Removal efficiency and mechanism of bio-treated coking wastewater by catalytic ozonation using MnO2 modified with anionic precursors
Article information
Abstract
In this paper, MnO2 catalyst were firstly prepared and modified by four kinds of anionic precursors (i.e., NO3−, AC−, SO42− and Cl−) through redox precipitation method. After that, bio-treated coking wastewater (BTCW) was prepared and employed as targeted pollutants to investigate the catalytic ozonation performance of prepared-MnO2 catalyst was investigated and characterized by the removal efficiencies and mechanism of the prepared bio-treated coking wastewater (BTCW), which was employed as the targeted pollutants. Specifically, the effects of specific surface area, crystal structure, valence state of Mn element and lattice oxygen content on catalytic activity of MnO2 materials were characterized by BET, XRD and XPS, respectively. Results showed that COD of BTCW could be removed 47.39% under MnO2-NO3− catalyst with 2 h reaction time, which was much higher than that of MnO2-AC− (3.94%), MnO2-SO42− (12.42%), MnO2-Cl− (12.94%) and pure O3 without catalyst (21.51%), respectively. So, MnO2-NO3− presented the highest catalytic performance among these catalysts. The reason may be attributed to a series of better physiochemical properties including the smaller average grain, the larger specific surface area and active groups, more crystal defect and oxygen vacancy, higher relative content of Mn3+ and adsorbed oxygen (Oads) than that of another three catalysts.
1. Introduction
Advanced oxidation processes (AOPs) that utilized free-radical reactions are considered as an effective and promising method for the degradation and mineralization refractory organic compounds in wastewater treatment. Many studies have been conducted on the degradation persistent organic compounds by AOPs technologies, such as photocatalysis process [1, 2], Fenton of Fenton-like oxidation [3, 4], ozonation [5, 6] and non-thermal plasma [7]. Among them, heterogeneous catalytic ozonation technology has been widely studied because they can generate more reactive oxygen species (ROS) to ensure highly efficient in degrading the refractory organic contaminants in wastewater. Among them, heterogeneous catalytic ozonation technology has been widely studied because they can generate more reactive oxygen species (ROS) to ensure highly efficient in degrading the refractory organic contaminants in wastewater [8–10]. Among heterogeneous catalytic ozonation technologies, catalytic ozonation using MnO2 catalyst has been widely used in wastewater treatment with the characteristics of complex composition, high organic matter concentration and recalcitrance [11–13]. In particular, Manganese dioxide (MnO2) has its advantages, such as unique Mn2+/Mn3+ redox cycle involving a single electron transfer, environmental friendliness and diverse crystallographic structure, which may help MnO2 been extensively developed for applications in heterogeneous catalytic ozonation technologies to produce active radicals [14–16]. Obviously, catalysts play a significant role in catalytic ozonation. Refractory organic pollutants could be efficiently degraded by MnO2 based catalyst owning the excellent physical and chemical properties. Some studies indicated that different preparation conditions have a certain effect on the catalytic performance of the catalyst, such as acid treatment [17], reactant ratio, reaction time and temperature [18], calcination time and temperature, precursor anions, etc. Although the precursor anions did not change the elemental composition of the MnO2 catalyst, it would largely affect the structural characteristics of the MnO2 catalyst and then generate different catalytic performance. Jung et al. [19] used various Mn2+ precursors to prepare MnO2 by a simple redox method, which were used to investigate the influence of Mn-precursors in the CO oxidation. The catalytic performance was arranged from high to low as: MnO2-SO42−, MnO2-AC−, MnO2-NO3−, MnO2-Cl−. Hwang et al. [20] used manganese nitrate and manganese acetate to prepare MnO2/TiO2 catalysts through a sol-gel method, concluding that the catalysts synthesized with manganese acetate showed high catalytic performance as a result of abundant MnO2 species and strong acid sites. Yue Yu, et al. [21] used manganese acetate, manganese nitrate, manganese chloride and manganese sulfate to prepare MnO2 catalysts on the toluene oxidation. The catalytic performance was arranged from high to low as: MnO2-AC−, MnO2-NO3−, MnO2-Cl−, MnO2-SO42−, which resulted in MnO2-AC−owned potential for practical applications. Pena et al. [22] observed that catalysts prepared using manganese nitrate yielded MnO2 and displayed enhanced catalytic performance than the corresponding catalyst prepared with manganese acetate. In fact, there remains on-going debate over how catalytic activity is influenced by the active phase of Mn-based catalysts prepared with different precursors. However, it is still unclear that the Bio-treated coking wastewater (BTCW) are degraded by catalytic ozonation using MnO2 catalyst modified with anionic precursors. So, it is interesting to know that the reaction mechanism and removal performance of BTCW is catalyzed by different MnO2 catalyst modified with anionic precursors.
