Cr(VI) removal using Fe2O3-chitosan-cherry kernel shell pyrolytic charcoal composite beads
Article information
Abstract
In this study, cherry kernel shell pyrolytic charcoal was synthesized (CKSC) and composite beads were obtained by blending this pyrolytic charcoal with chitosan and Fe2O3 nanoparticles (Fe-C-CKSC). Cr(VI) adsorption from aqueous solutions by Fe-C-CKSC composite beads and CKSC adsorbents was studied comparatively. The effects of Cr(VI) initial concentration, adsorbent dosage, contact time, pH and temperature parameters on Cr(VI) adsorption were investigated. Adsorption reached an equilibrium point within 120 min for CKSC and Fe-C-CKSC adsorbents. The maximum Cr(VI) removal was obtained at the initial pH value of 1.56 for CKSC and 2.00 for Fe-C-CKSC. The optimum adsorbent dosage was found to be 5 g/L for CKSC and 3 g/L for Fe-C-CKSC. Based on the Langmuir model, the maximum adsorption capacities were calculated as 14.455 mg/g and 47.576 mg/g for CKSC and Fe-C-CKSC, respectively. Thermodynamic and kinetic studies were performed. As a result of adsorption kinetics calculations, adsorption was found to be consistent with the pseudo second order kinetic model. Characterization of the synthesized adsorbents was performed by SEM, BET, FTIR and elemental analysis. This study has shown that low cost adsorbents CKSC and Fe-C-CKSC can be used in Cr(VI) removal from aqueous solutions.
1. Introduction
Heavy metal pollution in nature has natural causes as well as human causes. Volcanic activities, decomposition and erosion of minerals and rocks are some of the natural causes. Industrial activities such as mining, casting, electroplating and painting are the most important human factors. Nowadays, due to the increase in industrialization, the amount of heavy metals detrimental to the living organisms also increased. If heavy metals such as chromium, cobalt, lead, etc. are present in quantities above certain limits, they can cause adverse effects on the health of living organisms. For this reason, lowering the heavy metal concentrations below the certain limits is important to protect the health of humans and all other organisms [1].
Among these heavy metals, chromium exists in two forms as Cr(III) and Cr(VI). Cr(VI) is a much more toxic and carcinogenic species than Cr(III) form. Cr(VI) from soil and water environments can easily penetrate through the skin [2]. Maximum Cr(VI) concentration in potable water should be 0.05 mg/L according to the World Health Organization regulations [3].
Methods for removing heavy metals from aqueous solutions include adsorption, ion exchange, membrane filtration, chemical precipitation methods. Among these methods, chemical precipitation is the most commonly used method. But chemical precipitation does not completely remove metals and produces a large amount of toxic sludge that needs additional treatment processes. Other methods except adsorption have high operational costs [4]. So, adsorption is an attractive method for the removal of Cr(VI) due to its efficiency and simplicity of operation [5]. Besides, if low cost adsorbents are used, adsorption can be more economical than other methods [6].
Recently, scientific studies have been intensified on the use of low-cost adsorbents to remove heavy metals from water. Waste products that can be obtained abundantly from agricultural, industrial or food production processes can be used as low cost adsorbents [7]. Some waste products can be used directly as adsorbents owing to their high adsorption performance. However, some waste products need to be processed to improve their adsorption performance. Some of these processes may be to form charcoal, activated carbon or composites from these materials.
The cherry kernel constitutes 14.6% of the weight of the cherry fruit and as a result of food processes, is produced in large quantities as waste [8]. Pyrolytic charcoal was obtained from the cherry kernel shell which is an agricultural waste and obtained pyrolytic charcoal (CKSC) was used as adsorbent for Cr(VI) adsorption, in this study.
Pyrolytic charcoal can be obtained from substances of biological origin such as cherry kernel. In contrast to the chemical or physical activation used in the production of activated carbon, raw materials used in the production of pyrolytic charcoal are not modified with any chemical additives. The raw materials are only treated with pyrolysis in nitrogen atmosphere and at 400–1,000°C. In this way, the volatiles in the raw material are removed; the carbon ratio, surface area and porosity are increased. This also can improve the adsorption performance of the material [9, 10].
Chitosan is obtained by deacetylation of chitin. Chitosan has a high capacity of heavy metal adsorption due to the reactive hydroxyl (–OH) and amino (–NH2) groups present in its structure. However, chitosan has some disadvantages such as low-acid stability, deficient mechanical strength and low thermal stability. Therefore, to overcome these disadvantages, chitosan and composites may be formed with different materials [11].
Several studies have been carried out on magnetic adsorbents. Lingamdinne et al. [12] synthesized the biogenic magnetic inverse spinel iron oxide nanoparticles using seed extract of Cnidiummonnieri (L.) Cuss (CLC) as a precursor for the removal of Pb(II) and Cr(III) from aqueous solutions. Additionally Lingamdinne et al. [13] synthesized low-cost magnetized Lonicera japonica flower biomass for the sorption of Pb(II), Co(II) and Cu(II) from aqueous solutions. Jiang et al. [5] prepared magnetically separable millimeter-sized chitosan beads containing nanosized γ-Fe2O3 for the removal of Cr(VI). Karaer and Kaya [14] synthesized magnetic chitosan/activated carbon composite using Fe3O4 for the removal of methylene blue and reactive blue4. Recently, there has been a growing interest in the use of magnetic materials as adsorbents. The high performance of magnetic particles in the adsorption of dyes and heavy metals is the reason for this interest. An advantage of these materials is that the magnetic adsorbents can be easily recovered by means of an external magnetic field. Among these magnetic particles, Fe2O3 nanoparticles show high performance in the adsorption of many heavy metals and dyes [5, 15].
Although there are studies carried out on composite adsorbents of chitosan and magnetic adsorbents, no studies have been found on Fe-C-CKSC adsorbent for Cr(VI) removal in the literature. In this study, Cr(VI) adsorption efficiencies of the pyrolytic charcoal obtained from the cherry kernel shell (CKSC) and composite beads of this pyrolytic charcoal made of chitosan and Fe2O3 nanoparticle (Fe-C-CKSC) were investigated in synthetic solutions. The effect of various parameters such as pH, contact time, initial metal concentration was investigated to determine the optimum values of this adsorption process.
