Impact of Current Density, Operating Time and pH of Textile Wastewater Treatment by Electrocoagulation Process
Article information
Abstract
Treatment of textile wastewater by the electrocoagulation (EC) process is being investigated by this experimental study. The objective of this experiment is to observe the efficiency of the EC process in removing chemical oxygen demand (COD) and turbidity. In this experiment an iron electrode is used in the EC process, and different working parameters such as pH, current density and operating time were studied in an attempt to achieve a higher removal capacity. The results show that the maximum COD removal occurred at neutral pH at operating time 30 min. COD and turbidity removal reaches at maximum, with optimum consumption of electrodes, between current density 85–95 A/m2, and only trace amounts of metals were determined in the EC treated effluent.
1. Introduction
The textile industry uses large volumes of water in dyeing and finishing processes, and the resultant wastewater generally has a high chemical oxygen demand (COD) and turbidity. Wastewater from dyeing and finishing processes, with a COD concentration which may exceed 1,600 mg/L and a strong dark color, is categorized as a highly contaminated wastewater [1–3]. It is a significant source of environmental pollution. Textile wastewater is known to exhibit a strong color, a large amount of suspended solids, highly fluctuating pH, high temperature, and a high COD concentration [4]. Different combinations of treatment methods have been proposed for the treatment of wastewater arising from the textile industry. Techniques for the removal for coloring substances, including adsorption, precipitation, coagulation, filtration, and chemical oxidation, have been studied by many researchers [5–9].
Coagulation is one of the most important physiochemical operations used in water treatment, used for the aggregation of smaller particles into larger particles. Electrocoagulation (EC) is a water treatment process whereby an electric current is applied across metal plates to remove various contaminants from water. Coagulation occurs upon the application of a current, which sets the small particles in motion. EC is an effective means of reducing the residue of wastewater [10].
EC consists of pairs of electrodes which are arranged in pairs of two, with one serving as an anode and the other as a cathode. Based on the principles of electrochemistry, the cathode is oxidized (loses electrons), while the water is reduced (gain electrons), thereby making improving the treatment of the waste-water. When the cathode makes contact with the wastewater, the metal is emitted into the apparatus. When this happens, the particulates are neutralized by the formation of hydroxide complexes, which then form the basis of agglomerates. These agglomerates settle to the bottom of the tank and can be siphoned out through filtration [11]. The efficiency of EC is influenced by wastewater type, pH, current density, type of metal electrodes used (aluminum, steel, iron), number of electrodes, size of electrodes, and configuration of metals.
The inherent disadvantage associated with most chemical processes (activated carbon adsorption being an exception) is that they are additive processes. This problem is eliminated in the EC process.
In the EC process, when electrical current flows between two electrodes, coagulant is generated in situ by electrolytic oxidation of the anode material. With an iron anode, Fe(OH)2 or Fe(OH)3 is formed at the anode. The reaction mechanism of the iron electrode at the anode and cathode has been reported by many authors [12–16].
Mechanism 1
Anode:
Cathode:
Overall:
Mechanism 2
Anode:
Cathode:
Overall:
The Fe(OH)n(s) remains in the aqueous phase as a gelatinous suspension, which can remove the pollutants from the wastewater by either complex formation or electrostatic attraction, followed by coagulation. In the surface complex formation mode, the pollutant acts as a ligand (L) to chemically bind hydrous iron:
The per-hydrolysis of Fe3+ cations also leads to the formation of reactive clusters for wastewater treatment [16].