BTCW removal has been widely studied by catalytic ozonation due to the characteristics of strong toxicity, structural stability and low concentration of organic matter [23]. Liu et al. [24] synthesized a kind of CuFe2O4 nanocomposite as catalyst to investigate and evaluate the feasibility of the catalytic ozonation for the treatment of biologically treated coking wastewater. Their results showed that the total organic carbon removal efficiency in the catalytic ozonation was 2.9 times higher than that in the un-catalyzed ozonation. Zhang et al. [25] prepared different kinds of zinc ferrite catalysts for ozonation of BTCW, which showed that the presence of catalysts also generated a high removal efficiency for BTCW compared to non-catalytic ozonation, and the performance of the catalysts was primarily determined by their synthesis method defining the surface properties. However, few researchers studied the catalytic performance and mechanism of ozonation of BTCW with different precursors of manganese dioxide.
In this study, BTCW was selected as the target pollutant in this study, four kinds of MnO2 catalysts were synthesized from various Mn2+ precursors of manganese chloride, manganese nitrate, manganese acetate and manganese sulfate by redox precipitation method. Moreover, based on evaluating the catalytic performance of four MnO2 catalysts in the process of ozone oxidation for removal of bio-refractory organic pollutants in BTCW, the role mechanisms of different precursors on catalytic activities will be analyzed by XRD, TEM, BET and XPS, respectively.
2. Experimental
2.1. Synthesis of Catalyst
The reagents and chemicals were of analytical grade and purchased from national drug chemical reagents Co. Ltd (China). The redox-precipitation method is a typical catalyst preparation method by liquid phase preparation process for manganese-based catalyst, which has the advantages of simple preparation procedure and mild reaction conditions and thus has been becoming the research hotspot in recent years [21]. So, the redox-precipitation method is employed and selected as the synthetic method for MnO2 based catalysts in this study. Furthermore, the structure defects and the crystallinity of the catalyst can be controlled and improved its performance by changing the preparation conditions such as choice of reactant precursor, reactant ratio, pH, reaction time, temperature and calcination time, respectively [21, 26, 27]. Therefore, the above preparation conditions need to be comprehensively considered to manipulate the synthetic process of catalysts. The preparation process of four MnO2 catalysts are showed as follows. Firstly, 100 mL (0.45 mol/L) of solution A (manganese nitrate solution, manganese acetate solution, manganese sulfate solution or manganese chloride solution) was added dropwise into 75 mL (0.2 mol/L) of solution B (potassium permanganate solution, KMnO4), which was stirred magnetically for 15 min. After that, ammonia was used to adjust the pH of the mixture to 1.5. After crystallizing for 11 h, the product was collected by filtration and fully rinsed several times with deionized water to remove the remaining KMnO4. Finally, the collecting production were dried for 13 h at 105°C in the oven and calcined at 400°C for 4.5 h in the tubular furnace, and the catalysts denoted as MnO2-SO42−, MnO2-AC−, MnO2-Cl− and MnO2-NO3− were obtained, which were used to carry the oxidizing experiments and characteristic analysis, respectively.
2.2. Characterization of Catalysts
The atomic arrangement inside the crystal was determined by X-ray powder diffraction (model: D8 ADVANCE, Bruker, Germany). The XRD had monochromatic radiation Cu-Kα as the source of X-rays and operates at 40 kV, 30 mA. The surface compositions of the catalysts were determined by x-ray photoelectron spectroscopy (XPS), which was performed with ESCALAB250Xi system equipped with an Al-Kα excitation source and operated at 15 kW and 1486.6 eV. The spectra were fitted with Casa XPS software. Charging effects were corrected by adjusting the binding energy of C 1 s to 284.8 eV.
