Oxidization of hydrogen sulphide in biogas by manganese (IV) oxide particles
Article information
Abstract
Hydrogen sulphide is corrosive to most metallic equipment such as pipelines, compressors, gas storage tanks, engines, turbines and other units. It acts as a strong poison for fuel cells and its combustion leads to SO2 emissions. Due to the problems associated with hydrogen sulphide, it is critical to remove it from biogas before further processing. The removal of hydrogen sulphide from biogas using MnO2, which acts as a sorbent and catalyst, was investigated. The research was conducted in a full-scale agriculture biogas plant using chicken manure and maize silage as substrate. The manganese dioxide (manganese (IV) oxide) was derived from the waste products of a water conditioning system, after manganese removal from drinking water. The obtained results showed significantly better adsorption of hydrogen sulphide and faster regeneration of the bed compared to the bed filled with hydrated iron oxides. The H2S concentration in the treated biogas dropped from about 1,650 to 0–5 ppm.
1. Introduction
Biogas is a very important source of renewable methane. It is produced during anaerobic fermentation of biomass in the presence of anaerobic microorganisms. Anaerobic digestion is a series of metabolic processes conducted by various groups of microorganisms in different temperature and time intervals [1–5].
Before biogas can be used as a source of energy for generating e.g. heat and/or electricity, hydrogen sulphide must be removed. The amount of H2S in crude biogas usually ranges from 50 to 5,000 ppm, but can reach even 20,000 ppm (2%) in some cases [6, 7]. It is a colourless, flammable, malodourous, toxic gas in high concentrations [6, 8, 9]. The removal of H2S is critical to successful applications in combustion techniques, mainly to prevent problems such as corrosion of pipes, turbines and other units [7]. The required degree of purification of biogas depends on its intended use, e.g. in fuel cells or combustion engines. For trouble-free operation of combined heat and power units, the hydrogen sulphide concentration in the biogas must be lower than 0.01 to 0.03%v/v, depending on the equipment concerned [10].
In general, the H2S removal-methods can be classified as: physical using solubility in water and sorbents [11, 12], chemical based on chemical reactions [2, 13, 14] and biological using microorganisms [15–17] or a combination of these [18, 19]. As all the methods have advantages and disadvantages, new and improved solutions are investigated.
Jędrczak [20] discussed other methods of H2S removal from biogas, which are based on: dosing FeCl3 to a digestion chamber or air to biogas, absorption of chemicals binding H2S in solutions, absorption-oxidation process, biological methods with bio-filters and adsorption on activated carbon and alkaline iron oxidize beds. Sisani et al. [21], however classified H2S removal methods as wet/dry process and membrane process. The effect of dosing iron salts (in the form of FeCl2, FeCl3 or FeSO4) into the digester is to transform hydrogen sulphide into insoluble iron sulphide. This method is used for high concentrations of H2S and allows its content to be reduced to about 70 ppm. The advantage of this method is low operating and investment costs [7, 20, 22].
Though rapid and effective, the physical and chemical methods for H2S removal are costly and produce secondary wastes, which in turn gives rise to another pollution problem [19]. By using biological processes it is possible to avoid these problems. Besides, they can achieve a greater degree of desulphurization and generate by-products (S0) that can be used in other industrial processes [23]. Ho et al. [18] proposed a system, where the first stage is H2S oxidizing by ferric iron to generate S0, then the ferrous iron is oxidized by iron-oxidizing bacteria.
The direct dosing of air or oxygen into the digester was proposed in order to carry out both the production and desulphurization of biogas in a single unit. The process is based on biochemical reactions, i.e. under O2-limiting conditions sulphide-oxidizing bacteria oxidise sulphide to S0 [24–26].
The adsorption process is a promising technology, therefore much effort is being expended searching for new types of adsorbents, an important factor for the process efficiency. Adsorbents are porous solids with highly developed surface area available for adsorption; they are characterized by different surface properties.
