1. Introduction
The coastal areas have recently been worsened due to the industrial proliferation, urbanization, wide spheres of human activities, and aqua culturing. As a result large amount of pollutant including organic matters, untreated municipal sewage, industrial waste, heavy metals, chemicals, and hazardous compounds are accumulated on the bottom. These contaminants have caused harmful algal blooms, oxygen depletion, loss of aquatic life, and aesthetic problems [1, 2]. Furthermore, accumulated organic matter in the sediment layer produces lot of by-products such as hydrogen sulfide, ammonia, and phosphate and those are harmful for living organisms. In consequence it leads to mass mortality of marine organisms and brings much economic losses to aquaculture industries.
Many remediation techniques have been developed including physical method (dredging and aeration), chemical method and biological method (microbial activity) to remediate contaminated sediment in coastal area. In the chemical process, mainly solid peroxides, peroxy-hydrates are used to increase the growth of bacteria in the sediment and subsequently to degrade contaminated pollutant. Effective oxygenation is a critical factor in aerobic bioremediation of contaminated sediment. Solid compounds such as magnesium peroxide, calcium peroxide are an alternative source of oxygen in bioremediation [3, 4]. Magnesium peroxide and calcium peroxide are less water-soluble but release oxygen over prolonged periods without encapsulation. Several studies have reported that addition of magnesium peroxide in saturated soil resulted in increased concentrations of dissolved oxygen and enhanced the biodegradation of dissolved contaminants [5, 6].
Bioremediation is a microorganism facilitated transformation or degradation of contaminants into nonhazardous or less-hazardous substance. This technology relies on promoting the growth of specific microflora or microbial consortia on the contaminated sites that are able to perform desired activities. To maximize the process in bioremediation technologies, two main approaches have been explored: bio-stimulation, in which nutrients are added to stimulate the intrinsic organic compound degraders, and bio-augmentation, in which microbial strains with specific degrading abilities are added to work cooperatively with normal indigenous microorganisms [7].
While the physical methods and others are not reliable and short time solution than it is under searching of long term and effective solution with easy management. Therefore as one of the bio-remediation strategies, in this study, we apply and compare the performance of chemical agent and microbial agent to improve the sediment quality. It could give wide choice to detoxify the bottom of coastal area and contaminated location for advanced objectives.
2. Materials and Methods
2.1. Chemical Agent and Microbial Agent
Only chemical agent and four microbial agents were purchased from local sources having various bacterial species. On the basis of total viable count test (TVC), the best microbial agent was selected and was used for laboratory experiment. Later chemical agent and selected microbial agent component was requested from sources. The chemical agent consists of calcium oxides. The final microbial agent is a zeolite bio ball used in this experiments, consists different species of microorganism such as heterotrophic bacteria, consortium consisted of lactobacillus species, bacillus species, yeast, pseudomonas species, nitromonus species, and nitrobacter species.
2.2. Experimental Settings
Sediment samples were collected from Won-Mun bay, a southeast coastal area of Korea due to the sediment has been contaminated by large amount of aquaculture activity. The sediment was collected using a ponar dredge machine and sealed in white polyethylene containers, preserved in cool conditions before use. Three reactor bins were setup in the laboratory placing 1.5 L wet sediment samples in each. The applying chemical agent and microbial agents were used in the amount of 3% (v/v) into two reactor bin while 1.5 L of sediment without agent was used as a control. Sediment and agent were mixed well and the test was conducted in four days interval till 28 days of agent treatment.
2.3. Chemicals Analysis
The chemical oxygen demand (COD) was analyzed by the reducing agent of potassium permanganate followed by the iodometric titration method and the AVS was measured by the sulfide detection tube (Detector tube No. 201H; GASTEC, Japan) as follows: two grams of a sample were mixed with 2 mL concentrated sulfuric acid, which was continuously pumped into the tube, until the color of the tube changed [8]. The total nitrogen (T-N) was measured by oxidizing sample with potassium persulfate followed by passing through a cadmium-copper redox column and a spectroscopic absorbance analysis at 543 nm. The sediment sample was centrifuged at 3000 rpm for 5 min to collect supernatant and autoclaved for an hour in a closed bottle with the oxidizing potassium persulfate solution then total phosphate (T-P) was quantified by the ascorbic acid reduction method, in which the spectroscopic absorbance was measured at 885 nm. All spectroscopic analyses were done by UV Mini-1240 spectrophotometer (Shimadzu Corporation, Japan) [8]. Analytical grade chemicals were used in all experiment.
