1. Introduction
Food waste is organic waste discharged from various sources including food processing plants, domestic and commercial kitchens, and restaurants. Approximately 1.3 billion tons of food around the world is lost or wasted each year [1]. In South Korea, it was reported that approximately 4.9 million tons of various foods are wasted annually [2]. The disposal of food wastes has become a major concern and burden in South Korea because environmental law has prohibited the sanitary landfilling of food waste since 2005. Therefore, alternative methods to treat food wastes without causing secondary environmental problems have been an important issue for many years [3].
As food wastes contain various organic compounds, microorganisms can consume and reduce the amounts of food wastes. There is extensive research on food waste treatments using various microorganisms, such as aerobic and anaerobic fermentation [2–7]. Among the various microorganisms, bacteria are widely considered for food waste treatment because various bacteria are found in food wastes. During fermentation of food wastes, mesophilic and thermophilic bacteria, including Pseudomonas spp., Xanthomonas spp., Bacillus spp., and Stearothermophilus spp., are often found in food wastes [8–9]. Yi et al. [6] reported that thermophilic bacteria exhibit good activities to grow and decompose food wastes effectively because temperature increased to 50°C during fermentation. Shin et al. [5] reported hydrogen production from food waste using thermophilic bacteria. Kim et al. [10] isolated thermophilic Bacillus subtilis (B. subtilis) from various organic materials for food waste treatment.
There are several methods for preparing microbial starters such as freeze-drying, fluidized bed drying, and spray drying for industrial applications [11–13]. Recently, Lee et al. [14] developed and optimized a procedure for producing Saccharomyces and non-Saccharomyces yeast starters at the industrial level using an air-blast drying method. Following the protocols of the authors, this research attempted to develop air-dried bacterial cells that could potentially be applied to treat food wastes at the industrial level.
Equipment for food waste treatment was developed previously in our laboratory, and the optimum design temperature for the machine is high temperature greater than 50°C. This research was conducted with the goals of providing the best bacteria for use with this machine and manufacturing a starter of the bacteria under the running conditions at the commercial level. The specific objectives of the research were to 1) isolate and identify thermophilic bacteria responsible for food waste treatment; 2) investigate the capability of food waste treatment using Bacillus species; and 3) develop air-dried Bacillus starters for food waste treatment.
2. Materials and Methods
2.1. Isolation and Identification of Bacteria Screened from Food Wastes and Soils
Screening of bacteria for food waste treatment was conducted from various food wastes and soils obtained in the Cheonan area, Chungnam, South Korea. The food waste and soil samples (5 g) were mixed in 100 mL of peptone water, and 1 mL of the mixture was plated onto a TSA plate. The colonies formed after incubation at 45°C for 24 h were examined and differentiated by Gram staining. A total of 54 colonies were selected and tested for further experiments.
Each colony was tested for extracellular hydrolytic enzyme activities against starch (amylose), carboxymethyl cellulose (CMC), and protein using starch agar medium (Sigma-Aldrich, St. Louis, MO, USA), CMC medium (Sigma-Aldrich, St. Louis, MO, USA) and skim milk agar medium (Sigma-Aldrich, St. Louis, MO, USA), respectively. Each colony was inoculated on a respective medium and cultivated in an incubator at 45°C for 24 h. The enzyme activities were determined based on the growth and formation of clear zones on the agar plate [10, 15].
The isolated bacteria exhibiting three enzyme activities were further identified by comparing the 16S rDNA sequence with reference bacteria (Microgen Co., Seoul, South Korea) [5]. The identified Bacillus species were maintained by cultivating in TSB at 45°C for 48 h in a shaking incubator (200 rpm).
2.2. Preparation and Chemical Analyses of Standard Food Waste
The standard food waste was formulated according to the formula for standard food waste from the home established by the Korean Ministry of Environment (Guideline 2013–179). The detailed formula of the standard food waste is presented in Table 1. The food waste was prepared by grinding food materials using a blender and stored in a refrigerator prior to use.
