sativa L.) is the most important cereal grown in Thailand. High arsenic concentrations in soil and the use of irrigation water with high As levels may lead to elevated concentrations of arsenic in cereals, vegetables and other agricultural products in As contaminated areas [5]. Bari et al., 2008 found that increasing arsenic concentrations of both soil and irrigation water resulted in significantly increased arsenic concentrations in both rice grain and straw [6]. Human exposure to arsenic is mainly through the intake of drinking water and foods, such as rice grains, that contain elevated amounts of arsenic. Arsenic-contaminated rice could aggravate human health risk because it is consumed in large quantities especially in Asian countries.
In this study, 0.5, 2.5, 5 and 10% w/w of kaolin was studied as soil amendments in As contaminated soil. The effect of kaolin on arsenic accumulation in rice plants (Oryza Sativa L.) grown in arsenic contaminated soils was investigated.
2. Materials and Methods
2.1. Chemicals
Standard solution of arsenic, nitric acid (65%) and sulfuric acid were from Merck, Germany.
2.2. Preparation of Rice Plants
Rice plants (Oryza saltiva L.) age of 30 days was cultivated in pot containing uncontaminated soil under the planting condition with day light in green house until their roots grow for 1 cm and plant length about 30–40 cm with 6–10 leaves. The rice plant was watered by tap water.
2.3. Soil Preparation
2.3.1 Arsenic contamination soil preparation
Arsenic contaminated soil obtained from arsenic contaminated areas in Ron Phibun District, Nakhon Si Thamarat Province, Thailand. Their texture was that of sandy loam. Soil was sampled at 30 cm depth, air dried and sieved through 2 mm (No. 10) mesh to remove plant materials and stones. Composition of elemental-contaminated soil was analyzed by X-Ray Fluorescence Spectrometry (XRF) (S4 Pioneer; AXS Bruker, Karlsruhe, Germany) and the compositions are expressed as relative concentrations in the form of oxides.
2.3.2 Arsenic uncontaminated soil preparation
Uncontaminated soil was obtained from rice field in Si Sa Ket province, Thailand. Uncontaminated soil was air dried and sieved through 2 mm (No. 10) mesh to remove plant and stone.
2.4. Pot Experiment
Arsenic contaminated soil and kaolin were mixed together in the pots under 4 conditions: 0.5, 2.5, 5 and 10% w/w kaolin mixed with 1.5 kg of As contaminated soil compared to uncontaminated soil as a control (without addition of kaolin). Then water 1,500 mL was added in the pots. Each condition was replicated three times. Before planting, the soils were sampled from each pot for analysis of arsenic content. Rice plants were selected in similar size of shoot and length at 30–40 cm. Then the roots were washed several times by tap water to clean the adhering soil. The rice plant was planted as 6 plants per pot. Pots were kept in glasshouse (temperature 28–30°C) and watered daily by tap water. After 90 days growth, the rice plants were washed by tap water thoroughly and then with deionized water. Rice plants were cut and separated into 4 parts as roots, shoots, husks and grains. The samples of plants were dried at 60°C for 72 hr. Arsenic content in each part of plants was analyzed. In addition, the dry weight of plants was also measured.
2.5. As Concentration Analysis in Rice Plants
The harvested plants were washed with tap water, and rinsed with deionized water before being separated into shoots, roots, husks, and grains. Then, they were dried at 70°C for 3 days according to the method of Rahman et al. [7]. Soil and plant samples were digested with 1.0 mL of HClO4, 1.5 mL of H2SO4 and 4.0 mL of HNO3 following the heating block digestion procedure at temperature 150°C until a clear solution was obtained. The digested samples were diluted with deionized water and then filtered with filter paper Whatman No. 42. Total As concentration in plants and soil was determined by Hydride Generation Atomic bsorption Spectrometry (HG-AAS) (AA-6300; Atomic Absorption Spectrophotometer, Shimadzu, Japan) with detection limit at 0.2–0.8 ppb.
2.6. Data Statistical Analysis
Statistical analysis of the experimental data was performed using SPSS 21.0 (SPSS, USA) software. The statistically significant differences were determined by one way analyses of variance on ranks and two way ANOVA with p < 0.05.