2. Materials and Methods
2.1. Materials
In order to synthesize pyrolytic charcoal, cherry kernel was broken and the oily core was separated. Then, the shells that had been separated were subjected to pyrolysis process at 500°C pyrolysis temperature at 10°C/min heating speed. The pyrolytic charcoal obtained from cherry kernel shell (CKSC) in this way was used in laboratory work.
Chitosan, which was used as matrix to form composite beads with pyrolytic charcoal and Fe2O3, and glutaraldehyde, which was used for cross-linking chitosan, were purchased from Sigma Aldrich. Potassium dichromate, acetic acid, sodium hydroxide, hydrochloric acid and ethanol were purchased from Merck; Fe2O3 nanoparticles were purchased from Inframat.
2.2. Preparation of Adsorbents
Cherry kernel shell pyrolytic charcoal (CKSC), which was used alone and in composite beads as an adsorbent, was ground with Retsch RM 100 grinder, sieved with Retsch AS 200 sieve shaker and its particle size was reduced to less than 125 μm.
To prepare Fe2O3-chitosan-pyrolytic charcoal composite beads (Fe-C-CKSC), the method proposed by Karaer and Kaya [14] was used basically. Accordingly, 3.0 g of chitosan was stirred in 90 mL of 3% acetic acid solution for 12 h. 4.8 g of Fe2O3 nanoparticle was dissolved in 10 mL of 3% acetic acid solution and added to the mixture and stirred further for 2 h. Then, 3.0 g of pyrolytic charcoal was blended with 100 mL of 3% acetic acid solution and added to the mixture and stirred for additional 3 h. The mixture obtained was dropped into 500 mL of 3 M NaOH solution and the beads were formed. The beads were kept in the solution overnight. Then the beads were separated from solution and washed until a final pH value of 7. The purpose of synthesizing this composite is both to increase the adsorption capacity of pyrolytic charcoal and to facilitate the use of the adsorbent and separation of the adsorbent from the solution thanks to magnetic property of Fe2O3. Another advantage of Fe-C-CKSC composite is that it is synthesized in a cheap and easy way.
Scheme of preparation of beads and use of beads in adsorption was given in Additional file 1: Fig. S1.
2.2.1. Cross-linking of chitosan
Washed beads were stirred in 30 mL ethanol and 0.3 mL glutaraldehyde containing-solution at 70°C for 5 h and thus chitosan was cross-linked with glutaraldehyde. The beads were filtered and washed with ethanol to remove unreacted glutaraldehyde. Then the beads were washed until pH was 7 [11].
2.3. Characterizations of Adsorbents
The characterizations of CKSC and Fe-C-CKSC adsorbents were made with elemental analysis (LECO CHNS-932), Fourier Transform Infrared Spectroscopy (FT-IR) (Bruker Vertex 70), Scanning Electron Microscope/Energy Dispersive X-Ray (SEM/EDX) (Hitachi – SU 1510) and Brunauer, Emmett and Teller (BET) surface area analysis (Quantachrome – Quadrasorb Evo 4) [9, 14].
2.4. Batch Adsorption Studies
In order to determine the optimum conditions for adsorption, the adsorption experiments were performed as batch by varying adsorbent amount, initial Cr(VI) concentration, contact time, pH and temperature parameters. While explaining the mass transfer rate and mechanism in adsorption, realistic estimation of these optimum conditions is very important. 260 mg/L Cr(VI) stock solution was prepared by dissolving K2Cr2O7 in deionized water and Cr(VI) solutions for adsorption studies were obtained by diluting this stock solution with deionized water. For adsorption experiments, 10.0 mL of Cr(VI) solution was mixed with adsorbents. 0.1 M HCl and 0.1 M NaOH solutions and GLP 22 pH meter was used to adjust the solution pH. After the adsorption, CKSC was filtered out and Fe-C-CKSC was held with a magnet to remove the adsorbents from the solution. Cr(VI) concentration of the solutions was measured with UV-Visible spectrometer (Shimadzu UV-1700). The optimum result obtained in each parameter study was used in the next parameter optimization study [5].
The amount of Cr(VI) removed per unit adsorbent mass and the percentage of adsorption were calculated using Eq. (1) and Eq. (2), respectively.
Where, qe is the amount of the adsorbed substance (mg/g), C0 is the initial Cr(VI) concentration of solution (mg/L), Ce is the Cr(VI) concentration at the end of the contact time (mg/L), V is the solution volume (L) and w is the adsorbent amount (g).
2.4.1 Adsorption isotherms
Adsorption isotherms are very important for the design of adsorption systems. According to Freundlich isotherm, the adsorption is multilayer and the adsorbent surface is not homogeneous [16]. According to Langmuir and Scatchard isotherms, it is assumed that the adsorption is monolayer and the adsorbent surface is homogeneous [17, 18]. The linearity of the Scatchard curve supports the eligibility of the adsorption process to the Langmuir model [19]. The adsorption energy value calculated from the D-R isotherm informs which of the physical adsorption or chemical adsorption is more dominant at the adsorption mechanism [20]. According to the assumption of Temkin isotherm, the adsorption heat of all molecules in the adsorbent layers decreases linearly due to the adsorbent/adsorbate interactions [21].
2.4.2. Adsorption kinetics
The adsorption kinetics study gives information about the adsorption mechanism and the adsorption rate of the solute. The pseudo-first-order and pseudo-second-order kinetic models were studied [3].
2.4.3 Adsorption thermodynamics
The enthalpy change (ΔH ), the entropy change (ΔS ) and Gibbs free energy change (ΔG ) during the adsorption were calculated using the data obtained from the study of determination of the effect of temperature on adsorption. Eq. (3) and (4) were used to calculate these thermodynamic parameters [3].