Proximate analysis of standard food waste was conducted following AOAC standard methods [16]. The Hunter L, a, b color scale of standard food waste was determined using a CR-300 Minolta Chromameter (Minolta Camera Co., Osaka, Japan). The light source was illuminant C, and the white plate (CR-A43; L = 96.86, a = −0.02, and b = 1.99) provided by the manufacturer was used for calibration and background.
For determination of the pH and salinity of the standard food waste, 5 g of sample was added to 25 mL of distilled water, mixed for 1 h, and centrifuged for 10 min at 8,000 rpm. A pH meter (inoLab 720, inoLab, Seoul, South Korea) and a sodium chloride refractometer (DMT-20–1, Daeyoon Co., Seoul, South Korea) were used to determine the pH and salinity of the supernatant of the centrifuged wastes, respectively.
2.3. Treatment and Analysis of Standard Food Waste Using Identified Bacillus Species
The food waste treatment was conducted using each identified single Bacillus species as well as the mixture of Bacillus licheniformis (B. licheniformis) G1 and B. subtilis E1. The ratios of B. licheniformis G1 and B. subtilis E1 mixture were 1:0, 1:1, 1:2, 2:1, and 0:1 (v/v). An aliquot of 1 mL of fully grown single bacterial culture or bacterial mixture was inoculated into 100 g of sterilized standard food waste and cultivated in a shaking incubator at 45°C and 200 rpm for up to 8 d. The initial bacterial numbers of the standard food waste ranged from 104 to 105 CFU/mL. During cultivation, food waste samples were withdrawn at 0, 1, 2, 4, and 8 days and analyzed for solid content, bacterial growth, pH, and salinity. The bacterial numbers were counted by pour plating serially diluted food waste samples onto TSA and expressed as CFU/mL.
The solid content of the standard food waste was determined using the standard drying method established by the Korea Testing and Research Institute (KTR). An aliquot of 5 g of food waste was placed into a pre-weighed aluminum dish and dried in a drying oven at 105°C until it exhibited constant weight. After drying, the dish was re-weighed to calculate the solid content using following equation (Eq. (1)):
where W0 represents the weight of the dish (g); W1 represents the weight of the sample and dish before drying (g); and W2 represents the weight of the sample and dish after drying (g). The pH and salinity of the standard food waste culture were determined using a pH meter, as described in the previous section.
2.4. Preparation of Air-dried Bacterial Cell Powders
Skim milk (10%, w/v) and glucose (10%, w/v) were used as protective agents to evaluate the survival rate of the air-dried Bacillus cells. The protective solutions were sterilized at 121°C for 15 min prior to use. Two types of excipients, wheat flour (CJ Corp., Seoul, South Korea) and lactomil (composed of 89% lactose and 11% maltodextrin) (Seo Kang Dairy & Food Co., Ltd., Sacheon, South Korea) were used to process the bacterial cells into an appropriate powdered form.
B. licheniformis G1 and B. subtilis E1 were cultured in 100 mL TSB at 45°C for 24 h, collected by centrifugation at 3,000×g for 10 min, and rinsed twice with 0.85% NaCl solution. The collected cells were mixed with 0.5, 1.0, and 1.5 g of the excipients (lactomil and wheat flour, respectively) in the presence of 1 mL of protective agent solution. The mixed bacterial cell pellets were dried in an oven drier (HB-509C, HanBaek, Bucheon, South Korea) at 37°C until the moisture content of the dried cells was less than 10% [14]. After air-drying, the samples were analyzed immediately to determine their moisture content and survival rate and then stored at 4°C for later use.
2.5. Determination of Viability of Air-dried Bacterial Cells
The air-dried cell powders were reconstituted using sterilized distilled water until reaching their original volume before drying. Serially diluted samples were pour plated onto TSA plates and incubated at 45°C for 24 h. The survival rate of each sample was calculated as percent survival using the following equation (Eq. (2)):
where N represents the number of the viable cell count after air-drying (CFU/mL) and N0 represents the number of the viable cell count before air-drying (CFU/mL). The moisture content of the powders was determined following the AOAC standard method (16, AOAC, 1996).
