Simultaneous Removal of Gas and Dust by Activated Carbon Coated Electrode
Article information
Abstract
This study aimed to develop a new dust collecting system equipped with an activated carbon (A.C.) coated electrode. Before fabrication, pre-treatment of A.C. was performed to remove metal ions within the A.C. to enlarge its specific surface area. Then, pre-treated A.C., black carbon, polyvinyl acetate (PVAc), and methanol were mixed to make a gel compound, which was coated onto aluminum plates to fabricate electrodes. The optimal mixing ratio of A.C., black carbon, PVAc, and methanol was found to be 10 g: 2 g: 3 g: 20 mL. After fabrication, the electrodes were used in the batch-type experiment for NH3 and H2S removal. The reduction rates of the gases were high at the beginning and slowly reduced with time. Dust collection experiments were conducted in continuous flow, with various voltages applied. Compared to 5 kV, dust removal efficiency was 1.5 times higher when 10 kV was applied. Increasing the number of electrodes applied also increased the collecting efficiency. The correlation coefficient between actual collecting efficiency and trend line was higher than 99%. Consequently, the novel dust collection system equipped with A.C. coated electrode appears to be a promising substitute for existing dust-control devices.
1. Introduction
According to Seoul Air Environment Information System, the concentrations of annual average particulate matters (PM10) of Seoul, Gyeonggi, and Incheon have never satisfied the standard until 2009 since the air environment standard was strengthened to 50 μg/m3·yr in 2007. In 2010, Seoul satisfied the standard for the first time, with the level of 49 μg/m3·yr. Other than particulate matters, there are regulations on various contaminants, such as nitrogen dioxide, carbon monoxide, carbon dioxide, hydrocarbon, sulfur oxide, ozone, and others. The main source of these matters comes from exhaust fumes of vehicles [1]. These matters threaten human health as well as having adverse effects on other biological systems on the earth. In addition, it is essential to prepare measures to limit and mitigate the generation of exhaust fumes, because the number of vehicles is increasing together with the increasing human population across the world.
Electrostatic dust collection technology refers to air pollution preventing facilities which are mainly used in processing particles in the air pollutants listed above, and which include technology for the removal of dust using electrostatic power [2]. Gravity-based dust collection technology catches dust simply using gravity. It shows good efficiency for the removal of larger particles with low operating costs, though it is not suitable for the removal of smaller particles. Electrostatic dust collection technology shows higher dust collecting efficiency even for dust with a comparatively smaller diameter, when compared to other technologies, such as filtering dust collection, gravity dust collection, and centrifugal dust collection [3]. However, there are disadvantages; dust collection requires high energy and it is difficult to remove gaseous matters with an independent use of electrostatic system. Furthermore, it generates corona discharges which can produce nitrogen oxides which are designated as harmful gaseous matters [4]. In order to circumvent this disadvantage, wet electrostatic dust collection technology has been developed, but it also has problems, such as beading of cleansing water, dry spot formation on dust collecting plates, disposal of wastewater from cleansing water, and so on [5].
This study introduces a dust collection system based on the use of an activated carbon (A.C.) coated electrode. A.C. is characterized to carbon with large internal specific surface area and high absorptive power, which arises due to its porosity, which has been used for the absorption of pollutants for a long time. There have been many studies based on the use of electrical double layer capacitors (EDLC) due to their high specific surface area [6]. EDLC refers to a device to use electrical charges accumulated in electrical double layers generated between a solid electrode and an electrolyte. Although its energy density is lower than that of a battery, rapid charging and discharging can result in excellent characteristics in power density to load an instant power. In accordance, it was considered that the use of an A.C. electrode with high density of electrical charges may achieve the desired dust collection efficiency without any corona discharge, thus enduring an efficient energy usage, while preventing the generation of nitrogen oxides [7]. Given the characteristics of A.C. to absorb air pollutants, its use as an electrode in an electrostatic dust collection process was considered to be highly suitable for dust collection and for the removal of gaseous matters through a single process. In addition, the possible degradation of dust collecting efficiency due to no corona discharge was supplemented by introducing a gravity device.
This study aimed to introduce an A.C. coated electrode to an electrostatic dust collection process in an effort to identify dust collection efficiency and the removal of gaseous matters. For this purpose, an electrode was fabricated by coating A.C. onto an aluminum plate as a conductor. Then, the elimination and electrical resistance for the A.C. coated electrode were identified together with dust collection efficiency, based on the conditions of various voltage and different numbers of plates applied.