2.3. Catalyst Activity Test
The catalytic ozonation treatment of simulated BTCW was performed in a semi-batch reactor showed in Fig. S1. The fixed reactor adopted a double-layer design and the inner layer was the catalytic ozonation reaction layer fed with 1 L aqueous solution. The constant temperature of water bath pot was used to keep the stable temperature of the inner layer reaction. Ozone was generated by ozone generator (COM-AD-01, Germany) from oxygen with high purity. During the reaction, the gaseous ozone was fed through a porous diffuser at the bottom of the reactor with a flow rate of 2.0 L/min and the concentration of 2.6 mg/L. The tail gas produced in the experiment was absorbed by potassium iodide solution. In the typical catalytic ozonation procedure, 2 g catalyst was mixed with 1 L simulated BTCW (cyclohexanone: aniline = 20:1, pH = 7.2, 323 ± 1 K; the initial COD was 470 mg/L) in the reaction system. During the reaction process, the samples were taken to analyze at certain intervals.
3. Results and Discussion
3.1. Catalytic Ozonation Performances
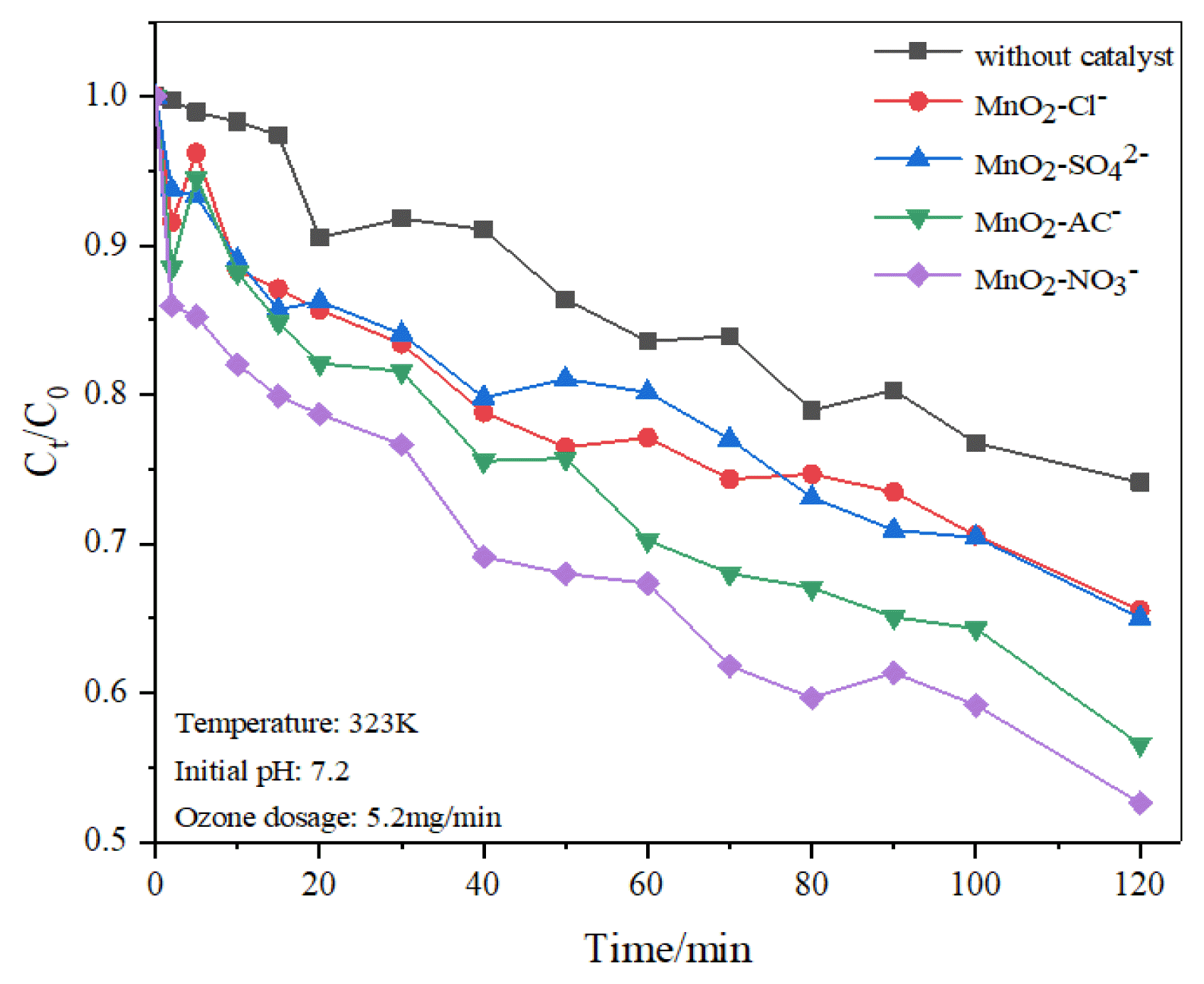
The degradation performance of BTCW by ozonation and catalytic ozonation is showed in Fig. 1. Compared with the alone ozonation process, it can be observed that the degradation rates of BTCW are greatly improved with the addition of the prepared-catalysts. The degradation rates are accordingly increased about 8.57%, 9.09%, 17.57% and 21.51%, respectively when MnO2-Cl−, MnO2-SO42−, MnO2-AC− and MnO2-NO3− are employed as the catalysts to enhance the ozonation efficiency on BTCW. The difference may be due to the different morphology and distribution of active components formed on the catalyst surface after calcination of different precursors. The catalytic reaction mechanism will be further discussed in part 3.2 through part 3.5. Reaction kinetics of four prepared-catalysts were further investigated to the catalytic ozonation performance. The mathematic equation of pseudo first-order and second-order kinetic model could be expressed as Eq. (1) and Eq. (2), respectively.