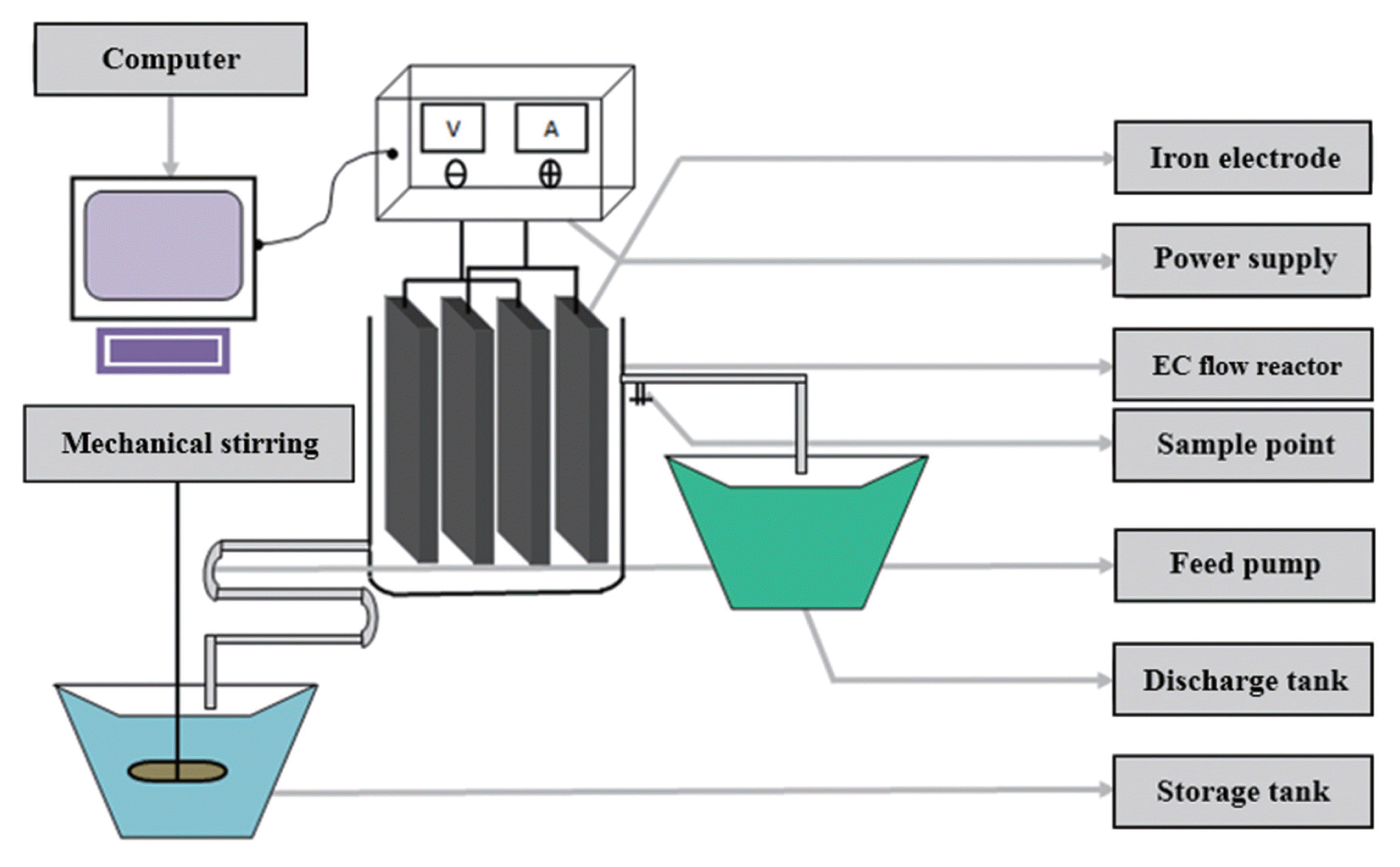
The present study is carried out at Intramex Textile Limited at Joydevpur, Gazipur, Bangladesh. Wastewater generated from pretreatment, dyeing and finishing are collected in a sewage tank within the industry before discharging. The collected wastewaters were treated by EC process shown in Fig. 1 for predetermined time interval and were analyzed for various parameters. In this experiment, the efficiency of EC process for the removal of residual color, COD and turbidity from textile wastewater have been elucidated.
2. Materials and Methods
2.1. Experimental Setup
There are ten iron electrodes placed in 1.5 cm distance between them and are parallel to each other with two power blades used in the experiment with the following specifications. Direct current (DC) is introduced into the chamber, the liquids then becomes a conductor between each and each blades allowing the DC current to pass freely throughout the chambers. The metal blades react to the current by releasing charged metal ions into the influent at an approximate rate of 0.40 kg per 10 m3 at a 60 min resonance time.