In these equations, KD is the thermodynamic equilibrium constant and is calculated as in Eq. (5). In addition, R (8.314 J mol−1K−1) is universal gas constant and T (K) is temperature.
3. Results and Discussion
3.1. Characterizations of the Adsorbents
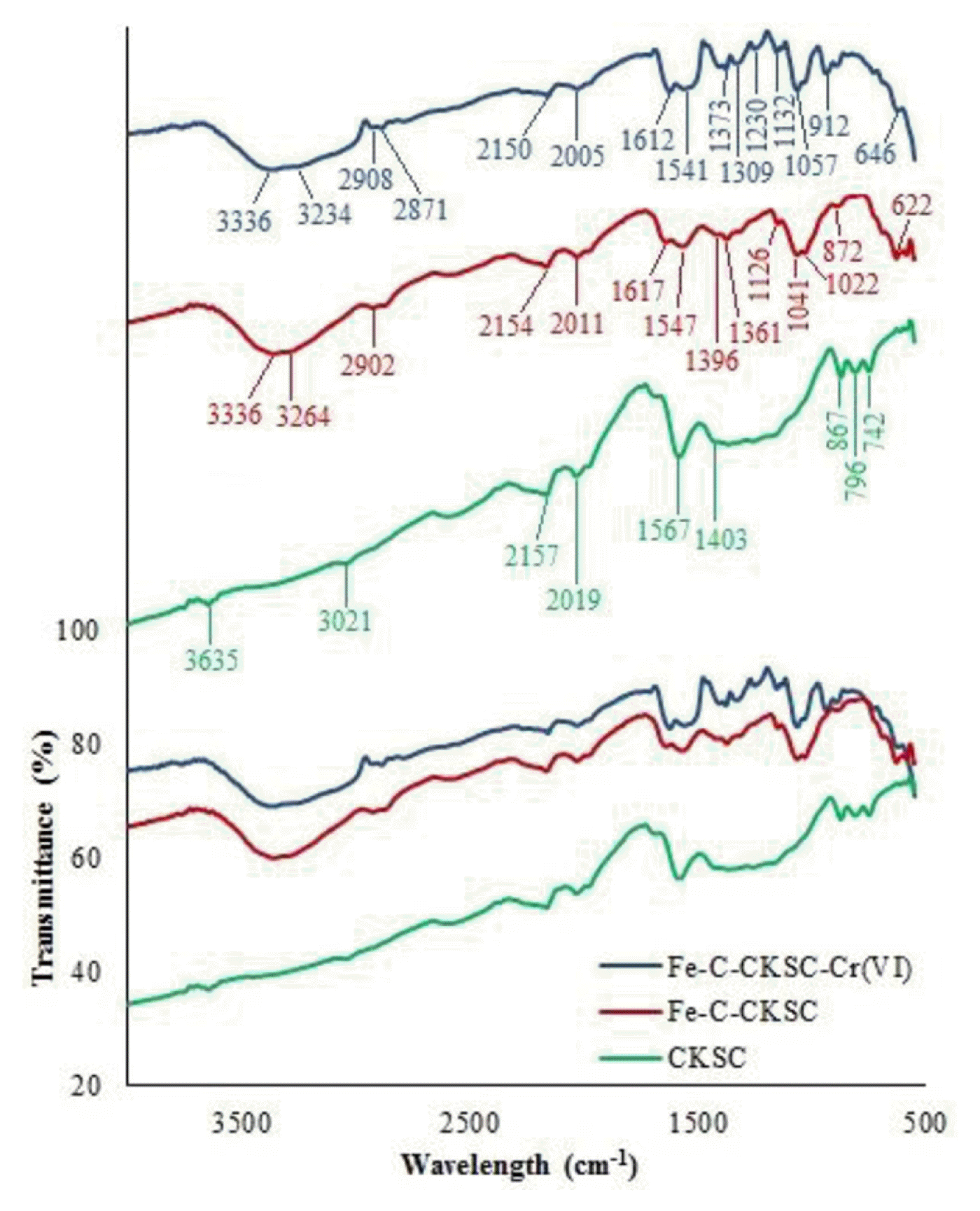
The FTIR spectra were obtained and are displayed in Fig. 1. For both adsorbents, the band in the range of 3,200–3,750 cm−1 is caused by O-H and N-H stretching vibrations [22, 23]. The band in the range of 2,850–3,021 cm−1 is due to the aliphatic C-H stretching [24]. The band in the range of 2,000–2,200 cm−1 is due to the triple bonds of alkynes and 1540–1570 cm−1 is due to the C=O and C=C aromatic vibrations in ring [25]. The band seen at 1396–1403 cm−1 indicates deformation stretching of the −CH, −CH2 and −CH3 functional groups [23]. The band at 740–875 cm−1 is due to C–H and CH=CH2 [26].
The band seen at the Fe-C-CKSC spectrum at 1,617 cm−1 is due to N-H bending of aromatic ring structures [17]. The band at 1,361 cm−1 is due to −CH vibrations in the alcohol group [27]. The characteristic band of chitosan at 1,126 cm−1 is attributed to the stretching vibration of the glycosidic (C-O-C) bonds [28]. The band at 1,041 cm−1 is due to the stretching vibrations of CH-OH bonds in the chitosan structure [22]. The band at 1,022 cm−1 is assigned to the stretching vibrations of C-N bonds of amino groups [29]. The band at 622 cm−1 is due to the stretching vibrations of Fe-O bonds [14, 30]. These results show that after the formation of the composite of the CKSC with chitosan and Fe2O3, there is an increase in the functional groups such as hydroxyl and amine.