3. Results and Discussion
3.1. Isolation and Identification of Bacteria from Food Wastes and Soils
Among the 54 isolated colonies tested for hydrolytic enzyme activities, 10 colonies exhibited at least one positive activity from the starch, CMC, and protein tests (Table 2). All selected bacteria possessed positive amylase activity, and five and seven bacteria exhibited positive CMC and protein hydrolytic activities, respectively.
Based on the 16S rDNA sequence comparison with reference bacteria, all the bacteria isolated from the food wastes and soils were identified as five different Bacillus species (Table 3). This research aimed to select thermophilic bacteria to treat food wastes. The result indicated that the growth temperature of 45°C under the anaerobic condition allowed Bacillus species to grow favorably compared to other bacteria. The identified Bacillus species isolated from the food wastes and soils were named B. licheniformis G1, Bacillus circulans (B. circulans) C2, B. subtilis E1, Bacillus vanillea (B. vanillea) F1, and Bacillus atrophaeus (B. atrophaeus) G2.
There are extensive reports indicating that Bacillus species are commonly isolated bacteria from food wastes and soils [3, 5, 17]. Sudharhsan et al. [7] identified Bacillus species isolated from spoiled food waste for amylase production. Kwon et al. [3] isolated six microorganisms from food wastes to treat food wastes and reported that one of the isolated microorganisms was identified as Bacillus amyloliquefaciens (B. amyloliquefaciens). Bacillus species are gram positive thermophilic bacteria that are often used for enzyme production, biomaterial production, and food waste treatment [2, 18]. Kim et al. [18] reported that three-stage anaerobic fermentation using Bacillus species reduced total chemical oxygen demand significantly. The identified bacterial species included B. subtilis, B. amyloliquefaciens, Bacillus alcalophilus, Bacillus polymyxa, and B. licheniformis, as well as some other species. Therefore, five Bacillus species including B. licheniformis G1, B. circulans C2, B. subtilis E1, B. vanillea F1, and B. atrophaeus G2 were selected as bacteria to treat food waste in this research.