2. Materials and Methods
2.1. Fabrication of Activated Carbon
Because of the massive metal ions contained in A.C., electrical resistance may occur when electric current is applied on to the A.C. coated electrodes. A.C. was pre-treated to prevent electrical resistance, by washing with acid solution to remove any metal ions which were present [8]. A.C. was washed with boiling water at 100°C and was then washed three times with boiling hydrochloric acid solution (0.1 N). After treatment with acid, it was washed again with distilled water and dried. The supernatant of acid washing was taken and the content of metal ions was identified through the inductively coupled plasma (ICP) analysis. Fig. 1 shows the procedure of A.C. pre-treatment.
The coating materials for A.C. plate electrode include A.C., black carbon, and polyvinyl acetate (PVAc). Acid-treated A.C. (derived from the palm tree) was mixed with black carbon in order to reduce electrical resistance [9], and then PVAc was added to support the coating on the electrode surface. The mixing ratio of A.C., black carbon, and PVAc was derived from previous studies [10]. In order to ensure an even coating onto the electrode surface and to prevent its removal, methanol was injected into the A.C. mixture. The amount of methanol injection was evaluated by trials, so that it dissolved the three materials and did not negatively affect the coating procedure.
After an A.C. plate was constructed at the optimal composition ratio, the internal specific surface areas of pre-treated A.C. and A.C., which is made as an electrode, were measured and compared.
2.2. Removal of Gaseous Matters
The performance of A.C. to remove gaseous matters was tested. The gases used in the experiment included NH3 and H2S, which were generated from ammonia water and sulfuric acid solutions, respectively. A.C. samples were taken from a plate electrode and were used for the batch-type experiment to remove the gases generated into a Tedlar bag. Gases of 150 ppmv NH3 and 5 ppmv H2S were injected to a Tedlar bag, and then, 0.05 g and 0.5 g of A.C. sample were respectively injected. The gas concentrations and amount of A.C. injected in each Tedlar bag were different because it was thought that NH3 removal would be rapid but that H2S removal would be relatively slowly removed. The gas concentrations were measured by a Gastech.
2.3. Experiment for Dust Collecting Performance
Experimental studies on the batch-type of dust collection in the past showed that the optimal distance between each plate was 1 cm, at 5 kV. In accordance, this study considered an electrostatic and gravity dust collection method together in fabricating a test device to remove dust, and the experiment was carried out in a continuous mode. For the gravity dust collection system, a reactor was designed based on the longitudinal direction. In addition, an electrostatic dusting collection system was equipped, and thus, both larger and smaller particles were removed electrostatically. Figs. 2 and 3 show the overall schematic diagram and the experimental system. Two air pumps were connected to a holding tank for flow control, while dust in high concentration was generated from a dust generator. A fluidized bed dust generator (Model 3211) of Kanomax in Japan was used.