Adsorption processes among others include absorption using raw zeolites or with e.g. TiO2 [27, 28], biochar [29–31], metal active sorbents [32–34] and activated carbon is one of the most suitable methods for removal of hydrogen sulphide from biogas. But, its high operational costs have promoted the search for low-cost alternative adsorbents using natural materials (wood, peat, coal, lignite) as well as industrial/agricultural/domestic wastes or byproducts, such as slag, sludge, fly ash or red mud [35–40].
Generally, activated carbon used for H2S removal is modified with chemicals or oxidative agents, to promote oxidation of H2S to elemental sulphur [22, 37, 39]. The desulphurization performance of different metallic oxides on activated carbon decreases in the order: Mn > Cu > Fe > Ce > Co > V. The desulphurization performance using metal oxides was markedly improved [41, 42].
For example, potassium permanganate (KMnO4) is a strong oxidizing agent, it can be used in sewers to reduce corrosion problems and odor development [12, 43], for toxin removal from water [44, 45], for biogas purification [46] as well as sulphide removal from air [47]. Manganese dioxide is also active in the removal of sulphide [42, 48–51]. It was furthermore found that H2S reacts significantly faster with KMnO4 than with MnO2 [52], but the complex reactions between KMnO4 and H2S are still not clearly understood [12].
The importance of MnO2 for hydrogen sulphide removal from biogas can be seen in the following reactions, where MnO2 can react with H2S non-catalytically (Eq. (1)) or catalytically (Eq. (2)) [12]
As an alkaline sorbent for SO2 removal, limestone, quick lime and hydrated lime or slaked lime are commonly used [50, 53, 54]. Positive impact of hydrated lime was observed on reducing hydrogen sulphide emissions [55], calcium-based sorbents were also used for H2S removal from hot coal gas [56]. Hydrated lime can be obtained from the calcination of abundant and low cost raw materials such as calcitic or dolomitic limestone. The calcination of these materials implies the formation of a porous structure resulting from the release of CO2 and H2O [57].
Zhang et al. [49] used a series of adsorbents based on manganese oxide supported on mesoporous MCM-41 at different calcinations temperatures for removal of H2S from natural gas at low temperatures. The results of desulfurization experiments have shown that the adsorbent calcinated at 550°C exhibited the best desulfurization performance, with Mn present in the form of Mn2O3. The saturated H2S capacity reached 28.65 mg/g at an adsorption temperature of 25°C.
Zhang et al. [51] studied the effect of preparation conditions on hydrogen sulphide removal activity of manganese dioxide-loaded activated carbon (AC) from model gas with a low concentration of H2S. The removal of H2S has been improved significantly after modification by manganese dioxide. A suitable H2S removal activity was obtained with adsorbent of MnO2/AC-1:1.
In the present study, the removal of hydrogen sulphide from biogas using manganese (IV) oxide and hydrated lime as an adsorbent in operating a biogas plant was studied. Beech wood chips were used as a structure material. The efficiency of removing hydrogen sulphide from biogas using the mentioned absorbent was investigated. To our knowledge, there are no studies relating to the removal of hydrogen sulphide using manganese oxide and lime, as well as wood chips as a structural material.
2. Material and Methods
The research was carried out in a full-scale agricultural biogas plant located at a broiler farm in the Subcarpathian region (Poland). The biogas plant works using chicken manure and maize silage as substrates under mesophilic conditions with a working volume of 2,500 m3 and hydraulic retention time of 52 days. The biogas plant produces about 100 m3/h of biogas. Hydrogen sulphide in the biogas ranges from about 1,000 to 3,500 ppm.
During experiments a new absorption bed consisting of manganese oxide and hydrated lime was used. The manganese dioxide applied during full scale experiments was a catalytic bed used to remove manganese from groundwater in a water treatment plant.