Where K1 is the pseudo first-order rate constant (min−1); K2 is the pseudo second-order rate constant ((mg/L)−1min−1); t represents the reaction time; C0 and Ct stand for the COD concentration at 0 min and t min, respectively.
The fitting curves and kinetic parameters of pseudo first-order and pseudo second-order reaction kinetics for different ozonation are showed in Fig. 2(a), (b) and Table S1. As showed in Table S1, the linear correlation coefficient R2 of the second-order reaction kinetics was significantly higher than that of the first-order reaction kinetics. Therefore, under the condition of ozonation and catalytic ozonation, the degradation process of BTCW was more in line with the pseudo second-order reaction kinetics. Fig. 2(b) and Table S1 show that the rate constants (K2) of catalytic ozonation of MnO2-Cl−, MnO2-SO42−, MnO2-AC− and MnO2-NO3− were calculated to be 0.00297, 0.00367, 0.00377, 0.00544 and 0.00625 (mg/L)−1min−1, respectively. The kinetic rate of MnO2-NO3− catalyst are almost two times of ozonation without catalyst. To sum up, four kinds of prepared-catalysts have different catalytic ozonation performance and nitrate modified MnO2 (i.e., MnO2-NO3−) has the highest catalytic ozonation activity on BTCW.
3.2. Specific Surface and Pore size Distribution
The results of BET surface area for different catalysts are showed in Table 1. Compared with the other three catalysts, MnO2-NO3− catalyst has the highest specific surface area, which is about 9 times of MnO2-Cl− catalyst and thus MnO2-NO3− catalyst has the best catalytic activities. Moreover, the total pore volume of MnO2-NO3− is also the highest among the four different MnO2 catalysts, which is beneficial to the adsorption and then increases the mass transfer rate between catalyst and BTCW [28]. So, the highest kinetic and removal rate through MnO2-NO3− catalyst are observed in Fig. 1 and Fig. 2, respectively.