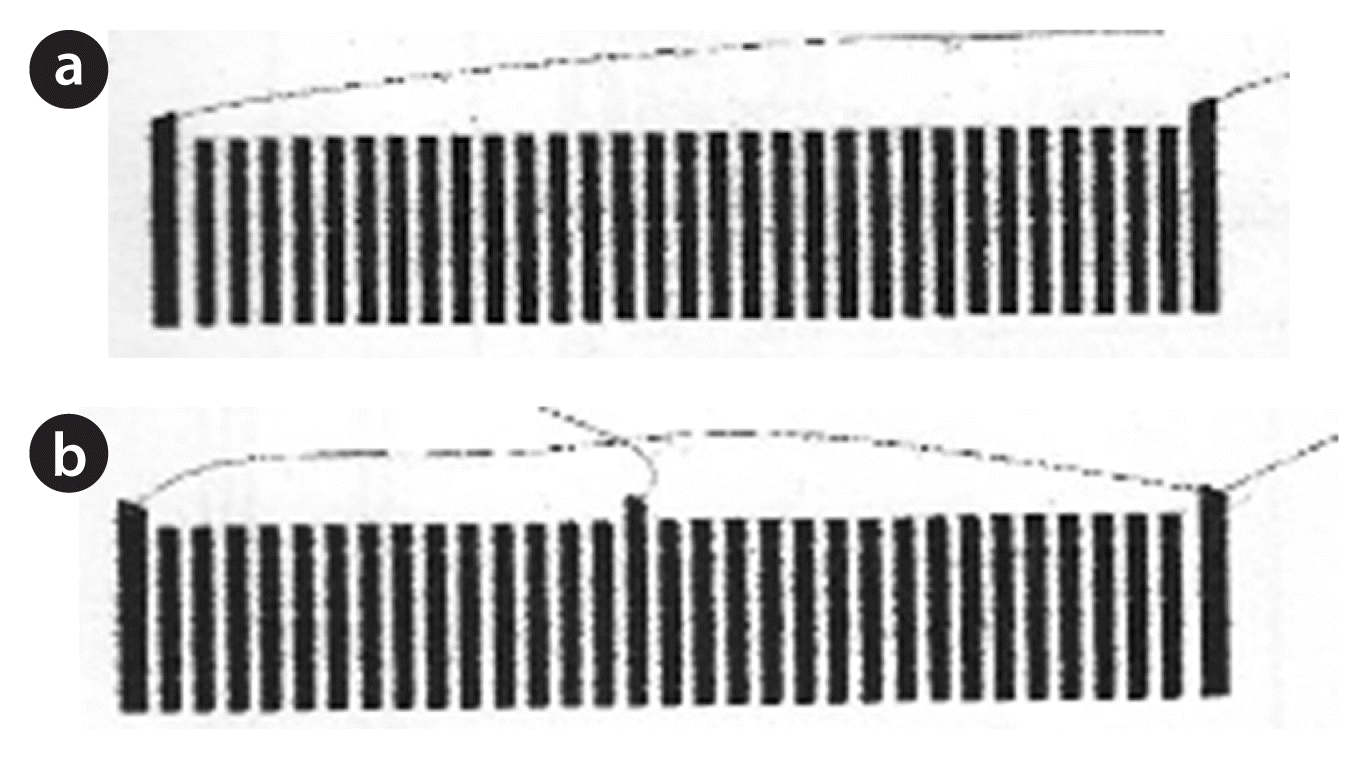
In the reaction chamber (capacity 280 m3) power blades are placed at opposite ends of the blade guides. Each power blade is connected to its appropriate supply line, and the power connection is cleaned with a wire brush. Although the reaction chamber is designed to accept three power blades, the power blades can be connected in different sequences, to provide two chamber configurations shown in Fig. 2, which is advantageous in cases of poor conductivity. In the case of a single connection, power cables are connected to the first and last power blades, but in case of double connections, power cables are connected to all power blades using electrical jump-leads. If the chamber is wired for single chamber connections (first and last) the current going through the water is the same as the reading on the meter. If the chamber is divided into two chamber connections, then the current going through the water will increase, and this is observed on the current meter. Non-conductive waters may need to have more chamber connections added, so as to increase the voltage in the system.