Fig. 4 shows the assembly of reactors after plate electrodes were inserted. A dust counter was installed respectively to the front and back of the reactor to measure the concentration of dust, where a digital dust monitor (Model 3442, Kanomax) was used. A 1.5-μm dust was selected for the experiment. The experiment was carried out at two voltage conditions, 5 kV and 10 kV with 1 cm of each electrode distance, a flow rate of 85 L/min, linear velocity at around 6 cm/s, and staying time at around 1.4 sec. The concentration of influent dust was about 3 to 4 mg/m3.
3. Results and Discussion
3.1. Preparation of Activated Carbon Coated Electrode
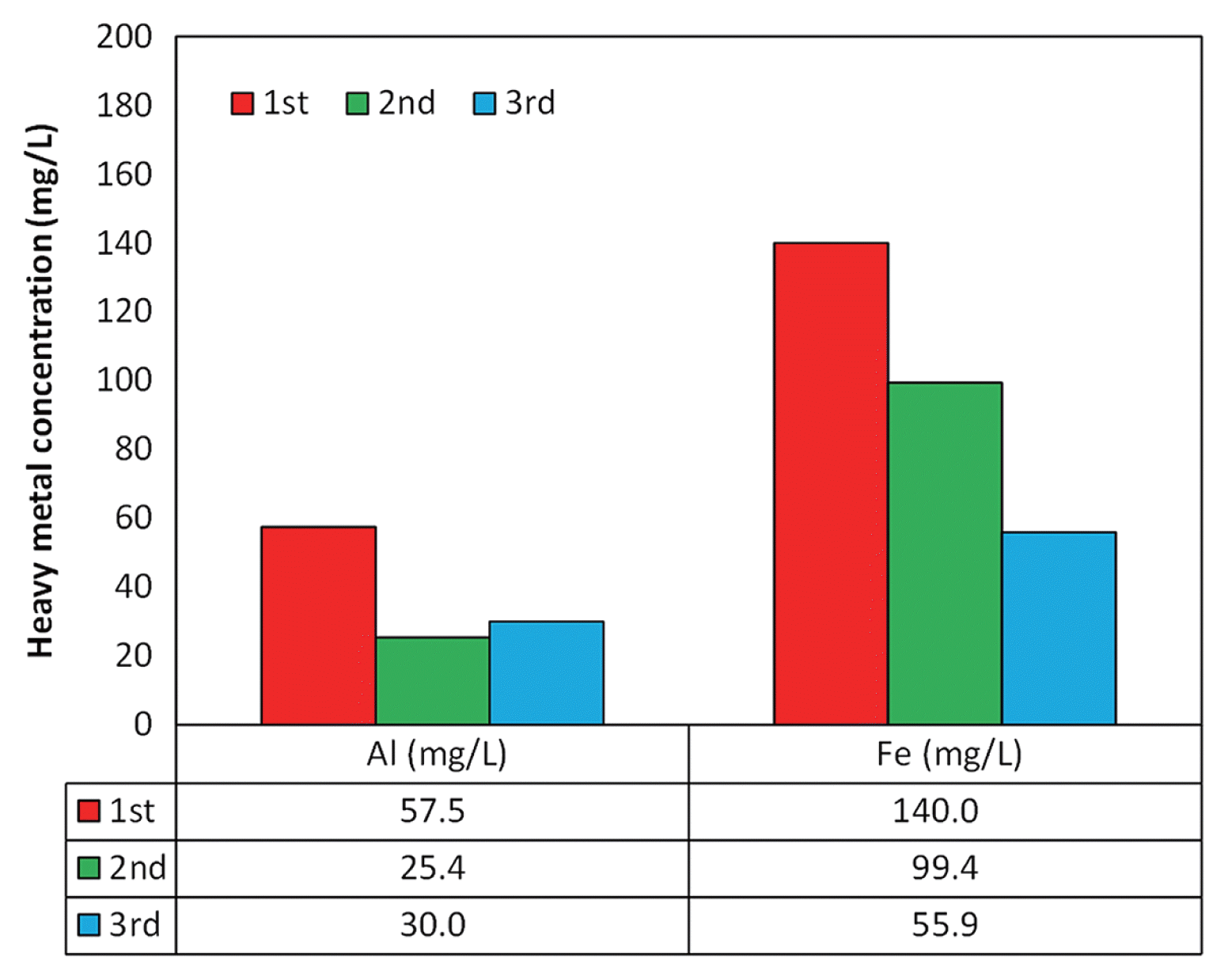
Pre-treatment of A.C. was conducted before the plate electrodes were made to increase the specific surface area of A.C. and to remove metal ions increasing the electric resistance of A.C electrode. Using 0.1 N HCl, acid washing was carried out three times and metallic properties of the supernatant were analyzed using ICP analysis. Fig. 5 shows the results of metal ion analysis. The main metals contained in A.C. were aluminum and iron. As a result of greater than triplicate acid washing, it was confirmed that the metal content was gradually reduced with washing. In addition, the specific surface area of A.C. after pre-treatment increased by 700 to 900 m2/g.
As the gaseous matters were expected to be captured by the pores of A.C., it was considered important to retain a high pore volume. However, PVAc was used as a binder agent to attach A.C., and while it has a suitable adhesiveness, it may clog the pores of A.C. Therefore, methanol was injected at a pressure of 1 bar, before the coating components for electrodes plate were mixed in an effort to form and keep the pore volume, and then the methanol was lost from the pores during the drying process.
Through the preceding studies, the proper ratio of A.C., black carbon, and PVAc appeared to be 10 g: 2 g: 3 g [10]. To be attached evenly on the plat surface, the coating materials should become a type of slurry with the proper use of solvent. Methanol, ethanol, and acetone were used and the optimum amount for the injection was investigated. Among them, only methanol appeared to dissolve PVAc properly. To the coating mixture at the ratio of A.C., black carbon, and PVAc (10 g: 2 g: 3 g), gradient methanol solutions were applied to find out optimum injection amount as a solvent: 5, 10, 15, 20, and 25 mL. As a result, when 20 mL of methanol was injected, the slurry was formed with a suitable texture and uniform coating was observed over an aluminum plate. In addition, cracking on the coating surface did not occur and the uniform surface remained after drying. When 25 mL of methanol was injected, the slurry became to watery and did not attach to the plate. Consequently, 20 mL of methanol was found to be the optimal injection amount.
Fig. 6 shows A.C. plates which were fabricated from A.C., black carbon, PVAc, and methanol at the proper composition ratio. The resulting A.C electrode was a very firm construction, and the coating was not eliminated even by scratching. As such, it was determined to be suitable for use in a dust collection system.
3.2. Removal of Gaseous Matters
One of the reasons for the introduction of the A.C. plate electrodes to electrostatic dust collecting technology is that A.C. shows excellent performance to absorb gaseous pollutants. Accordingly, experiments in a batch-type were carried out in a Tedlar bag by taking A.C. from the fabricated A.C. plate electrodes.