According to the FTIR spectrum of Fe-C-CKSC after Cr(VI) adsorption, the band appeared at 1,361 cm−1 before adsorption shifted to 1,373 cm−1. The band at 1,396 cm−1 shifted to 1,385 cm−1 by increasing its intensity. The band at 872 cm−1 transformed to band of 912 cm−1 and its intensity increased. The band at 622 cm−1 shifted to 646 cm−1 by decreasing its intensity. According to the elemental analysis results, C%, H%, N% and O% (+*Fe% − for Fe-C-CKSC) in the CKSC structure are 85.20%, 2.93%, 1.42% and 10.45%, respectively, and in the Fe-C-CKSC structure are 37.41%, 3.26%, 2.42% and *56.91%, respectively (*The value of 56.91% indicates the total amount of oxygen and iron in the structure). After the formation of composite with pyrolytic charcoal, chitosan and Fe2O3, carbon content in the structure decreased. In addition, nitrogen and hydrogen contents also increased. Due to the oxygen and iron in chitosan and Fe2O3 structures, the amount of oxygen and iron in the structure increased after the formation of Fe-C-CKSC composite. These data indicate that functional groups such as hydroxyl, amine are increased in the structure after composite formation with chitosan and Fe2O3.
SEM images of CKSC and Fe-C-CKSC adsorbents before and after adsorption are shown in Fig. 2.

SEM images: (a) and (b) CKSC before adsorption, (c) CKSC after adsorption, (d) and (e) Fe-C-CKSC before adsorption, (f) Fe-C-CKSC after adsorption.
When Fig. 2: (a), (b) and (c) are examined, it can be seen that CKSC surface, which was flatter before adsorption, was filled after Cr(VI) adsorption and the particulate structure formed on the surface.
It can be seen in Fig. 2(d) that particle size of Fe-C-CKSC is approximately 1 mm. Although the particle size of the adsorbent was thought to have a negative effect on Cr(VI) adsorption capacity, Cr(VI) adsorption capacity of Fe-C-CKSC was found to be much greater than CKSC (below 125 micrometer). On the other hand, bead structured adsorbent has ease of application and easy separation from solution. Besides, it can be seen in Fig. 2: (d), (e) and (f) that the absence of any deviation from homogeneity on the surface of Fe-C-CKSC shows that the chitosan and iron molecules are homogeneously distributed. In addition, before Cr(VI) loading, there are smaller particles on the surface of the adsorbent, whereas after Cr(VI) loading, larger particles are formed on the surfaces and the gaps in the surface are filled. This supports the occurrence of the adsorption of Cr(VI) on the surface of the adsorbent occurs.
The results of the EDX analysis of CKSC and Fe-C-CKSC adsorbents are given in Fig. 3.
EDX analysis results: (a) EDX spectrum of CKSC before adsorption, (b) EDX spectrum of CKSC after adsorption, (c) distribution map of elements on surface of CKSC after adsorption, (d) distribution map of chromium on surface of CKSC after adsorption, (e) EDX spectrum of Fe-C-CKSC before adsorption, (f) EDX spectrum of Fe-C-CKSC after adsorption, (g) distribution map of elements on surface of Fe-C-CKSC after adsorption, (h) distribution map of chromium on surface of Fe-C-CKSC after adsorption.
With the EDX analysis, the presence of carbon and oxygen in the structure of both adsorbents and the presence of iron in the structure of Fe-C-CKSC adsorbent were confirmed. These results are also corroborating elemental analysis results. As a result of the analysis of the samples after adsorption, chromium was found in the structures and this showed that the chromium was retained by the adsorbents. It can also be seen from the map data that chromium was distributed homogeneously to the adsorbent surface.
As a result of BET surface area analysis, surface area of CKSC was determined as 224.148 m2/g and surface area of Fe-C-CKSC was determined as 31.286 m2/g.
3.2. Adsorption Studies
3.2.1. Effect of adsorbent amount
Adsorbent dose in adsorption experiments were kept between 1.0–20.0 g/L for CKSC and 1.0–6.0 g/L for Fe-C-CKSC. In order to determine the optimum amount of adsorbent for adsorption, 55 mg/L of the Cr(VI) solution adjusted to pH 2.0 was added to different amounts of adsorbents and the mixtures were stirred for 2 h. Effect of the amount of adsorbent on the percentage of Cr(VI) removal and adsorption capacity is shown in Fig. 4(a) and (b).

(a) Effect of adsorbent amount on the adsorption for CKSC, (b) Effect of adsorbent amount on the adsorption for Fe-C-CKSC (adsorption conditions for adsorbent amount study: concentration of Cr(VI) 55 mg/L, solution pH 2, contact time 120 min, temperature 25°C) (c) Adsorption isotherms and change of Cr(VI) removal percentage for CKSC (d) Adsorption isotherms and change of Cr(VI) removal percentage for Fe-C-CKSC (Adsorption conditions for isotherm study: Adsorbent amount 5 g/L for CKSC and 3 g/L for Fe-C-CKSC, solution pH 2, contact time 120 min, temperature 25°C).
According to the graphs, when the amount of adsorbent was increased, the percentage of adsorption was increased and the amount of substance retained per unit adsorbent mass (qe) decreased. After reaching a certain value, increasing the amount of adsorbent did not affect the adsorption percentage much. As the amount of adsorbent was increased, the increase in the percentage of adsorption was due to the increase in the number of active sites on the adsorbent surface. As the amount of adsorbent was increased, the decrease in the amount of substance retained per unit adsorbent mass was due to the decrease in the amount of adsorbate per adsorbent particle.
As a result of the experiment, optimum adsorbent amounts were determined as 5 g/L for CKSC and 3 g/L for Fe-C-CKSC. According to these values, for the adsorption of Cr(VI) ions, the efficiency of Fe-C-CKSC adsorbent appears to be higher [24].
3.2.2. Adsorption isotherms
The adsorption of Cr(VI) at different concentrations plays an important role in determining the adsorption capacity of the adsorbent. Cr(VI) solutions adjusted to pH 2.0 at different concentrations (8, 25, 58, 80, 98, 131, 158, 171, 191 mg/L) were added to the optimum amounts of adsorbents and the mixtures were stirred for 2 h. The equilibrium relationships between the adsorbents and Cr(VI) were evaluated using Freundlich, Langmuir, Scatchard, Dubinin-Radushkevich (D-R) and Temkin isotherm models [16, 18–21]. Adsorption isotherms and effect of the concentration on the percentage of Cr(VI) removal are depicted in the Fig. 4(c) and (d).