3.3. Crystal Phase
In order to analyze the crystalline structure of the different MnO2 catalysts, the XRD studies were carried out in this section. The XRD patterns of MnO2 prepared by different precursor anions (NO3−, AC−, SO42−, Cl−) are presented in Fig. 3. The MnO2-SO42− and MnO2-Cl− catalyst have the characteristic diffraction peaks at around 2θ = 28.2°, 37.5°, 42.9° and 56.7°, and these diffraction peaks are assigned to the (110), (101) and (211) reflections of β-MnO2 (JCPDS No.24-0735 or JCPDS No.81-2261). The MnO2-NO3− catalyst has the diffraction peaks at around 2θ = 37.7°, 42.8° and 56.9°, and these diffraction peaks are assigned to the (101), (111) and (211) reflections of β-MnO2 (JCPDS No.24-0735 or JCPDS No.81-2261). The MnO2-AC− catalyst has characteristic diffraction peaks at around 2θ = 12.9°, 18.2°, 28.6°, 37.4° and these diffraction peaks are assigned to the (110), (200), (310), (121) and (301) reflections of α-MnO2 (JCPDS No.44-0141 or JCPDS No.72-1982). From the above XRD results, it can be seen that the different anions affect the crystallization and phase transition of catalysts to some extent [21].
According to the principle of Voigt single line method [29], the average size and lattice distortion of MnO2 grain could be calculated from the diffraction peak parameters of the crystal plane. The calculation results are showed in Table 2. The average grain size of MnO2 material was arranged from high to low as: MnO2-Cl−, MnO2-SO42−, MnO2-AC−, MnO2-NO3−, and the micro strain capacity was arranged from high to low as: MnO2-NO3−, MnO2-AC−, MnO2-SO42−, MnO2-Cl−. Some studies indicated that the crystal defect was the main cause of lattice distortion, which might promote the migration of adsorbed oxygen, and the migration of adsorbed oxygen played an important role in the decomposition and transformation of organic matter, and finally affected the catalytic performance of the catalyst [30, 31]. Therefore, although all the four MnO2 materials have some crystal defects, the MnO2-NO3− catalyst has the smallest grain size and the largest lattice distortion, which might be also a reason that this catalyst has the best catalytic activities in the process of ozonation for removing organic pollutants.
3.4. Surface Chemical Compositions
Oxygen vacancy is one of the most common crystal defects, which has significant influence on the catalytic ozonation performance of MnO2 materials. The presence of Mn3+ on the surface of MnO2 material is an important reason for the occurrence of oxygen vacancy, and its reaction mechanism is showed as follows [32]:
Where Δ represents oxygen vacancy, so a higher Mn3+ relative content indicates that there are more oxygen vacancies in MnO2 material.
In order to analyze the influence of four precursor anions on the valence state of Mn on the surface of MnO2, Peak-splitting fitting was performed on energy spectrum (narrow sweep) of four MnO2 materials through XPS PEAK41. Fig. 4(a) illustrated Mn 2p XPS spectra of MnO2 prepared by different precursor anions. Due to existing a fixed energy interval and peak area ratio between Mn2p1/2 and Mn2p3/2 in MnO2, in this article, only Mn2p3/2 was analyzed in detail. It could be seen from Fig. 4(a) that MnO2 materials prepared by four anions could be divided into two characteristic peaks in Mn2p3/2, and the binder energy of the peak at 641.8ev and 643.3ev was attributed to Mn3+ and Mn4+, respectively. As was showed in Table 3, the Mn3+ relative content of MnO2-NO3− was the highest at 44.27%, the Mn3+ relative content of MnO2-Cl−, nO2-SO42−and MnO2-AC− was 34.94%, 36.11% and 39.26%, respectively. Moreover, the MnO2-NO3− catalyst had the highest molar ratio of Mn3+/Mn4+ on the surface. Therefore, MnO2-NO3− had the most oxygen vacancy, which would promote the conversion of ozone to reactive oxygen species (ROS) and beneficial to the activation and migration of oxygen in the gas phase [33].