2.4. Total Viable Count (TVC) Test
Bacterial growth, using chemical and microbial agents was monitored by the total viable counts (TVCs) test. Marine agar plates were used in this method as a culture medium. Plates were prepared by suspending 55.1 g of marine agar in one liter distilled water. The solution was autoclaved at 121°C for 15 minutes. The prepared solution was poured into petri dishes under a laminar flow hood. The sediment sample of 40.0 g was centrifuged at 3000 rpm for 5 min to collect supernatant and a one milliliter aqueous suspension of the microbial population was taken for serial dilution. Then one milliliter solution from each dilution level was spread on the agar medium surface. The plates were air dried and then incubated at 25°C for 3 days. After incubation, the numbers of bacterial colony were counted visually for each plate in CFU/mL.
3. Results and Discussion
3.1. Chemical Oxygen Demand (COD) State Improvement of Sediment
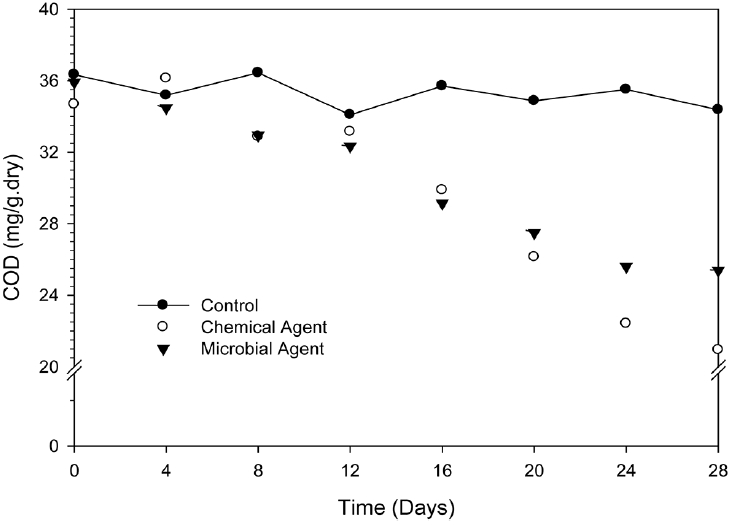
The analyzed COD value in this study is shown in Fig. 1. The average COD value was found in 35 mg/g in control sample. Treatment of chemical agent and microbial agent was shown the changes of COD value in different line. As shown in Fig. 1, the COD values have decrease with the increasing time until 28 days of studying period. The changed in COD was compared with control in entire discussion. Microbial agent was changed the COD value by 1% at initially (0 d; after mixing with sediment) and it was produced by 29% at 28 days of treatment. On the other hand, chemical agent was changed the COD value by 5% at initially whereas this change was with 40% after 28 days of treatment. COD value was found as altered in control sample by 5% at experimental duration. The declining of COD was not following same trend for microbial application when it was gradually decline fashion with time. Fig. 1 depict that the chemical agent treated samples were shown higher COD than control sample at 4 days (4% deviation). It might be due to the microbial adaptation/adjustment with new environment. However the COD value was declined sharply after 16 days and reach from 34 mg/g to 20 mg/g at 28 days by chemical agent whereas microbial agents reach from 36 mg/g to 25 mg/g at 28 days. The chemical agent activity to improve the sediment quality from pollution was praiseworthy. The chemical agent treatment until 28 days, the degradation rate of pollutant was entering into the acceptable level by means of chemical oxygen demand altered from 34 mg/g to 20 mg/g. Contaminant decay is often assume to follow first-order kinetics. Obtained rate constants were as 0.001/day, 0.014/day, and 0.02/day for control, chemical agent and microbial agent respectively.
In this study, after treatment of mediator, small deviation (4%) was observed at 4 days compared with control, it might be due to the microbial adaptation/adjustment with new environment. Then COD value decreased with increasing time indicating the existence of active microbial consortium. Since the microbes are present in the sediment the decreasing rate was greatly improve because microbes absorbed organic pollutant as the energy source. Microbes extracts energy via energy yielding biochemical reactions mediated by the microbial oxidoreductases enzymes to cleave chemical bonds and to assist the transfer of electrons from a reduced organic substrate (donor) to another chemical compound (acceptor) [9]. During such oxidation-reduction reactions, the pollutants are finally oxidized to harmless compounds.