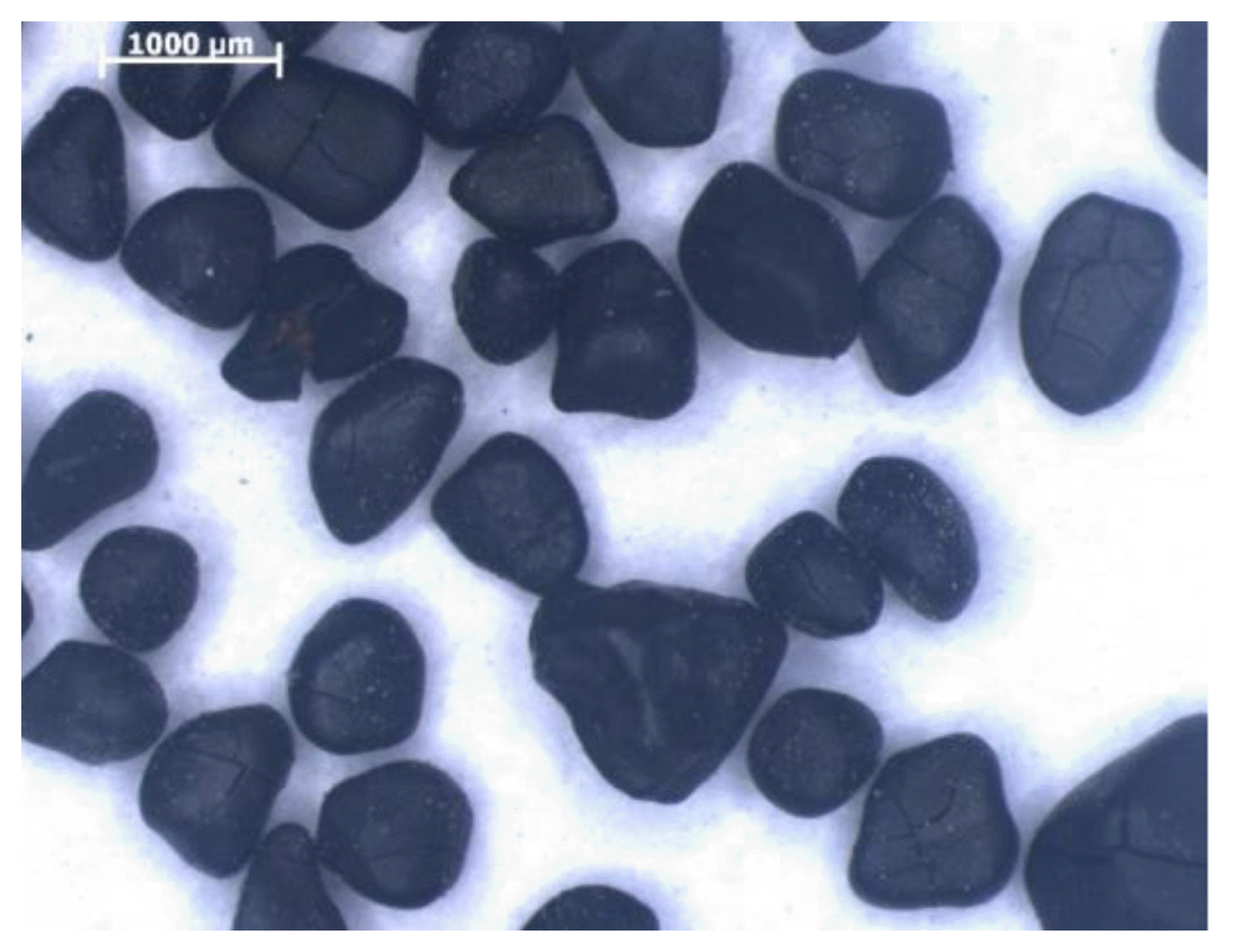
The chemical composition of the catalytic bed used is shown in Table 1. Its structure is presented in Fig. 1. The MnO2 used in the adsorber had numerous cracks (fissures), which caused an increase in the active oxide surface.
The absorber bed for the biogas plant was made by mixing manganese (IV) oxide and hydrated lime, the components were mixed in the ratio two to one (by weight), respectively. Mixing manganese (IV) oxide with lime in a ratio of 2 to 1 results from the preference for using oxidizing properties over sorption properties. Alkali (calcium hydroxide) binds hydrogen sulphide which has acidic properties, the following reactions takes place (Eq. (3) and (4)):
Then calcium sulphide is oxidized (Eq. (5)):
The following reactions occur in an aquatic environment (Eq. (6) and (7)):
The large surface area of the catalyst and catalytic agent promotes the oxidation of hydrogen sulphide.