Furthermore, two types of surface oxygen species were identified according to the O 1s spectra in Fig. 4(b). The peaks were located at 529.6–529.8ev and 531.4–531.5ev corresponding to lattice oxygen (Olat) and adsorbed oxygen (Oads), respectively [34]. Olat was the oxygen in the crystal structure, and the different relative contents of Mn3+ and Mn4+ of the four catalysts led to the difference in the binding energy of lattice oxygen. The Oads was the oxygen on the crystal surface, mainly including •OH, •O−, •O2− and other active groups, which had an important impact on the catalytic performance of MnO2 [35]. The adsorbed oxygen had higher mobility than that of surface lattice oxygen, so adsorbed oxygen played more important role in oxidation reactions than lattice oxygen [24, 36]. In this work, the MnO2-NO3− catalyst contained the highest surface adsorbed oxygen ratio Oads/Olat at 0.41, which was corresponding to its highest content of active groups on its surface, and had the highest catalystic activities among four MnO2 catalysts.
3.5. Morphology Formation of Catalyst
The XPS energy spectra (wide sweep) of MnO2 prepared by different precursor anions (NO3−, AC−, SO42−, Cl−) were presented in Fig. 5. In terms of the MnO2-SO42− and MnO2-Cl− and MnO2-NO3− catalyst, they all contain the elements of Mn, O and C, but the MnO2-AC− catalyst contains the elements of K, Mn, O and C, and the difference is mainly related to the crystal type of the catalyst. From the above XRD results, it can be seen that the MnO2-SO42− and MnO2-Cl− and MnO2-NO3− are all β-MnO2 crystals, while the catalyst MnO2-AC− is α-MnO2.
α-MnO2 is the most common crystalline form of manganese oxide, and the internal channel structure of α-MnO2 is of [2×2] type and the basic structural unit is [MnO6], which stacks in the form of hexagonal density. In the process of α-MnO2 crystal formation, cations often enter into the pores due to its large channel radius, and the ion radius of K+ is similar to the channel size of α-MnO2 crystal, so K+ can play the role of template in the synthesis of α-MnO2 [37, 38]. Additionally, Studies had showed that appropriate K+ concentration could stabilize the [2×2] tunnel structure of α-MnO2, and is a key factor in the formation of α-MnO2 [39]. Therefore, the MnO2-AC− containing K+ is considered to be α-MnO2. However, H+ and K+ have a competitive relationship for occupying the [2×2] tunnel site because of the proton exchange under the conditions of high acidity in this study [40]. The addition of weakly alkaline ion would lead to the decrease of H+ concentration and K+ dominates competition between K + and H + in the tunnel structure. The high concentration of H+ will greatly reduce the chance of K+ occupying the active site of [2×2] tunnel, therefore, the highly acidic conditions are conducive to the generation of [1×1] tunnel structure of MnO2, which might be the reason that the MnO2-SO42−, MnO2-Cl− and MnO2-NO3− are β crystal forms and the constituent elements do not contain K ions.
3.5.1. MnO2-AC−
According to the characterization analysis of XRD and XPS, MnO2-AC− is α-MnO2 crystals. In addition, Fig 3 shows that the intensity of XRD diffraction peak of MnO2-AC− is weaker than that of MnO2-SO42− and MnO2-Cl−. Studies have showed that the smaller the intensity of the diffraction peak, the weaker the crystallinity and the smaller the grain size, which is more conducive to the selective adsorption of some organic molecules [41]. This is why the catalytic activities of MnO2-AC− catalyst is stronger than that of MnO2-SO42− and MnO2-Cl− catalyst. However, the catalytic activities of MnO2-AC− catalyst is weaker than that of MnO2-NO3− catalyst. The reason for this phenomenon may be that AC−, which has the largest ionic radius among the four anions, limits the diffusion rate of the precursors Mn2+ and Mn7+, and then results in a reduction in the redox reaction rate.
3.5.2. MnO2-Cl−
MnO2-Cl− halargest grain size among the three β crystal catalysts. This might because the Stokes radius of Cl− was the smallest among the four precursor anions, and it could be uniformly adsorbed on the (110), (101), (111) and (211) crystal plane [42]. The presence of Cl− reduced the surface energy of the crystal plane, resulting in a low growth rate of the crystal plane, which ultimately led to a large exposed area of the crystal plane and a strong diffraction peak intensity. Therefore, MnO2-Cl− has the largest grain size, the best crystallinity and the fewest crystal defects. In addition, the MnO2-Cl− has the smallest specific surface area (14.683 m2/g), the smallest pore volume (0.0013 cm3/g) and a lowest ratio of Mn3+/Mn4+ (0.54). The above reasons result in poor catalytic performance of MnO2-Cl−.