Fig. 1 depicts the decreasing efficiency of chemical agent (40%) at 28 days is 1.5 times greater than microbial agent (29%). High performance of chemical agent might be explained as, chemical agent (oxygen releasing oxides) continuously input molecular oxygen in presence of water, which is helping the internal micro-organism to enhance the efficiency. Kwon et al. [10] reported that the generating molecular oxygen helps to form more oxidative environment for the residing microorganisms. On the other hand external microbial agents are also competing with internal micro-organism to enhance the efficiency. These organisms absorbed organic pollutant as energy source and surface of the pollutant is surrounded by the attached organism. Thus a film is formed around the pollutant by the microbes, and then the biodegradation of absorbed pollutant is continued as the enzymatic metabolism of microorganism proceeds.
3.2. Acid Volatile Sulfide (AVS) State Improvement of Sediment
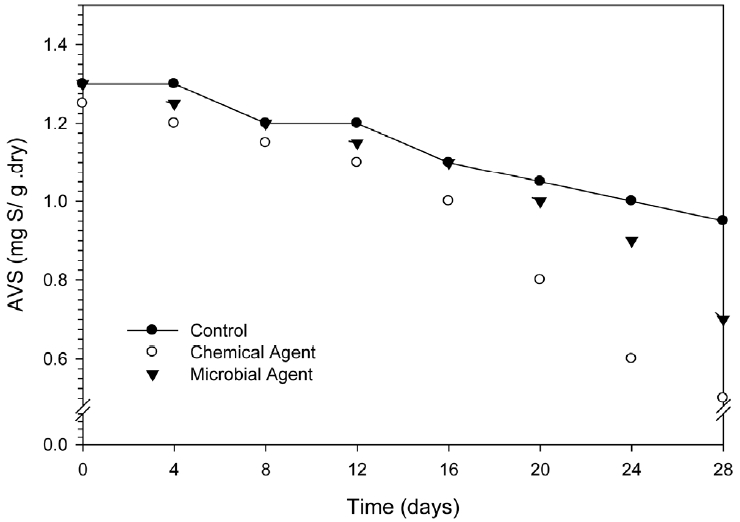
The mean AVS value was found in 1.13 mg/g of collected sediment as natural condition. The changes of AVS in control and treated sample over time have shown in Fig. 2. As it is presenting, the AVS was changed with time in all samples. The control sample was out of agent application when it also shown the AVS declining from 1.13 mg/g at initially (0 d) to 0.95 mg/g at 28 days. The percentages of this declining were changed when the sediment treated by agents. The change of AVS was found of 27% in control, 46% in microbial agent treated sediment and 60% in chemical agent treated sediment during 28 days of study period. The AVS change in control with time implying the inherent effects of sediment and might be due to the activity of residing microbial community. Subsequently the increasing of this percentage was likely due to the effect of agent application. However the effect of microbial agent and chemical agent was different. As the effect of agent, the microbial agent was very active and reduced by 46% of AVS. On the other hand the activity of chemical agent was gradually increasing until 28 days of studying period (60%). This finding specifies that probable the chemical agents provide more congenial environment to increase internal microbial growth compare with microbial agent. In fact microbial agent include as a new microbial member only into the sediment microbial community instead of chemical change. But the chemical agent provides the multiplication opportunity of the internal microbial community by changing pH and other parameters. Obtained rate constants were as 0.012/day, 0.033/day, and 0.020/day for control, chemical agent and microbial agent respectively.
In anaerobic sediments, sulfate reduction by anoxic bacteria leads to the formation of sulfides, which complexes some cationic metals and thereby influences the toxicity of these metals to benthic organisms. Hydrogen sulfide is formed from the dissimilarly reduction of sulfate and the decomposition of amino acids such as cysteine [11]. Hydrogen sulfide then reacts with ferrous iron to form the amorphous FeS which comprises the majority of AVS. The oxidation of AVS (e.g. H2S and FeS) can be achieved by oxidants such as O2, NO3−, MnO2 and FeOOH [12–14]. Thus, oxygen produced by the chemical agent can dominant the oxidation of AVS than microbial agent.