3.2. Characteristics of Standard Food Waste
The characteristics (color, pH, and salinity) of the standard food waste prepared according to Table 1 are presented in Table 4. The appearance of the ground standard food waste was a yellowish paste. The ranges of pH and salinity of the standard food waste were considered to be favorable for a growth media for Bacillus species. The proximate analysis of the standard food waste also indicated that the standard food waste could serve a good medium for growth of Bacillus species (Table 5).
3.3. Food Waste Treatment Using Identified Bacillus Species
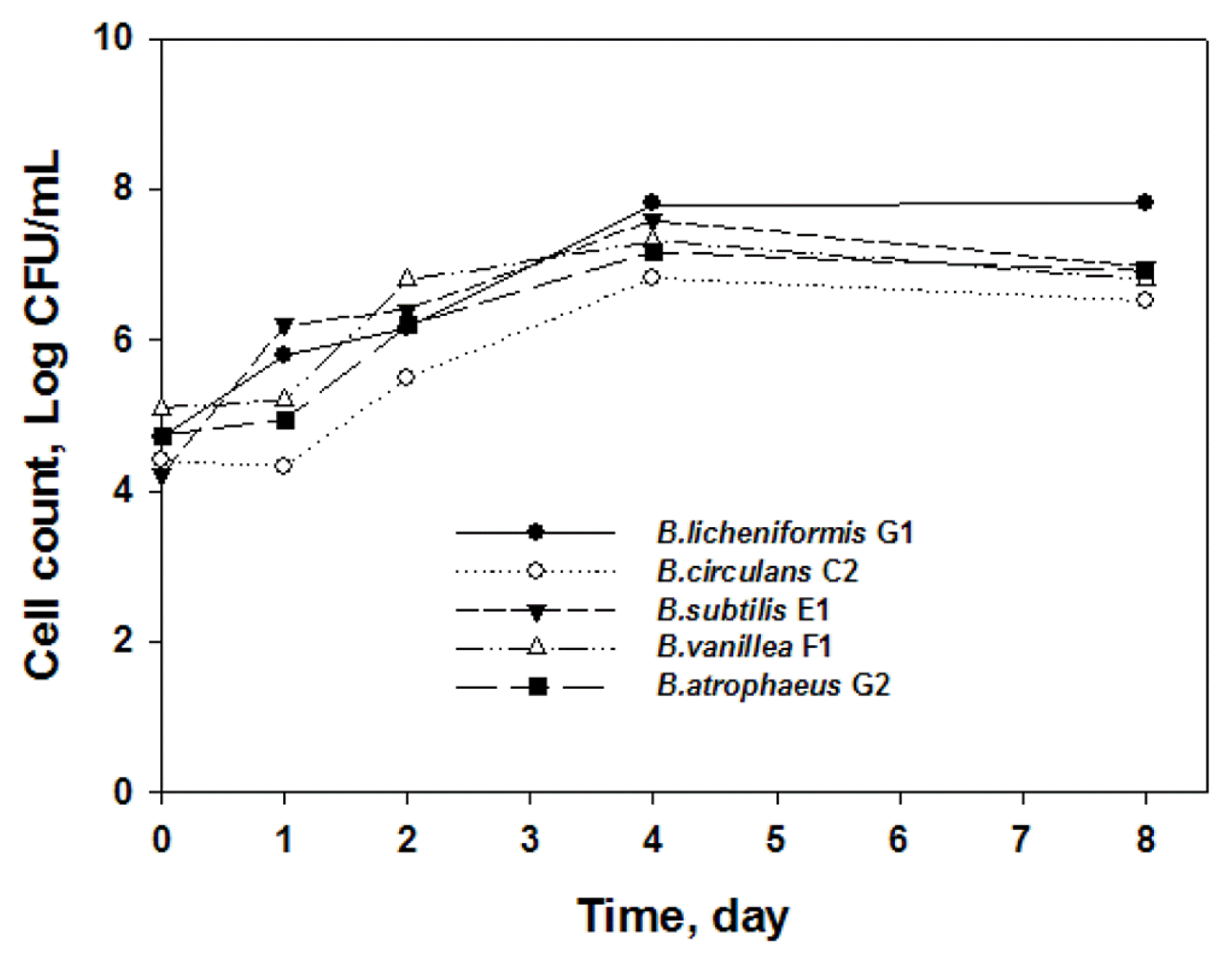
The growth curves of the identified Bacillus species waste exhibited a similar pattern, as presented in Fig. 1. There were no significant differences in cell count among the tested Bacillus species during the 8-day cultivation in the standard food waste. The cell counts of each Bacillus species increased with the number of cultivation days until day 4 and then exhibited a plateau until day 8. Bacillus species, as thermophiles, are known to grow rapidly during the early stage of cultivation in food wastes [4, 8]. Choi et al. [4] reported that the bacterial counts of Bacillus species increased rapidly up to 108–109 CFU/mL during 3 to 4 d of cultivation in organic food wastes. Kim et al. [11] also identified B. subtilis as a fast-growing bacteria responsible for hydrolyzing organic compounds in food waste. In this research, it was observed that B. licheniformis G1 attained the highest cell count among the identified Bacillus species during the 8-day cultivation (~108 CFU/mL). The results indicated that the standard food waste was an appropriate medium for Bacillus species growth and treatment without any modifications.