3. Results and Discussion
In this study, As contaminated soil contained As concentration 578.83 mg/kg. The results conformed to the study of Chintakovid et al., 2008 that the arsenic concentration in soil at the contamination site was set at 417.76 μg/g [8]. The elemental analyses indicated the main minerals in As-contaminated soil as Si, 53.20%; Al, 8.61%; Fe, 1.79%; K, 0.34%; Ti, 0.72; Ca, 0.61%; P 0.06 %; Na, 0.07%; As, 0.01%. The soil was analyzed for its physical and chemical properties using standard methods [9]. pH of arsenic contaminated soil, uncontaminated soil and soil amendments in distill water ratio 1:1 were 7.09, 6.52 and 4.90. Plant growth can influence on As accumulation such as organic acids lead to higher As accumulation [10]. The chemical characteristics of kaolin affected for As accumulation in plants [4, 11, 12]. Kaolin contained high composition of Al2O3 as 42.4 % w/w [13]. The As contaminated soil had pH 7 that was suitable for planting the rice plant [14]. The internal distribution of As in plants are in apoplast and the symplast. In rice about 60% of the total plant As was located in the apoplast of the roots [15]. Cellular uptake of arsenate is mediated by phosphate transporters [16]. Another detoxification mechanism used by plants is the efflux of arsenic from the plant cell [17].
3.1. Effect of Kaolin on As Accumulation in Rice Plants
The effect of kaolin at 0.5, 2.5, 5 and 10% w/w on As accumulation in roots, shoots, husks and grains of rice plants shown in Fig. 1(a)–(d). The result showed that As concentration in rice roots was decreased significantly when increasable added kaolin from 0.5 to 10% w/w in As contaminated soil (Fig. 1(a)). As concentration in rice roots with 0.5, 2.5, 5 and 10% w/w kaolin addition were 532.2499, 509.1041, 491.7891 and 480.1966 mg/kg, respectively. The decreasing of As concentration in rice shoots when dosage of kaolin addition increase from 0.5, 2.5, 5 and 10% w/w was shown in Fig. 1(b). As concentration in rice shoots were 111.06, 93.50, 85.67 and 72.76 mg/kg. As concentration in rice husks with 0.5, 2.5, 5 and 10 w/w kaolin addition was 0.01, 0.01, 0.008 and 0.007 mg/kg that lower than the control (Fig. 1(c)). The result of As concentration in grains was conform to the As concentration in roots, shoots and husks. Fig. 1(d) shown that also decreased with the increasing dosage of kaolin from 0.5 to 10% w/w. As concentration in rice grains were 0.004, 0.004, 0.004 and 0.003 mg/kg, respectively which lower than the control about 36, 41, 47 and 56%, respectively. The result indicated that As concentration in part of roots, shoots, husks and grains of rice plants was significantly decreased with the increasing dosage of kaolin addition from 0.5% to 10% w/w. Kaolin includes high component of Al2O3 42.4% w/w. Al2O3 could form with As in soil, decrease available As that effect on the decreasing of As accumulation in rice plants. These results were conform with the results of Jeong et al., 2007 who indicated that the rate of As(V) adsorption was found to be higher with high dosages of Al2O3 to As(V) [18]. Based on studies of activated alumina and aluminum-loaded Shirasu zeolite, the As(V) adsorption mechanism of Al2O3 can also be considered a ligand exchange process between As(V) and the hydroxide groups that also effect on As bioavailable uptake into rice plants [19, 20]. The pH of arsenic contaminated soil with 0.5, 2.5, 5.0 and 10%w/w kaolin were 7.04, 7.03, 7.01 and 6.99, respectively, compared to the control about 7.08. The pH values of the soils did not change significantly when amended with kaolin. Arsenic tends to adsorb with Al2O3 at acid neutral pH and with desorbing at alkaline pH [21]. The pH values of the soils was neural that proper to adsorb of arsenic with Al2O3. According to Xu et al., 2002 who indicated that activated alumina used in the pH range of 5.5–8.5 preferred OH− to H2AsO4− [19]. The results shown that arsenic uptake in rice plants decreased with the increasing of adsorption of As and Al2O3 that conducted by the raising dosage of kaolin and neural pH. Kaolin is a good adsorbents because it is non-hazardous materials, easy availability and low cost. Therefore, it indicated that kaolin might be a potential amendment for As stabilization in contaminated soil [4].