2.2. Analytical Method
The pH of the solutions was measured using a bench top pH meter (model HI 2211; Thermo Scientific, Waltham, MA, USA). Conductivity was measured using a WTW conductivity meter (Model 82362; WTW Wissenschaftlich-Technische Werkstätten, Weilheim, Germany). The color of the wastewater samples was analyzed using a HACH DR/2010 spectrophotometer (HACH, Loveland, CO, USA). COD was determined using potassium permanganate as oxidizing agent, following a standard method which is detailed elsewhere. Turbidity was determined using a Shimadzu model UV-160 (Shimadzu, Kyoto, Japan) double beam spectrophotometer.
3. Results and Discussion
The characteristics of wastewater in both the equalization tank and the outlet of the effluent treatment plant (ETP) are shown in Table 1. Wastewater was collected from Intramex Textile Limited on August, 2011. All the data presented in the tables and figures in this experiment are the mean values of eight test samples. The experiment was carried out at room temperature. Color, pH, turbidity, total dissolved solids, total suspended solids, biochemical oxygen demand, COD were analyzed using the United States Environmental Protection Agency methods [18].
3.1. Effect of Current Density
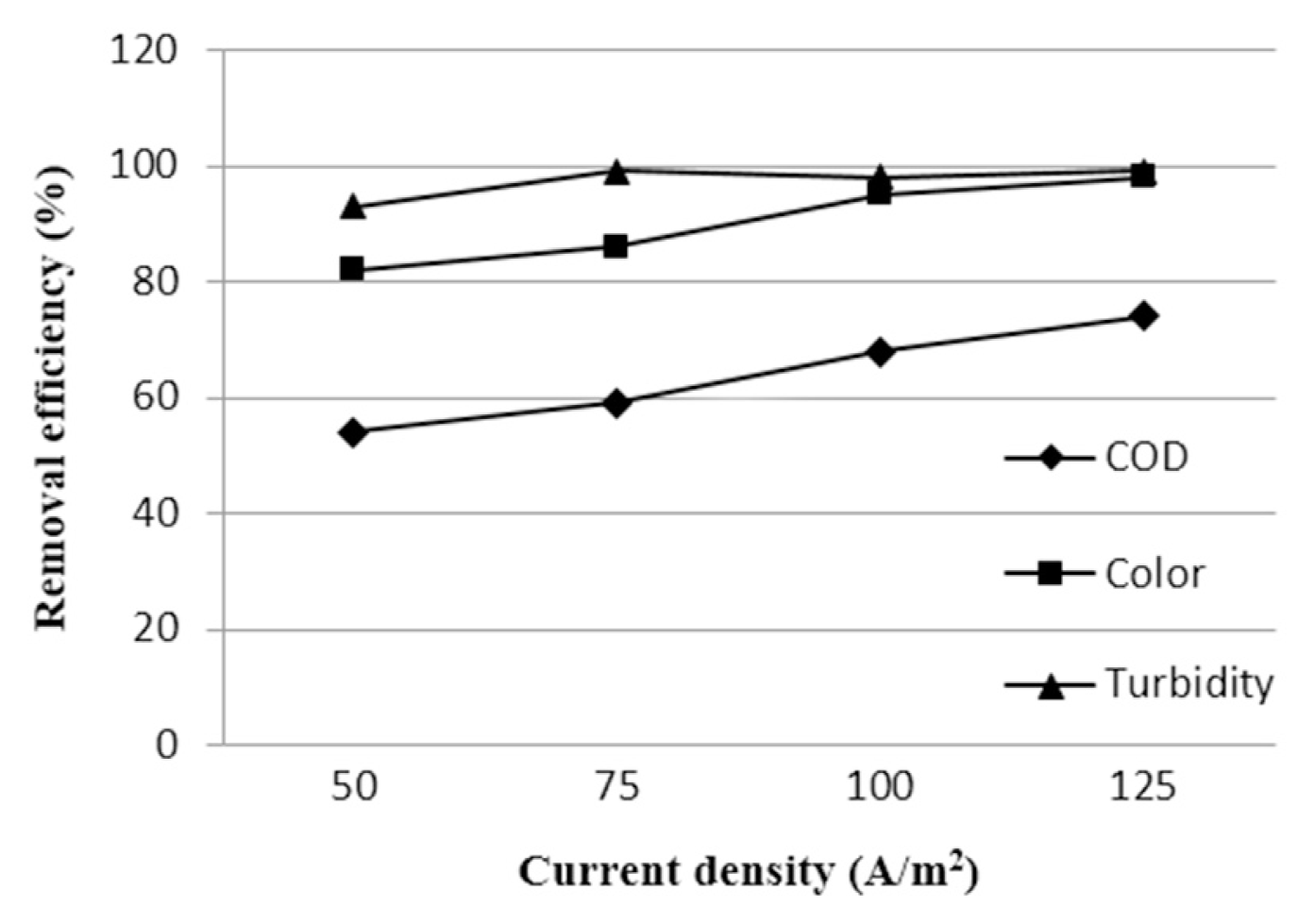
The effect of current density on COD, color and turbidity removal percentages is illustrated in Fig. 3. With the increase of current density from 50 to 125 A/m2 removal efficiency also increases. This is due to the higher amount of ions produced on the electrodes promoting destabilization of the pollutant molecules. From Fig. 3 it is found that, turbidity removal percentage maintains a plateau from current density 75 to 125 A/m2. Conversely, COD removal percentage shows a gradual upward trend, which is almost same for