It can be seen in Fig. 4: (c) and (d) that when the initial Cr(VI) concentration was increased, the amount of substance retained per unit adsorbent mass (qe) increased and the percentage of adsorption decreased. The reason of the decrease in the percentage of adsorption as concentration increases is that the amount of adsorbate per each active site on the adsorbent surface increased, because amount of adsorbent is constant. Thus, as the concentration increases, the amount of adsorbate that cannot be adsorbed by the adsorbent also increases. The reason for the increase in the amount of substance retained per unit adsorbent mass is that the amount of retaining substance increased at the active sites. This allows the adsorbent to approach its maximum capacity.
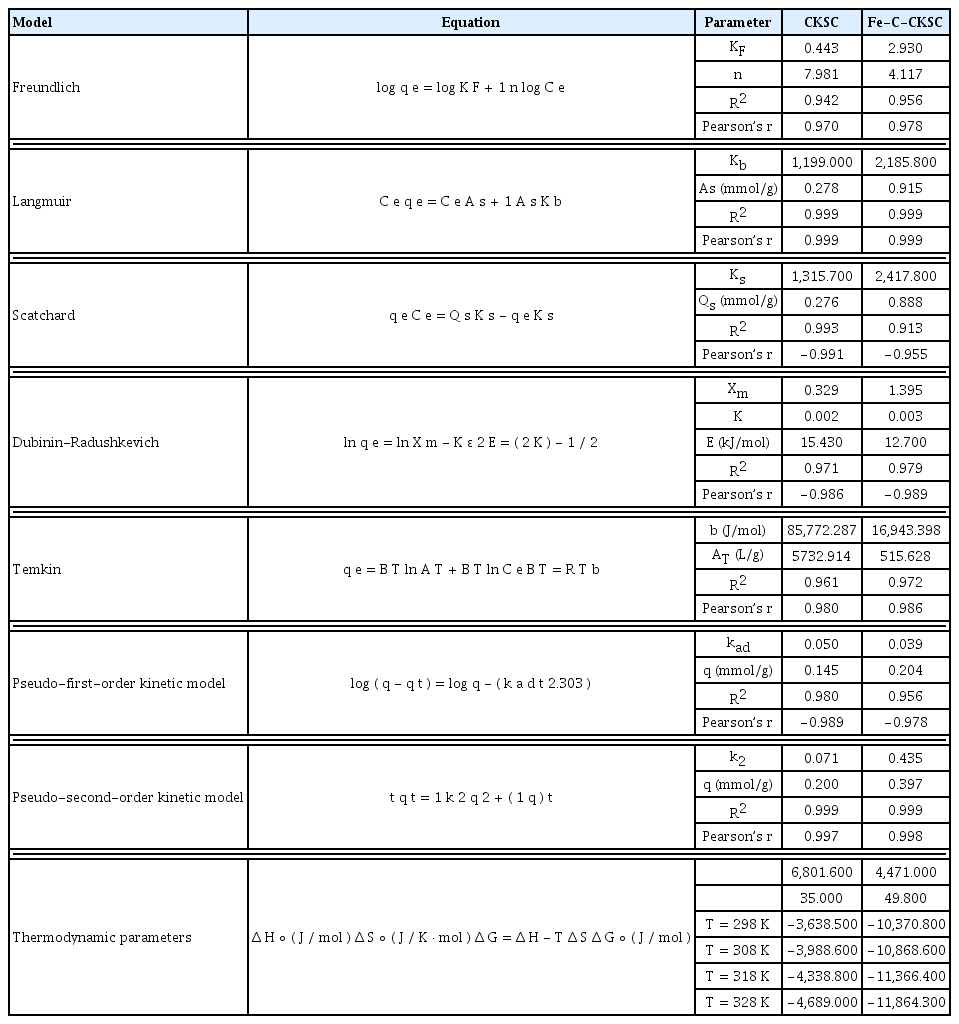
The linearized forms of isotherms and the results of Freundlich, Langmuir, Scatchard, Dubinin-Radushkevich (D-R) and Temkin isotherm analyses calculated for the adsorption of Cr(VI) on CKSC and Fe-C-CKSC from aqueous solutions are shown in Table 1. Isotherm graphics are shown in Fig. 5.

Freundlich, Langmuir, Scatchard, Dubinin-Radushkevich and Temkin isotherms plot for the adsorption of Cr(VI) onto CKSC and Fe-C-CKSC.
The highest values of R2 were obtained when the experimental data were fitted into Langmuir model for both adsorbents. Therefore, it can be said that the adsorption mechanism is generally single layer and adsorbent surface is homogeneous [17, 18, 31]. The R2 values for Freundlich isotherm were found to be higher than 0.94. This indicates that physical adsorption also took place in the process. The R2 values for Scatchard isotherm were found to be higher than 0.91. This data supports the conclusion that the adsorption is compatible with the Langmuir isotherm. The R2 values for Dubinin-Radushkevich (D-R) isotherm were found to be higher than 0.97. This indicates that the mechanisms that are effective in adsorption can be determined by this model. Adsorption energy (E) values calculated with D-R isotherm indicate that adsorption mechanism is chemical. The R2 values for Temkin isotherm were found to be higher than 0.96. This indicates that the heat of adsorption of all molecules in the layer decreases linearly [21, 31]. Pearson’s r correlation coefficients of isotherm models are given in Table 1.
The maximum adsorption capacities of Cr(VI) according to the Langmuir isotherm model were 14.455 mg/g (0.278 mmol/g) for CKSC and 47.576 mg/g (0.915 mmol/g) for Fe-C-CKSC. As can be seen from these values, the adsorption capacity of Cr(VI) ions of Fe-C-CKSC adsorbent is 3 times more than the adsorption capacity of CKSC. In addition, maximum adsorption capacities (Qs) calculated from the Scatchard model supporting the conformity of the Langmuir model were found to be close to these values. Similar results are also available in the literature [12, 22]
Adsorption energies (E), which are one of the parameters of D-R isotherm, were found to be 15.430 kJ/mol for CKSC and 12.700 kJ/mol for Fe-C-CKSC. These values in the range of 8–16 kJ/mol indicate that complex formation and ion exchange are more effective in the adsorption mechanism. The D-R model is usually used to explain adsorption on the heterogeneous surface based on the pore filling mechanism with Gaussian energy distribution. [31, 32].