3.5.3. MnO2-SO42−
Among three β crystal catalysts, the (110) crystal plane diffraction peak of MnO2-SO42− is widened. This phenomenon might be attributed to the fact that the SO42− ion has a large radius and can only preferentially adsorbed on the (111) crystal plane with the lowest Mn4+ density, and then the steric hindrance effect is formed [29]. Therefore, the growth of the crystal along the crystal plane is restricted, and the (110) crystal plane with higher Mn4+ density becomes the preferential growth plane, which eventually leads to the phenomenon of broadening the diffraction peak of the (110) crystal plane. In addition, the diffraction peak intensity of MnO2-SO42− is weaker than MnO2-Cl− but stronger than MnO2-NO3−. Therefore, MnO2-SO42− has low crystallinity and some crystal defects, which in turn show good catalytic activity. However, SO42− interacts with Mn2+ in the system, and its larger ionic radius reduces the diffusion rate of the reactants, thereby reducing the rate of product formation, so the product obtained is also less than that obtained under other conditions [43].
3.5.4. MnO2-NO3−
The diffraction peak intensity of MnO2-NO3− was the weakest and the (110) crystal plane disappears. The above phenomenon indicates that MnO2-NO3− has low crystallinity, imperfect crystal growth, and many crystal defects. Complementing this, the XPS characterization results indicate that the MnO2-NO3− has the highest ratio of Mn3+/Mn4+ (0.79) and the most oxygen vacancies. At the same time, XRD characterization shows that the average grain size of MnO2-NO3− is the smallest (11.9 nm), which leads to the smallest crystal plane spacing and grain gap. This will inevitably result in the largest specific surface area of the MnO2-NO3− (132.303 m2/g), which is consistent with the BET characterization results. Also, XPS characterization shows that the surface of MnO2-NO3− has more adsorbed oxygen (such as surface hydroxyl groups), and crystal defects can further promote the migration of adsorbed oxygen with a higher content of the catalyst surface, while the migration of adsorbed oxygen plays an important role in the decomposition and conversion of ozone, which ultimately affects the catalytic performance of the catalyst. So, MnO2-NO3− exhibits the best catalytic activity.
4. Conclusion
Four MnO2 samples were prepared by the redox precipitation method using KMnO4 and various Mn2+ precursors for catalytic ozonation. The different anionic precursors forming structure form of MnO2 and its catalytic ozonation performance on simulated BTCW were studied. The results showed that anion precursors played a great role in controlling the morphology of the product MnO2, but it did not work in the catalytic performance. The surface chemical composition, content, specific surface area and crystal structure of four MnO2 materials prepared by different precursor anions (NO3−, AC−, SO42−, Cl−) were significantly different. Among them, MnO2-NO3− had the smallest average grain size, the largest crystal defect, the highest relative content of Mn3+, the most oxygen vacancy, the highest relative content of adsorbed oxygen (Oads), the most surface active groups and the largest specific surface area. The combined action of these factors finally improved the catalytic ozonation performance of MnO2-NO3−. After 2 h catalytic ozonation, the COD removal rates by MnO2-NO3− reached 47.39%, which was 3.94%, 12.42%, 12.94% and 21.51% higher than that of MnO2-AC−, MnO2-SO42−, MnO2-Cl− and pure O3 systems, respectively.
Supplementary Information
Acknowledgment
This study was supported by the Fundamental Research Funds for the Central Universiies (2019XKQYMS78) and China Postdoctoral Science Foundation (Grant No. 2019M650131).
Notes
Author Contributions
D.B. (M.D. student) and D.D. (M.D. student) conducted all the experiments and wrote the manuscript. Z.J. (Associate Professor) and G.SJ. (Associate Professor) wrote and revised the manuscript. H.SL. (Associate Professor) revised the manuscript.