Figs. 7 and 8 show the results from experiments to remove NH3 gas and H2S gas. As shown in Fig. 7, NH3 gas was rapidly removed and was absorbed in A.C. The reaction speed dropped quickly after about 10 sec. Fig. 8 shows that the initial response was very fast in the removal speed of H2S as in the case of NH3. Likely, A.C. showed very high removal efficiency for both gaseous matters.
3.3. Dust Collection Efficiency
In the experiments for dust collection, voltages of 5 kV and 10 kV were applied into the system and the results were compared. Increasing concentrations of dust were applied so that the relationship between dust collection efficiency and the numbers of electrodes could be identified. Each module contained 11 A.C. coated electrodes at a 1-cm distance from each other.
Figs. 9 and 10 show the experimental results of dust collection when 5 kV and 10 kV were applied, respectively. The level of influent dust was about 3,000 to 4,000 μg/m3 and the effluent dust levels were reduced down to about 2,000 μg/m3. The subsequent removal efficiency appeared to be about 40% to 50%. As shown in Fig. 10, when 10 kV of voltage was applied, 65% to 70% of removal efficiency appeared at 2,500 to 4,500 μg/m3 of influent dust concentration, reaching 1,000 to 1,500 μg/m3 of effluent concentration. Consequently, efficiency increases 1.5 times higher with a 10 kV application compared to 5 kV.
Fig. 11 shows the results from the experiments per number of dust collecting modules. The experiments per number of dust collecting modules were carried out under the same conditions, except for the number of dust collecting modules. Flow rate was 50 L/min, as controlled by an air pump and dust-containing air was generated by a dust generator at a speed of 35 L/min. Voltage was 10 kV and the distance between electrodes was 1 cm. The linear velocity under the condition was 6 cm/s and the retention time per module was about 1.4 sec. The concentration of influent dust was set to about 3,000 to 4,000 μg/m3. Dust removal efficiency increased as the number of modules increased. The average removal efficiencies were 67.5% for a single module, 76.5% for two modules, 84% for three modules, and 88% for four modules. The concentration of effluent dust was 330 μg/m3 which results in a removal efficiency of about 90%. Regarding that regulation of dust level is 50 μg/m3, about 98.6% of removal efficiency is required when initial dust concentration is 3,400 μg/m3

Dust removal efficiency at different number of dust collecting modules at 10 kV (a) 1 module, (b) 2 modules, (c) 3 modules, and (d) 4 modules.
Fig. 12 shows correlations between the number of electrodes used in the dust collecting experiment and the dust collection efficiency. As 11 electrode plates were inserted into a single dust collection module, the corresponding number of electrode plates which were used became 11, 22, 33, and 44 pieces, by increasing module numbers. As a result, a relational equation between the number of dust collection modules and the removal efficiency was revealed:
The correlation coefficient R2 of the relational equation with the experimental data was 0.9945, showing a very high correlation. About 90 pieces of electrode plates are expected to be necessary to show 99% of dust collecting efficiency if other conditions are the same when this trend line is extended.
About 90% of removal efficiency was not so surprising when four dust collection modules equipped with 44 electrode plates were used. However, regarding that the dust particle size used in this experiment was the smallest among JIS industrial dust standard, 90% of removal efficiency is thought to be high. In addition, the initial influent dust was maintained at relatively high levels of 3,000 to 4,000 μg/m3, 6 to 7 times higher than levels used in other studies. The influent dust level for this study was set relatively high in order to evaluate the performance of dust the collection system at extreme conditions. It is also planned to test lower dust condition, such as 500 μg/m3, and in addition, extended experimental designs will be applied with the use of influent dust in various particle sizes and with various mixing ratios in the near future.
4. Conclusions
This study developed a new dust collection system by introducing A.C. coated electrodes to supplement the existing electrostatic dust collecting technology. Metal ions were removed and the specific surface area was enhanced through the pre-treatment of A.C. The optimal mixing ratio of A.C., black carbon, PVAc, and methanol for coating materials on A.C. plate electrodes was determined, and subsequently, electrodes were fabricated. Experiments for the removal of gaseous matters and dust collection were carried out with the fabricated electrodes. In the batch-type gas removal experiment, high concentrations of both NH3 and H2S gases were removed easily during the initial 10 sec. then the reaction rates dropped, and gradually decreased over time. From the dust collection studies, it was confirmed that dust collection was favorable in the conditions of higher voltages (10 kV rather than 5 kV), and the collection efficiency becomes higher when a greater number of electrodes are applied. As high as 90% dust removal was achieved, with high influent dust concentration (3,000 to 4,000 μg/m3), which shows promising performance, even for higher concentrations of dust collection.
Acknowledgments
This research was supported by a grant from a Strategic Research Project (2013-0048-1-1) funded by the Korea Institute of Construction Technology.