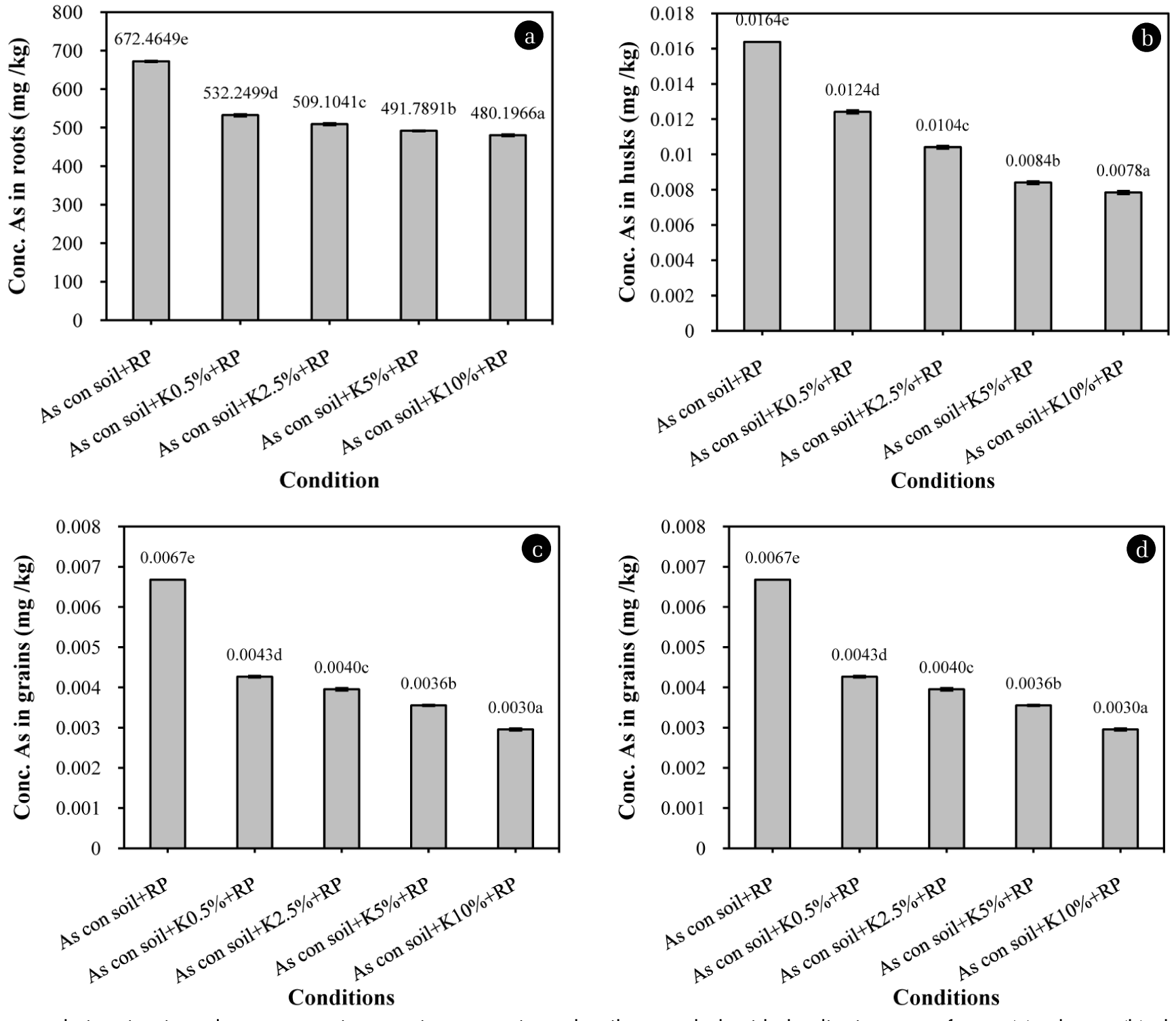
As accumulation in rice plants grown in arsenic contaminated soil amended with kaolin in part of roots(a), shoots (b), husks (c) and grains (d) of rice plants grown in arsenic contaminated soil amended with kaolin. bars represent S.D. of three replicates, and the different letter above column indicates a significant difference at p<0.05 according to two way ANOVA.
3.2. Effect of Kaolin on Dry Weight of Rice Plants
The dry weight of rice plants grown in arsenic contaminated soils increases with the increasing of kaolin addition from 0.5 to 10% w/w (Fig. 2). The results indicated that kaolin could raise the growth of rice plants. The dry weight of rice plants was the highest at 6.67 g/pot when added kaolin at 10% w/w that higher than the control 13%. The rise of dry weight was probably attributed to the highest As concentration formation with kaolin at this dosage [4].
4. Conclusions
The results showed that kaolin might be a potential amendment for As stabilization in contaminated soil. Kaolin addition increased rice plants dry weight and reduced As accumulation in rice plants, which mainly could be attributed to the formation of stable crystalline Al oxides bound As that decreased the available As in soil with decreased As accumulation in rice plants. Additionally, kaolin is inexpensive chemicals and has a high potential as a soil amendment. Rice plants grown in As contaminated soil amended with kaolin in this experiment was safety for eating according to Australian Food Standard that established a permissible limit maximum for grain arsenic concentration of 1.0 mg/kg (National Food Authority, 1993) and the Maximum Contaminant Level (MCLs) for inorganic arsenic in rice grains was set at 0.15 mg/kg in China (Chinese Food Standards Agency, 2005). For further study the effect of soil amendments and microorganisms on arsernic accumulation in rice plant (Oryza sativa L.) grown in arsenic contaminated soil will be study.
Acknowledgement
This research was supported by Center of Excellence on Environmental Health and Toxicology, Thailand.