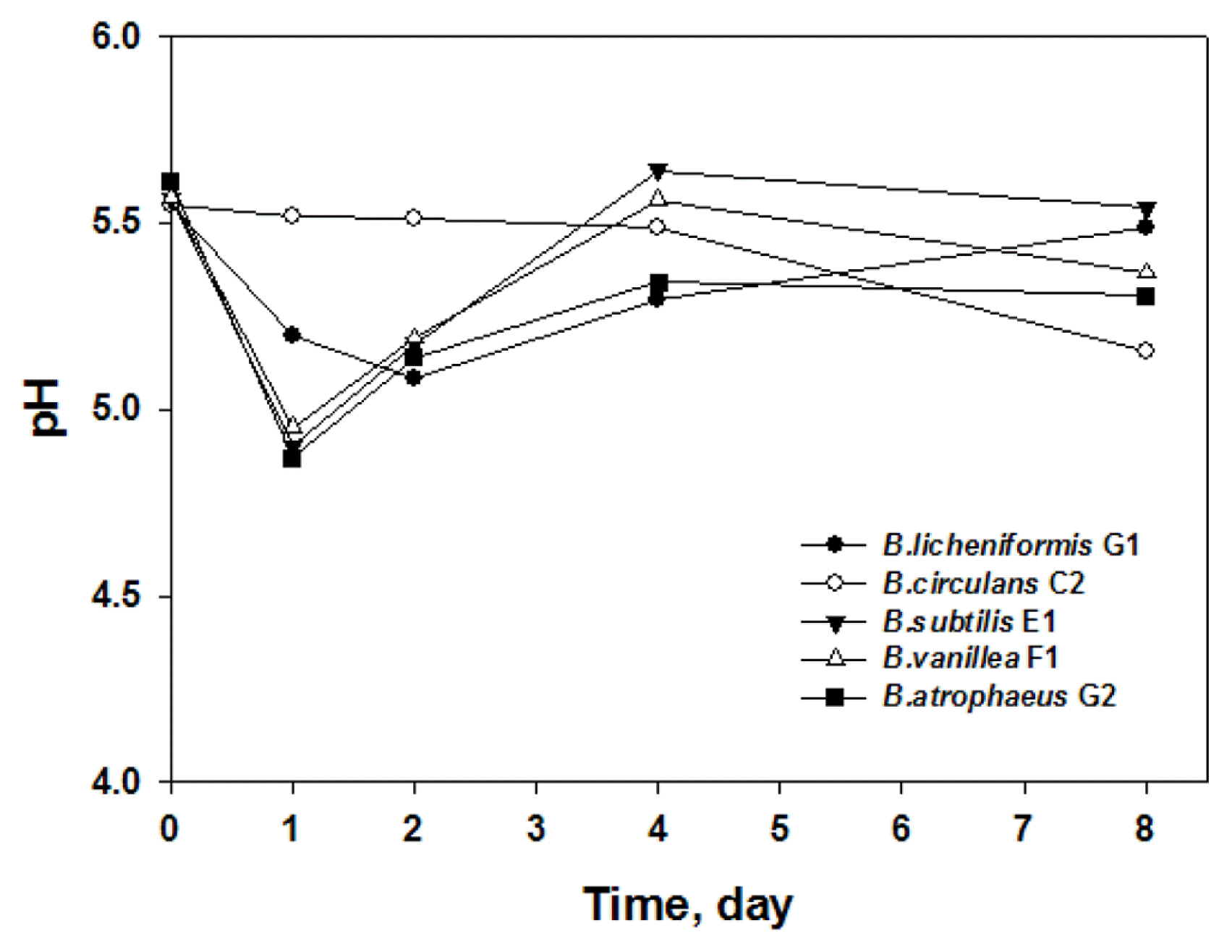
As the Bacillus species, except for B. circulans, grew in the standard food waste, the pH value of the food waste decreased initially but then increased gradually until day 4 of cultivation and finally reached a plateau (Fig. 2). This result is in good agreement with the result of Kim and Lee’s report [10], in that the pH of the media decreased initially but then increased as B. subtilis grew and stabilized in the media.