Values of b constant related to adsorption potential calculated from Temkin isotherm are 85.77 and 16.94 kJ/mol for CKSC and Fe-C-CKSC, respectively. These values greater than 8 indicate that there is strong cohesive forces between adsorbents and Cr(VI) and that chemical adsorption mechanism is the dominant mechanism in the adsorption process [21, 31]. According to these results, it can be said that homogeneous distribution of functional groups on the surface of the adsorbents causes a homogeneous binding energy distribution.
When the correlation coefficients (R2) of the adsorption isotherms were compared, all of the R2 values were found to be greater than 0.9. Therefore, it is thought that more than one mechanism is effective on adsorption. When the results of all the isotherm models were evaluated, it was seen that complex formation, ion exchange and electrostatic attraction were the predominant mechanisms on the adsorption of Cr(VI) with CKSC and Fe-C-CKSC. In addition, it was also seen that physical adsorption was occurred.
Table 2 shows the maximum adsorption capacities of CKSC, Fe-C-CKSC and some adsorbents that are recommended for Cr(VI) adsorption in the literature. It can be seen that when chitosan is used alone, its Cr(VI) adsorption capacity is 35.6 mg/g [33]. This value is lower than the Cr(VI) capacity calculated for Fe-C-CKSC. The interest of Fe-C-CKSC to Cr(VI) ions was found to be higher than CKSC. Fe-C-CKSC can be used as an effective adsorbent in the removal of Cr(VI).
3.2.3. Testing of kinetic adsorption models
55 mg/L Cr(VI) solutions adjusted to pH 2.0 were added to the optimum amounts of adsorbents and the mixtures were stirred during different contact times. Effect of the contact time on percentage of Cr(VI) adsorption is given in Fig. 6(a).
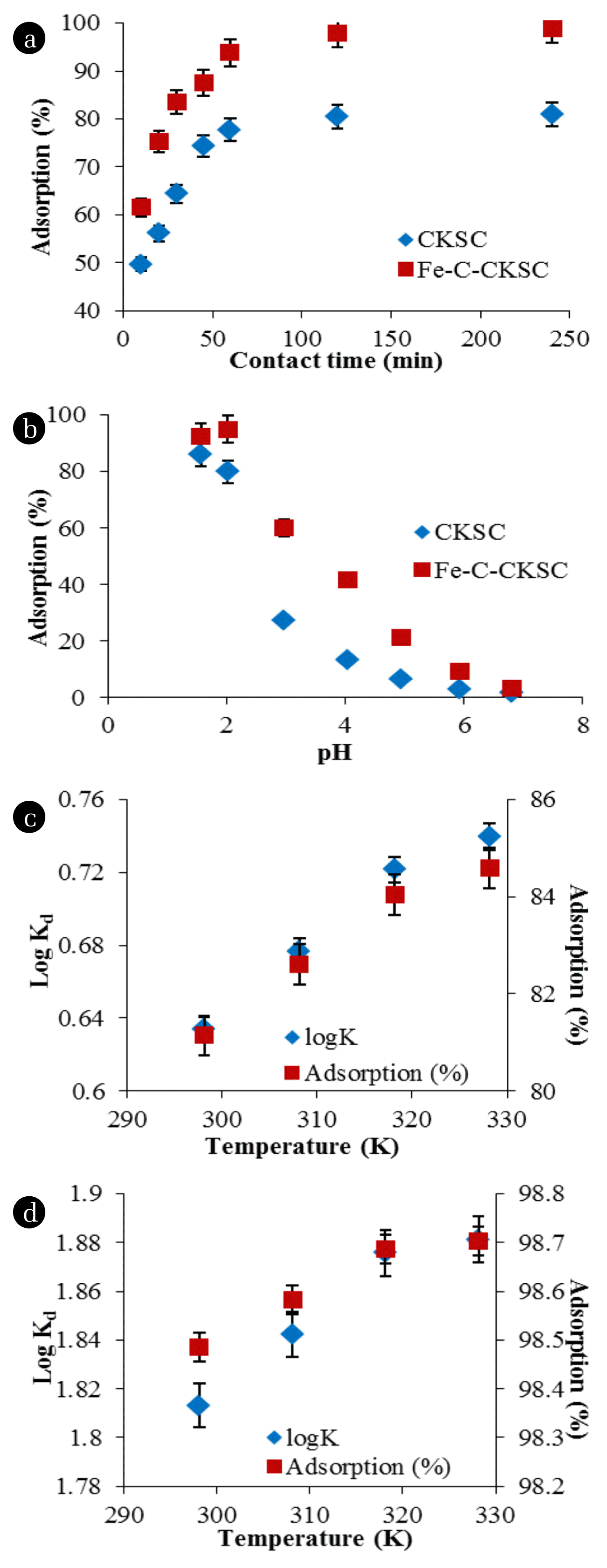
(a) Effect of contact time on the adsorption (Adsorption conditions: adsorbent amount: 5 g/L for CKSC and 3 g/L for Fe-C-CKSC, concentration of Cr(VI): 55 mg/L, solution pH: 2, temperature: 25°C) (b) Effect of pH on the adsorption (Adsorption conditions: adsorbent amount: 5 g/L for CKSC and 3 g/L for Fe-C-CKSC, concentration of Cr(VI): 55 mg/L, contact time: 120 min, temperature: 25°C) (c) Effect of the temperature on the adsorption for CKSC, (d) Effect of the temperature on the adsorption for Fe-C-CKSC (Adsorption conditions for temperature study: adsorbent amount: 5 g/L for CKSC and 3 g/L for Fe-C-CKSC, concentration of Cr(VI): 55 mg/L, pH: 2, contact time: 120 min).