color removal efficiency. From the experiment, it was also found that if the current density exceeds 95 A/m2 then electrodes start to decay quickly, which will result in a limited life time of electrodes. In addition, the removal percentage of color and turbidity is not so significant after the current density of 95 A/m2. Increasing the electrical power reduces the lifespan of the electrodes (current drawn is directly proportional to voltage input). Hence, a current density of 85–95 A/m2 is optimal for higher removal efficiency. So, it is very important to ensure optimum current density supply in the EC process.
3.2. Effect of Operating Time
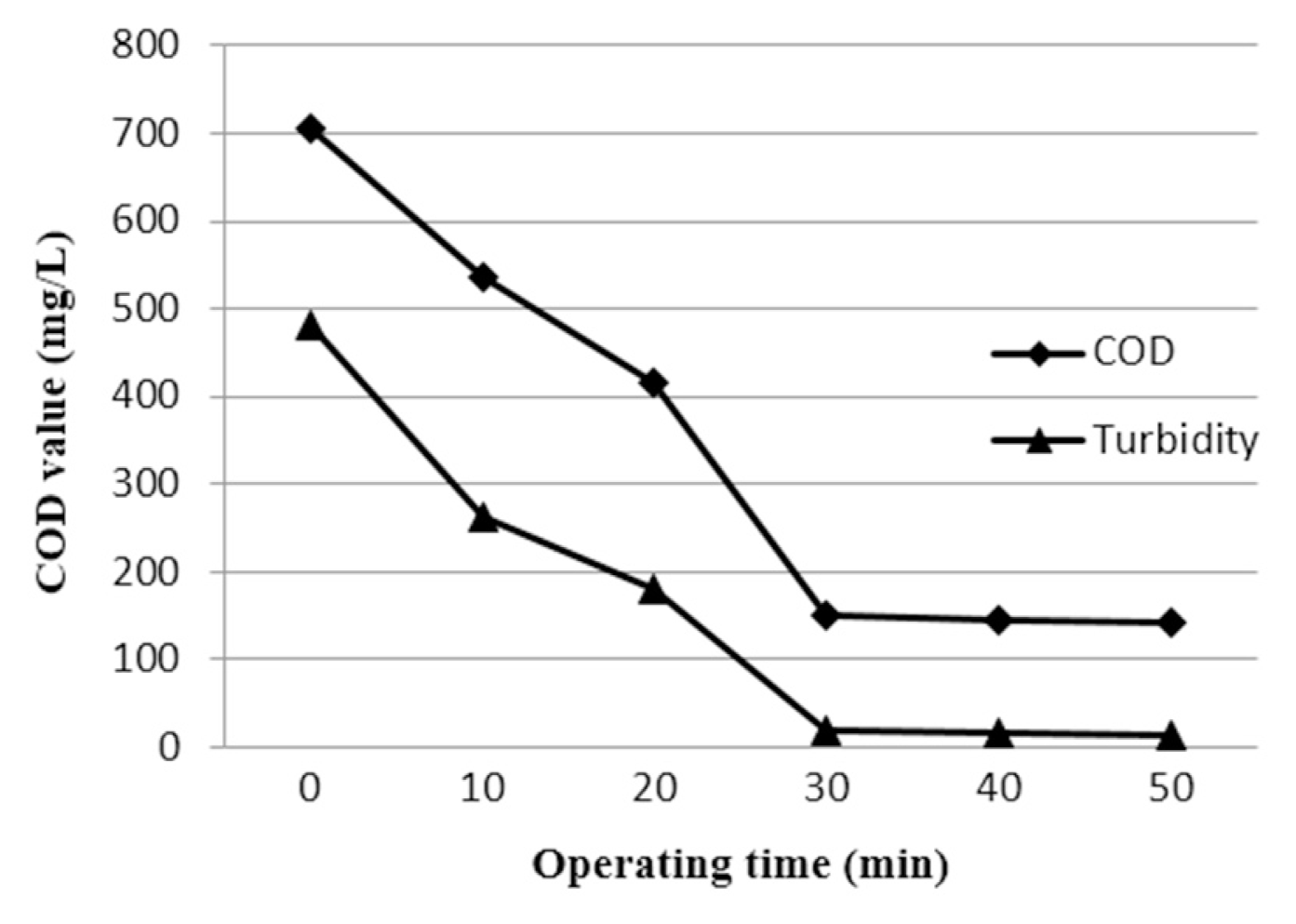
The effect of operating time was studied at a current density 100 A/m2. Fig. 4 depicts the effect of operating time on the COD and turbidity removal efficiency. By changing the operating time from 10 to 50 min the removal of COD and turbidity increases from 23.97% to 79.86% and 45.21% to 96.88%, respectively. In this process, EC involves two stages: destabilization and aggregation. The first stage is usually short, whereas the second stage is relatively long. Metal ions, as destabilization agents, are produced at the anode thorough electrochemical reactions. This result in a low charge loading when the operating time is shortened, whereupon the metal ion (Fe3+) dosage was found to be insufficient to destabilize all colloidal and finely suspended particles. Thus, the COD and turbidity removal efficiencies were not high. Results show that the maximum efficiency of the EC process was obtained at a treatment time of 30 min, and a further increase in treatment time did not result in any significant improvement in the removal efficiency of the studied parameters.
3.3. Effect of pH on COD Removal