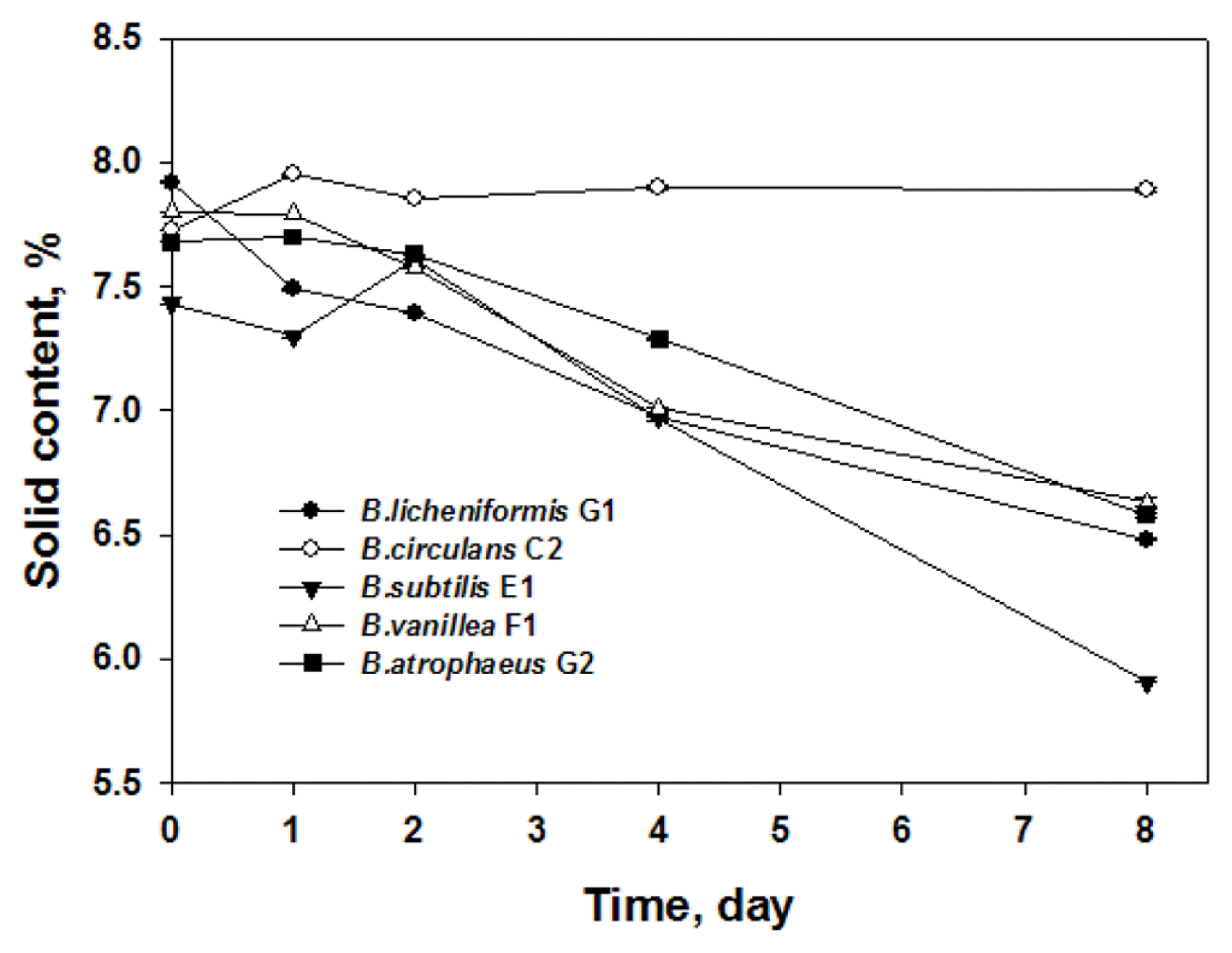
The changes in solid contents of the standard food waste cultivated with the Bacillus species are presented in Fig. 3. The Bacillus species, except for B. circulans, significantly reduced the solid content of the food waste during the 8-day cultivation. B. circulans did not reduce the solid content during the entire cultivation time. This could be attributable to the fact that B. circulans did not grow well in the standard food waste, as presented in Fig. 1. The reductions in solid content caused by the Bacillus species other than B. circulans in this research were somewhat similar to each other and exhibited linear reduction patterns during the 8-day cultivation time.