3.3. Total Nitrogen (T-N) Situation Improvement of Sediment
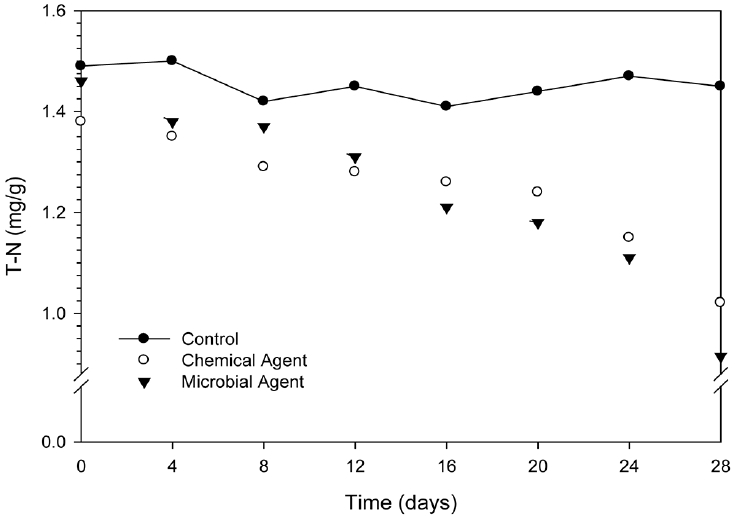
Measuring T-N concentration in this study is presented in Fig. 3. The average T-N concentration was measured 1.45 mg/g in control sample, where no agent was mixed. The changes of T-N concentration were measured in four days interval after the treatment of chemical agent and microbial agent. As shown in Fig. 3, the T-N concentration has decreased with the increasing time until 28 days of studying period. Microbial agent was decreased the T-N concentration by 2% at initially (after mixing with sediment) and it was found by 37% at 28 days of treatment. On the other hand chemical agent was changed the T-N concentration by 7% at initially and it was decreased by 26% at 28 days of treatment. T-N concentration was decreased by the both agent but microbial agent showed better improvement after 12 days of treatment compared with chemical agent at the studied period. Finally chemical agent reach from 1.38 mg/g to 1.02 mg/g (26%) and microbial agents reach from 1.46 mg/g to 0.91 mg g (37%). Obtained rate constants were as 0.0008/day, 0.009/day, and 0.015/day for control, chemical agent and microbial agent respectively.
After 28 days of mediators, T-N concentration declined from 1.38 mg/g to 1.02 mg/g by chemical agent (26%) and from 1.46 mg/g to 0.92 mg/g by microbial agent (37%). Although T-N declining percentage is slow, we assume that few numbers of nitrifying bacteria might be present in the sediment, degrade the nitrogenous compounds as the nitrification-denitrification process. In this process nitrogenous compound is oxidized to nitrite and subsequently it is oxidized to nitrate under aerobic condition by the separate species of nitrifying bacteria. Heinz [15] reported that the bacteria Nitromonas, Notrosospira, Nitrosolobus, Nitrosoviria and Nitrococcus perform the oxidation of NH4+ to nitrite while Nitrobacter, Nitrosospira and Nitrococcus participate in the oxidation of nitrite to nitrate. Denitrification is then reduction of nitrate to nitrite and further on to nitrogen under anoxic conditions by the catabolism of heterotopic bacteria. The pH increases during denitrification and the optimal (pH: 7–9) pH for denitrifying bacteria is in the same range as typical for other heterotrophic bacteria. The effect of other environmental factors such as temperature and nutrient requirements are in general about the same for denitrifying bacteria as for aerobic heterotrophic bacteria. The nitrification rate is strongly dependent on the oxygen concentration. Previously discussed that chemical agent inherently generates more oxygen in presence of water, in a consequence T-N removal performance of chemical agent might be better than microbial agent but result indicates opposite performance. It may be caused by the growth of active nitrifying-denitrifying microorganism.