Beech wood chips about 10x5x1 mm were used as a structure material. The total content of chips in the desulfurizer was 90%. Basic information about the hydrogen sulphide removal process is provided in the Table 2.
Removal of hydrogen sulphide from biogas depends not only on the concentration of MnO2 but also on the size of its grains. The smaller the grains, the larger the biogas flow resistance through the bed, but the more efficient the removal of hydrogen sulphide. To improve the efficiency of hydrogen sulphide removal air was introduced into the gas space in the digester. The volume of air was measured using a rotameter. The oxygen content in the fermentation chamber as well as at individual levels of the desulfurizer was determined using a gas analyzer. Hydrogen sulphide measurements were carried out using a portable gas analyzer (GA5000, Geotech). The analyzer had ATEX II 2G Ex ib IIA T1 Gb (Ta= −10°C to +50°C), IECEx and CSA quality certifications and UKAS ISO 17025 calibration certificate. The system allows determination of oxygen and hydrogen sulphide in the following ranges O2: 0–25%, and H2S: 0–5,000 ppm. Calibration of the device was performed before experiments using calibration gases −1,000 ppm H2S, while oxygen sensor was performed by synthetic air.
The removal efficiency of hydrogen sulphide was determined using the following Eq. (8):
where cinlet – H2S concentration in raw biogas before adsorption bed, coutlet – H2S concentration in biogas after passing the adsorption bed.
3. Results and Discussion
Fig. 2 presents concentrations of hydrogen sulphide in biogas and their dependence on air content in the digester. The concentration of hydrogen sulphide in raw biogas ranged from 1,080 to 1,850 ppm. The H2S content in the upgraded biogas after treatment ranged from 2–640 ppm, depending on the oxygen concentration in the digester. The efficiency of hydrogen sulphide removal depending on the oxygen concentration is also shown in Fig. 2. It was observed that as the concentration of oxygen in the fermentation chamber increases, the efficiency of hydrogen sulphide removal also increases. For 0.5% concentration of oxygen, the efficiency was about 41%. Increasing the oxygen concentration to 0.9% the efficiency of H2S removal is more than doubled. For oxygen concentrations from 1 to 1.9%, the removal efficiency was approaching 99.5%.