As a result of the experiments, since the adsorbent surface was completely filled, it was seen that the adsorption reached equilibrium within 120 min. Cr(VI) adsorption percentages at the end of 120 min of contact time were found to be 80.5% for CKSC and 97.9% for Fe-C-CKSC [41].
In order to determine rate and rate constants of Cr(VI) adsorption with CKSC and Fe-C-CKSC, pseudo-first-order and pseudo-second-order kinetic model equations were used. Kinetic models were tested to investigate adsorption mechanisms such as mass transport and chemical reaction processes. Calculated kinetic model constants, adsorption capacities and correlation coefficients are given in Table 1 [31].
Table 1 shows the calculated adsorption capacity (q) values. At the end of 120 min of contact time, the experimentally determined adsorption capacities (qe) were found to be 0.192 mmol/g for CKSC and 0.388 mmol/g for Fe-C-CKSC.
When the correlation coefficients of kinetic models are compared, it is seen that the correlation coefficient of the pseudo-second-order kinetic model is greater than the correlation coefficient of the first order kinetic model. In addition, the adsorption capacities calculated from the pseudo-second-order-kinetic model equation is closer to the experimentally determined adsorption capacities. Therefore, the pseudo-second-order model was accepted as a useful model for the kinetic studies. This indicates that the chemical adsorption is more effective in the adsorption mechanism [31, 42]. The values of Pearson’s r calculated for the pseudo second-order model, has been found higher than that of pseudo first-order model (Table 1).
3.2.4. Effect of solution pH on adsorption
55 mg/L Cr(VI) solutions adjusted to different pHs were added to the optimum amounts of adsorbents and the mixtures were stirred for 120 min. Effect of pH on percentage of Cr(VI) adsorption is given in Fig. 6(b).
As a result of the experiments, it is seen that the adsorption percentages were the highest at the pH values of 1.56 for CKSC and 2.00 for Fe-C-CKSC. Adsorption percentages at these pH values are 86% for CKSC and 95% for Fe-C-CKSC. When the pH increased to 2.97, the percentage of adsorption decreased to 27% for CKSC, and to 60% for Fe-C-CKSC, and the percentage of adsorption decreased as the pH increased [35].
The reason why adsorption is efficient at low pH is the protonation of the functional groups such as −NH2 and −OH on the adsorbent surface at acidic pHs. The Cr(VI) ions are retained by the positively charged adsorbent surface due to the electrostatic attractions, because Cr(VI) present in the solution as anionic components such as HCr2O7−, HCrO4−, CrO42− and Cr2O72− [41]. In addition, the reduction of Cr(VI) to Cr(III) which is present in solution as cationic form is also very low at low pH. Therefore, the total amount of chromium in the solution is considered to be equal to the amount of Cr(VI). However, the increase of pH also increases the reduction of Cr(VI) to the positively charged Cr(III). Adsorption of Cr(III) species onto protonated CKSC and Fe-C-CKSC surface decreased due to the fact that the opposite charges repel each other [43].
The mechanisms of removal of Cr(VI) by Fe-C-CKSC include mainly electrostatic attraction between protonated adsorbent surface (−OH2+ and −NH3+ groups) and Cr(VI) species, ligand exchange of Cr(VI) species and re-sorption of Cr(III) reduced from Cr(VI) via chelation. −OH groups of chitosan can supply electrons for Cr(VI) reduction. Hence, a part of reduced Cr(III) can interact with −NH2 groups via chelation. Reactions of electrostatic attractions can be written as Eq. (6) and (7), reactions of ligand exchange and chelation mechanisms can be written as Eq. (8) and (9), respectively [44]. The scheme of adsorption mechanism was given in Additional file 1: Fig. S2.
Point of zero charge (pHPZC) is the pH at which the charge on the adsorbent surface is zero. While the total surface of the adsorbent is negatively charged above this pH, it is positively charged below this pH. pHpzc of CKSC and Fe-C-CKSC were found to be pH 6.5. Based on the pHPZC value found, the CKSC and Fe-C-CKSC surfaces will be charged negatively when the solution pH > 6.5 and positively charged when pH < 6.5. The adsorption of Cr(VI) with CKSC and Fe-C-CKSC is more efficient at pH < pHPZC. Predominant Cr(VI) species are H2CrO4, HCrO4−, CrO42− and Cr2O72− at acidic pHs. Considering the adsorption between the positively charged surface and the anionic Cr(VI) ions, at acidic pH values, excess H+ ions are present on the adsorbent and the adsorption of the anionic Cr(VI) ions takes place with these suitable centers. As shown in additional file 1: Fig. S3, a point where the surface charge is zero is reached with increasing pH. The adsorption of Cr(VI) ions with CKSC and Fe-C-CKSC is very low at pH > pHPZC. At basic pH values, since the surface and Cr(VI) ions are negatively charged, adsorption of Cr(VI) ions is prevented. In high pH values, the removal of Cr(VI) ions from the aqueous solution is difficult due to the propellant electrostatic power between the negatively charged adsorbent surface and Cr(VI) ions. Therefore, the adsorption was higher at lower pH values and the optimum pH value was found to be 2. Similar results are also available in the literature [6]. The original pH of Cr(VI) ions is about 4.93 and adsorption tests were carried out after the pH was reduced to 2 to determine the other optimum parameters.
3.2.5. Thermodynamic interpretation
55 mg/L Cr(VI) solutions adjusted to pH 2 were added to the optimum amounts of adsorbents and the mixtures were stirred for 120 min at different temperatures. Effect of the temperature on Cr(VI) adsorption is given in Fig. 6(c) and (d).
It can be seen in Fig. 6(c) and (d) that when the temperature was increased, the percentage of adsorption increased. This indicates that the adsorption is endothermic. The increase in adsorption with temperature may indicate chemical interactions between the adsorbent and the adsorbate.
The increase in temperature increased the percentage of Cr(VI) adsorption, but this increase is negligible. Since operating the adsorption at high temperatures would cause high costs, 25°C was chosen as the optimum temperature.