3.4. Total Phosphate (T-P) State Improvement of Sediment
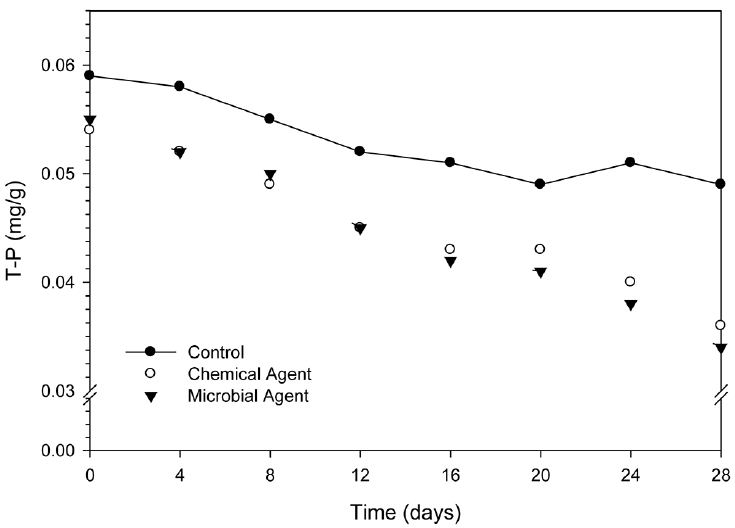
Measuring T-P concentration in this study under laboratory condition is presented in Fig. 4. The average T-P concentration was measured 0.05 mg/g in control sample. Treatment of chemical agent and microbial agent was shown the changes of T-P concentration in different volume. As shown in Fig. 4, T-P concentration has decreased with the increasing time until 28 days of studying period. For control sample, the T-P concentration was decreased by 16% at 28 days compared with initial concentration. At the initial period (0; day) both agents decrease almost same percentage of T-P concentration (8% for chemical agent and 7% for microbial agent). The initial T-P concentration was not too high value but it was gradually decreased by the treatment of mediators. The chemical agent shows remarkable efficiency till 12 days of treatment, and then microbial agent altered the efficiency fashion. Comparatively, decreasing efficiency of T-P concentration was found 38% by microbial agent and 33% by chemical agent. Microbial agent was achieved 5% more removal efficiency than chemical agent at 28 days of treatment. Obtained rate constants were as 0.007/day, 0.014/day, and 0.017/day for control, chemical agent and microbial agent respectively.
T-P concentrations in sediment were decreased from 0.054 mg/g to 0.036 mg/g (33%) by the treatment of chemical agent and from 0.055 mg/g to 0.034 mg/g (38%) by the treatment of microbial agent. Nutrients releasing from sediment is highly complex, involving chemical and biological process. Chemically, phosphorus removal is done by the precipitation of phosphate and coagulation of particulate phosphorus using a metal salt of calcium, aluminum or iron [16]. Biologically, phosphorus removal offers an alternative to chemical treatment methods that has a potential for reduced sludge production. In this process, phosphorus removal is performed by phosphate accumulating micro-organisms that have the ability to accumulate phosphate over and above what is required for growth. Iron, aluminum and especially calcium have been reported to precipitate phosphate in biological phosphorus removal processes [17]. Moritimer [18] reported that under high reduced condition ferric ion is reduced to soluble ferrous ion and this lead to the liberation of phosphorus. Morgenroth and Wilderer [19] reported that the removal of phosphorus could be limited in a biofilm system due to domination of the biofilm by heterotrophic bacteria utilizing organics, thus decreasing the metabolism of phosphate accumulating microorganism. Thus phosphate might be removed by the adsorption of microorganism. Kim et al [20] also reported that high pH may change the sediment properties, thus affecting phosphate decreasing through the combination with metals such as iron, aluminum and calcium.