Several authors have indicated that pH is an important factor influencing the performance of the EC process [1, 19–23]. Generally, the pH of the medium changes during the operating process and this change depends on the type of electrode material and on initial pH. On the other hand, the EC process exhibits some buffering capacity, especially in alkaline medium, which prevents large changes in pH and decreases the pollutant removal efficiency [24].
The effect of varying the initial pH on COD removal efficiency is presented in Fig. 5. From the figure it is seen that COD removal is higher at neutral pH, than at pH values of 5–6. The formation of ferrous ions and their posterior oxidation to ferric ions cause the precipitation of Fe(OH)3, and it is likely that this oxidation by oxygen becomes higher as the pH increases. The higher value of removal efficiency at neutral pH may be due to the formation of Fe(OH)3 and this compound may be responsible for the removal of the major part of the impurities in textile wastewater.
4. Conclusions
The purpose of this study was to evaluate the effectiveness of EC for the treatment of textile wastewater. It was observed that initial pH, operating time and current density significantly affect the removal of impurities from wastewater. Removal of COD is higher at neutral pH. Operating time till to 30 min gives better result in removing maximum COD. From current density 85 to 95 A/m2, removal percentage of color, COD, and turbidity is recorded as the highest, with optimum consumption of electrodes. In contrast, the EC treated effluent was relatively free of metals. This makes the EC unit an excellent choice for effluent treatment where irrigation is being considered.
Acknowledgments
This work was supported by the Kongju National University (No. 2011-0938).