The thermodynamic parameters were found with the slopes and intercepts of the plots of logKd versus 1/T using Eq. (3) and (4) and these parameters were shown in Table 1.
The positive value of the enthalpy change (ΔH°) confirms that the adsorption is endothermic [40]. The positive value of the entropy change (ΔS°) indicates that during the adsorption process the irregularity on the adsorbent and solution interface increased [45]. The fact that the Gibbs free energy (ΔG°) is negative indicates that adsorption is spontaneous at higher temperatures than −78.9°C for CKSC and −183.3°C for Fe-C-CKSC [46].
3.3. Desorption and Reuse Studies
The repeatability of the adsorption process was also investigated. Desorption studies are required to recover Cr(VI) and to ensure the re-use of adsorbents. The stripping of Cr(VI) was carried out using 0.5 M NaOH solution. Cr(VI) ion loaded on CKSC and Fe-C-CKSC was shaken with 50 mL NaOH for 24 h. To determine the re-usability of the adsorbents after desorption, the adsorbents were removed from the solution and washed with deionized water. The washed and dried adsorbents were re-used for the adsorption process, while the filtrate was analyzed for the desorbed Cr(VI) ion. The concentration of desorbed Cr(VI) ion was calculated by measuring. The percentage of desorption was determined from the data. The adsorption-desorption cycle was repeated three times using the same sorbent. When the cycle was repeated for the fourth time, the mechanical stability for the Fe-C-CKSC adsorbent was reduced, the adsorbent slightly disintegrated and iron leakage was observed. Additional file 1: Fig. S4 shows the removal capacity of adsorbents after using the same adsorbents three times. Cr(VI) desorption capacity of Fe-C-CKSC was found to be 93.65%, while CKSC was 85.13%. The data calculated from the experiments showed that the synthesized Fe-C-CKSC had a better adsorption property in repeated applications.
4. Conclusions
In this study, cherry kernel shell pyrolytic charcoal (CKSC) and composite beads (Fe-C-CKSC) were synthesized and Cr(VI) adsorption from aqueous solutions with synthesized Fe-C-CKSC composite beads and CKSC adsorbents was studied comparatively. Fe-C-CKSC composite beads consisted of CKSC, chitosan and Fe2O3.
Optimum adsorbent dosages were found to be 5 g/L for CKSC and 3 g/L for Fe-C-CKSC. Equilibrium adsorption data were correlated with Langmuir, Freundlich, Scatchard, D-R, and Temkin isotherm models. The adsorption equilibrium data fitted well with Langmuir isotherm model. Maximum adsorption capacities of adsorbents are calculated as 14.455 mg/g for CKSC and 47.576 mg/g for Fe-C-CKSC with Langmuir isotherm model. It was seen that removal of Cr(VI) was higher at acidic pH than at higher pH. The optimum pH values are 1.56 for CKSC and 2.00 for Fe-C-CKSC. The adsorption reached the equilibrium at the end of contact time of 120 min. In addition, the adsorption kinetics was found to be more compatible with the Pseudo-second kinetic model. The results showed that experimental data with Pearson’s r values were better defined by the pseudo-second order kinetic model. Pearson’s r values were calculated as 0.997 and 0.998 for CKSC and Fe-C-CKSC adsorbents, respectively. These values are greater than the values calculated for Pseudo-first-order kinetic model. Thermodynamic calculations showed that the adsorption was endothermic and had spontaneous nature.
As a result of isotherm studies, kinetic calculations and thermodynamic calculations, it can be concluded that the chemical adsorption was more dominant in the adsorption mechanism.
FTIR, SEM and EDX analyzes for the characterization of adsorbents showed that Cr(VI) was loaded onto the surfaces of adsorbents. As a result of BET surface area analysis, surface area of CKSC was determined as 224.148 m2/g and surface area of Fe-C-CKSC was determined as 31.286 m2/g.
This study showed that cherry kernel shell pyrolytic charcoal-chitosan-Fe2O3 composite can provide high adsorption efficiency for removal of Cr(VI) from aqueous solutions.
Supplementary Information
Acknowledgments
We express our thanks to the Konya Technical University Scientific Research Foundation, which has financed the project (18101015/2018), a part of which is presented in this study. We would like to thank our colleague Prof. Dr. Yakup KAR, from Iskenderun Technical University, for his support in the preparation of pyrolytic charcoal in this study.
Nomenclature
ΔG
Gibbs free energy change (J mol−1)
E
A dsorption energy (KJ mol−1)
Qs
Maximum adsorption capacity (mmol g−1)
ΔH
Enthalpy change (J mol−1)
Fe-C-CKSC
Fe2O3-chitosan-cherry kernel shell pyrolytic charcoal composite
q
Amount of adsorbed substance per unit adsorbent mass (mmol g−1)
ΔS
Entropy change (J mol−1)
K
D-R isotherm adsorption energy constant
qe
Amount of the adsorbed substance (experimental) (mmol g−1)
ɛ
Polanyi potential
Kb
Constant about adsorption enthalpy
qt
Amount of adsorbed substance per unit adsorbent mass at any time t (mmol g−1)
As
Maximum adsorption capacity (mmol g−1)
KD
Thermodynamic equilibrium constant
R
Universal gas constant (J mol−1k−1)
AT
Temkin isotherm constant (L g−1)
KF
Constant about adsorption capacity (mmol g−1)
T
Temperature (K)
BT
Temkin constant related to adsorption temperature
Ks
Scatchard isotherm binding constant
t
Time (min)
C0
Initial Cr(VI) concentration of solution (mmol)
kad
Pseudo first order kinetic model rate constant
V
Solution volume (L)
Ce
Cr(VI) concentration at the end of the contact time (mmol)
k2
Pseudo second order kinetic model rate constant
w
Adsorbent amount (g)
CKSC
Cherry kernel shell pyrolytic charcoal
n
Isotherm constant, expressed as the heterogeneity factor
Xm
Maximum adsorption capacity of adsorbent (mmol g−1)