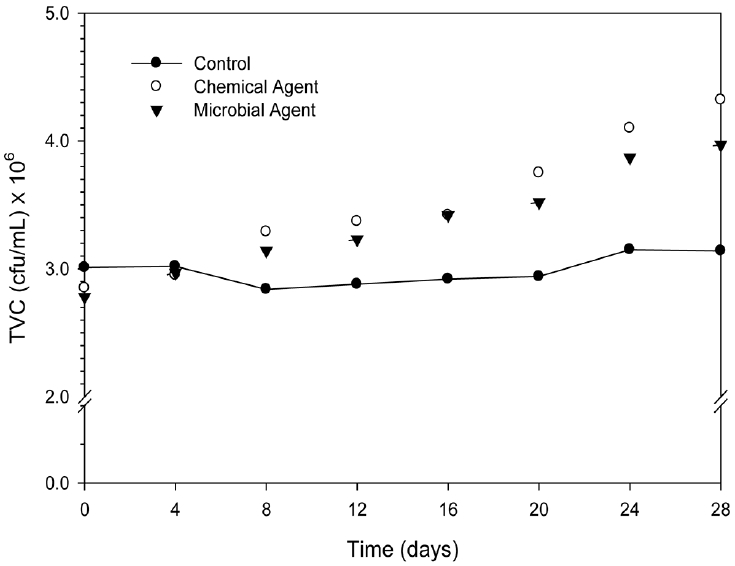
3.5. Growth of Microbial Population
The microbial growth rate was observed for all studied samples. The average growth was found to be 3 × 106 CFU/mL in control bin within the 28 days of study. Growth rate of microbial community in different bin with time was shown in Fig. 5. Microbial treated sediment was shown lower microbial growth than control from the starting time to 4 days. Overall microbial agent treated sediment has shown discrete microbial growth in the entire studied period. As we mention earlier, the adaptation/adjustment of added microbial community with current microbial community might be the possible cause except any chemical change in environment. Chemical agent treatment has shown increasing fashion from the starting time to 28 days. The maximum microbial growth was found as 3.15 × 106 CFU/mL for control, 3.97 × 106 CFU/mL for microbial agent, and 4.32 × 106 CFU/mL for chemical agent at 28 days of experimental duration. The growth percentages were found to be 4% in control, 43% in microbial agent treated sediment and 52% in chemical agent treated sediment. Results clearly indicate that the chemical agent was efficient than microbial agent to growth of microbial community.
3.6. Decay of Major Environmental Indicators Identified through a Reaction Kinetics
A normal reaction rate with a reactant (A) only can be expressed as
Where ‘k’ denotes a rate constant and ‘n’ stands for a reaction order. Through an algebraic manipulation, Eq. (1) turns into Eq. (2), which is
Linear regression gives a straight line with ‘k’ and ‘n’ values, which fits best to the experimental points. Table 1 shows the kinetic parameter values as results of the optimization procedure. The final reaction order was −1.6, which means that the decay reaction does not significantly depend on pollutants’ concentration but time. Judging the calculated ‘k’ values, the chemical treatment was more effective than the microbial one meanwhile, for the other three pollutants, the bio-treatment was slightly better or very much comparable to the chemical agent.
4. Conclusions
The agents have been applied in the purpose of elucidating there performance in contaminated sediment collected from south east coast Korea (Won-mun bay). The coast is get large quantity of aquaculture elements. The chemical agent is calcium oxide whereas the microbial agents are resides of some microbial community those are able to afford congenial decomposable environment. Therefore in this study it was shown that the reduction of COD by as much as 40% for chemical agent and 29% for microbial agent in 28 days. With the same treatment AVS decreased by 60% for chemical agent and 46% for microbial agent. T-N also decreased by 26% for chemical agent and 37% for microbial agent. T-P also removed by as much as 33% for chemical agent and 38% for microbial agent. Therefore the chemical agent is more efficient to declining COD of 11%, AVS of 14%, in comparison to the microbial agents and microbial agent reduced T-N of 11%, and T-P of 5% more than chemical agent. This research results demonstrated that the residing bacterial growth in contaminated sediment were enhanced by the addition of agents. Between the evaluated agents in this research, chemical agent had the greatest stimulatory effect on bacterial community growth. The observed in bacterial growth occurred at 28 days, suggesting that chemical agent could be expected agent. Among the bacterial community possibly COD and AVS reducing bacteria was dominated over T-N and T-P reducing bacterial community. Generated oxygen by chemical agent might facilitate congenial bacterial growth environment for selected species instead of altogether. Microorganism number, activity, specific species is also significant factor, as these directly affects the degradation of pollutants. The results suggest that the chemical agents could be better choice instead of selected microbial agent to improve sediment quality. Decay kinetic analysis shows that decay rate of any pollutant was not affected much by the present concentration of the pollutant, and the chemical agent was more adequate to reduce COD by 1.32 times.