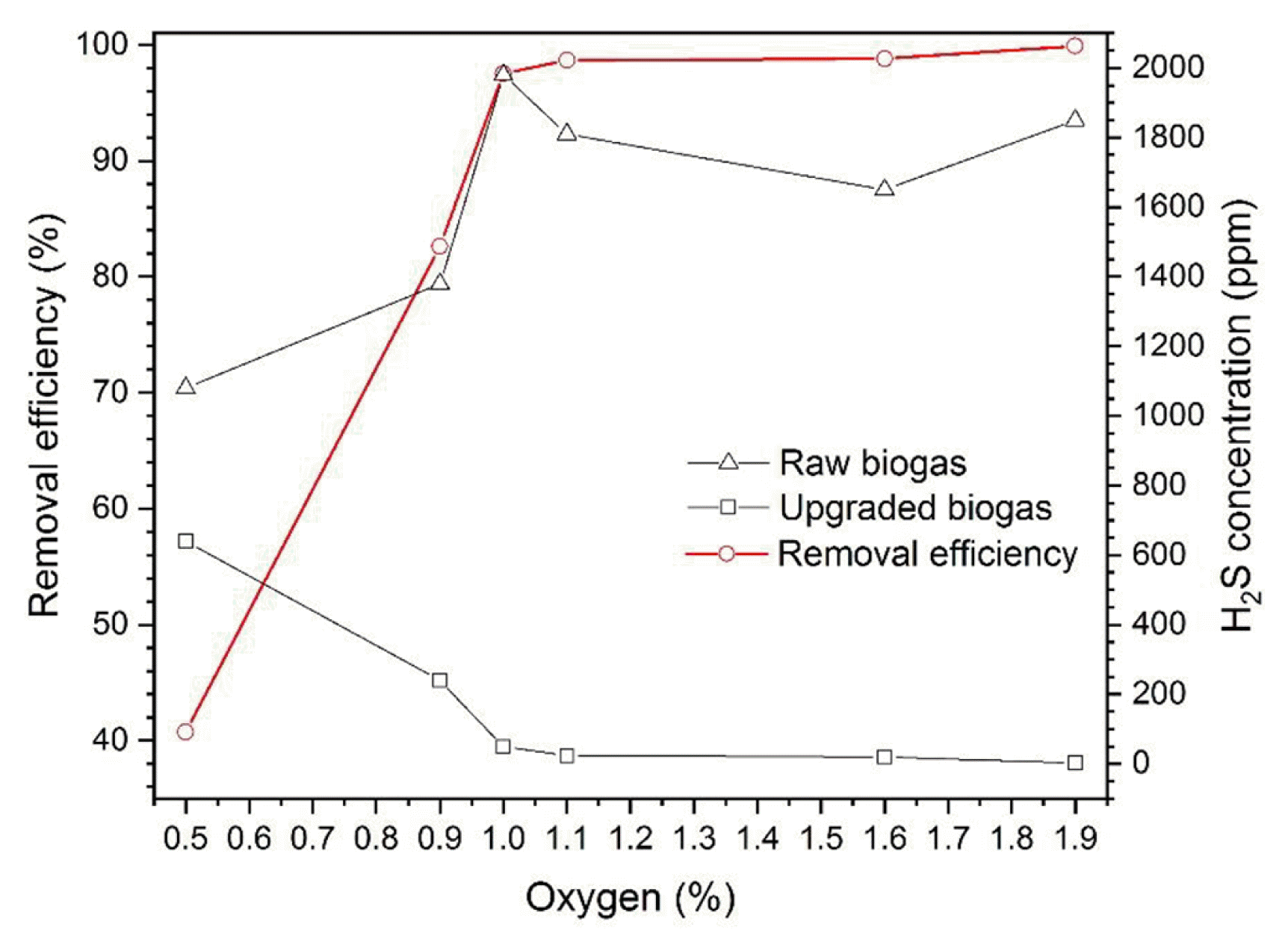
Hydrogen-sulphide removal-efficiency from raw biogas depending on variable air concentrations in testes biogas streams.
During the removal of hydrogen sulphide from biogas, intermediate sulphur compounds are produced: S, S2, SH, HS2, H2S2, SO, S2O, HSO, HOS, HSO2, HOSO2, (HSO)2, SO2, SO3 [16].
The generated elemental sulphur after half a year bed operation, limited the access of hydrogen sulphide to the catalyst and hydrogen sulphide concentration at the outlet increased to 45 ppm. The adsorbent bed should therefore be replaced. The sulfur content of the bed after depletion of sorption properties is up to 50 g/kg bed. The bed with precipitated sulphur is shown in Fig. 3. Accumulation of sulfur was observed from the side of biogas impact deeper into the deposit, but Goldnik and Turek [12] assumed that sulphur is distributed all over the adsorption bed.
4. Conclusions
There are many technologies solving the problem of hydrogen sulphide separation from biogas, but because of the high cost of investment and operation, the profitability of biogas production is significantly reduced. In this regard the presented experimental results seem very interesting. Adsorbent with manganese oxide, hydrated lime and wood chips were used to remove hydrogen sulphide from biogas. The adsorbent used proved to be very effective; almost 100% efficiency of hydrogen sulphide removal from biogas was obtained. The materials used to compose the adsorption bed are cheap and readily available, which makes such systems of biogas purification economically feasible.
Acknowledgment
This research has been supported by the European Regional Development Fund within the Interreg South Baltic Programme 2014–2020, under project no. WASTEMAN STHB.02.02.00-0131/17 and Provincial Fund for Environmental Protection and Water Management in Gdansk under project no. WFOŚ/D/748/20/2018.
Notes
Author Contributions
I.K. (Ph.D Student) carried out the experiments, discussed the results and contributed to the final manuscript. J.C. (Dr. Sc. Eng.) carried out the experiments, discussed the results, supported manuscript writing. A.C. (Professor) supervised the project, discussed the results and supported manuscript writing and verification.