The greatest reduction among the tested Bacillus species was achieved by B. subtilis E1. There are extensive reports on effective waste treatment at high temperatures using Bacillus species [3, 6, 15]. Yi et al. [6] reported that the most thermophilic bacterial species isolated from food wastes were Bacillus species and that the Bacillus species exhibited degradative enzyme activities and effectively decomposed food wastes. The addition of a microbial mixture containing Bacillus species into food waste has been shown to reduce soluble solids, COD, and BOD significantly [3]. Lee et al. [15] also identified B. subtilis as one of the major bacteria responsible for food waste treatment and reported that B. subtilis effectively converted the food waste into a valuable feedstuff by fermentation.
Considering their capabilities for reducing solid content as well as their growth in standard food waste, B. licheniformis G1 and B. subtilis E1 were selected as the optimum Bacillus species. B. licheniformis and B. subtilis are known to be attractive industrial bacilli because of their high growth rates, ability to secrete numerous hydrolytic enzymes, and GRAS status [19]. B. licheniformis strains were reported to be multifunctional and multi-enzyme-producing bacteria that can degrade diverse substrates and grow under various environmental conditions [19]. A proteomic analysis of exoenzymes secreted by B. licheniformis revealed the presence of diverse proteases that can lead to greater diversity of breaking of peptide bonds to degrade substrates. Meng et al. [20] reported that B. subtilis produced cellulase to degrade carboxymethyl cellulose, rice straw, corn stover, soluble starch, and wheat bran. Because of their strong proteolytic and cellulolytic activities, B. subtilis and B. licheniformis exhibited stronger capabilities to degrade food wastes.
When the mixture of B. licheniformis G1 and B. subtilis E1 was used for treatment, the solid content of the standard food waste was reduced more than when the waste was treated with a single Bacillus (Fig. 4). The greatest reduction of solid content was achieved by the 1:1 ratio mixture of the two Bacillus species (28.5%). This result might be attributable to synergistic effects of the multifunctional enzymes excreted by B. licheniformis G1 and B. subtilis E1 in the food waste.
3.4. Preparation and Viability of Air-dried Bacterial Cells
For the preparation of the air-dried Bacillus cells, including B. licheniformis G1 and B. subtilis E1 cells, commercial skim milk and glucose were selected as protective agents based on the results reported by Lee et al. [14]. These authors obtained the highest viability when 10% skim milk was applied to yeast cells. The selection of excipients is very important for generating a stable powdered form and shape to improve the stability and quality of the final product [21]. From among the many excipients described in the literature, lactose and wheat flour were selected as excipients in this research based on the popularity of these two materials.
The bacterial cell counts and survival rates of B. licheniformis G1 and B. subtilis E1 cells incorporated into selected amounts of either lactomil or wheat flour are summarized in Table 6. For B. licheniformis G1, lactomil exhibited a higher survival rate than wheat flour in general, and the highest survival rate (95%) was achieved when 1.0% lactomil was used. This survival rate is comparable with the survival rate (91%) of yeast with lactomil reported by Lee et al. [14]. Therefore, lactomil was thought to be the more appropriate excipient for B. licheniformis G1. For B. subtilis E1, there was no significant difference by excipient type or amount added. However, wheat flour showed a lumpy form after air-drying; on the contrary, lactomil produced an easily collectable and impalpable powder after air-drying. Based on the survival rates and the properties of the powdered forms, lactomil (1.0 g) was selected as the appropriate excipient for Bacillus species in this research.
4. Conclusions
Thermophilic bacteria isolated from food wastes and soils were identified as five different Bacillus species. The isolated Bacillus species were able to grow in a standard food waste mixture at high temperature, resulting in significant decreases in the solid content of the food waste. The highest reductions in solid content were achieved by B. licheniformis G1 and B. subtilis E1. Synergistic effects were also observed when a 1:1 ratio mixture of B. licheniformis G1 and B. subtilis E1 was used. Air-dried Bacillus cell powders were developed for use as starters for food waste treatment. Lactomil was a suitable excipient for producing the air-dried Bacillus cell powders, exhibiting a 95% survival rate. Overall, this research suggested that the air-dried Bacillus cells isolated from food wastes could be applied to reduce food wastes at the industrial level.