1. Introduction
Recently, the recycling of waste biomass has been widely studied to decrease the concerns on the environment and to increase health of the people. There are a lot of waste biomass such as food waste, sewage sludge, muck, domestic waste and waste livestock blood. Waste livestock blood is generated during the bloodletting process in slaughtering, and the amount of waste livestock blood generated annually has increased in parallel with increased meat consumption, resulting from higher incomes and living standards. In Korea, the blood of cattle is mostly consumed as food, and the one of chickens and ducks is recycled with other byproducts, whereas the blood of pigs is mainly disposed of as waste. The annual amount of blood generated from the slaughter of pigs has increased each year [1]. A total of 14,039,960 pigs were slaughtered in Korea in 2012, which is a 29.6% increase from the previous year, and the annual amount of waste livestock blood generated from pigs is nearly 42,000 tons. At present, it is prohibited by the Enforcement Regulation of the 1996 Protocol and Treaty of Marine Pollution (MARPOL) to dump any waste livestock blood into the ocean. Also waste livestock blood may be treated for disposal on land but separate purification treatment facilities must be established, which are costly installations. Thus, there is a need to develop technology for the treatment or recycling of waste livestock blood with minimal cost.
Waste livestock blood mainly consists of the liquid plasma and the solid constituents, such as red blood cells (RBCs). The plasma contains proteins such as albumin, globulin and fibrinogen, and the RBCs are mainly comprised of proteins called hemoglobin [2]. Hemoglobin, which accounts for the highest proportion of proteins in the blood, is a low molecular compound. It is relatively easier to convert these into amino acids compared to other proteins. On the other hand, in contrast to the other proteins, which exist in the plasma, hemoglobin is present inside the RBCs but it is obstructed by the cell wall. Thus, in order to recycle waste livestock blood, there is a need to prepare the proteins and turn them into a usable form through hemolysis, in which the RBCs are destroyed allowing for the leakage of hemoglobin molecules.
In spite of the efforts to recycle waste livestock blood into alternative resources, there are many problems due to the fact that the process can be exposed to a variety of pathogenic bacteria, depending on the slaughtering environment, with a high possibility of contamination by the bacteria present in the intestinal canal, such as Salmonella spp., Escherichia coli, Shigella spp. and Yersinia enterocolitica [3]. Such microbial contamination is expected to cause unpleasant odor during the process of recycling waste livestock blood and even disease transmission. Therefore, there is a need for countermeasures to prevent or eradicate this contamination.
Ultrasound, which lies outside the human hearing range, refers to a sound wave with a frequency of greater than 20 kHz. Ultrasonic wave causes a cavitation phenomenon, and ultrasonic frequency has a significant impact on the cavitation bubble. In particular, the destructive cavitation effect is known to occur from the lower frequency range than the higher frequency range. Also, irradiation of ultrasonic wave in the low frequency range (under 40 kHz) at a high intensity effectively destroys biological cells and enables the elution of soluble organic substances [4, 5]. The findings of a study on ultrasonic treatment of sludge showed that it caused a discharge of the intracellular materials, especially proteins, into the aqueous solution [6]. In addition, studies have shown that ultrasonic treatment can be used as a physical depolymerization method for high molecular compounds, in which a high degree of depolymerization was observed [7–9]. Thus, ultrasonic cavitation is expected to not only cause the solubilization of proteins in the blood, but also to kill various bacteria which may be present in the blood. However, a number of factors affect the extent of cell destruction, such as ultrasonic frequency, ultrasonic density, ultrasonic treatment time and temperature, as well as many others [10, 11], and these must be taken into consideration prior to ultrasonic treatment.
In this study, we aimed at solubilizing blood proteins with the application of an ultrasonic treatment system as the pretreatment for waste livestock blood. For this purpose, the optimum conditions were determined by varying the ultrasonic frequency, ultrasonic density and ultrasonic treatment time, and by observing the changes in the solubilization rate as well as examining the molecular weight distribution and changes in the particles before and after ultrasonic treatment.
2. Materials and Methods
2.1. Materials
The blood used in the experiment was that obtained from the pigs, and was obtained directly from an operational slaughterhouse. In order to collect pure waste livestock blood without foreign matter, the bloodletting section in the slaughter process was used, and the collected waste livestock blood was transported to the laboratory within 30 min to ensure its freshness. Because coagulation starts immediately from the bloodletting process, the transported waste livestock blood was homogenized using a grinding apparatus and was divided into 1 L samples for refrigerated storage prior to the batch experiment. All experiments were completed within 1 week after the sample collection date in order to minimize variations in results caused by sample deterioration.
2.2. Method for Ultrasonic Treatment
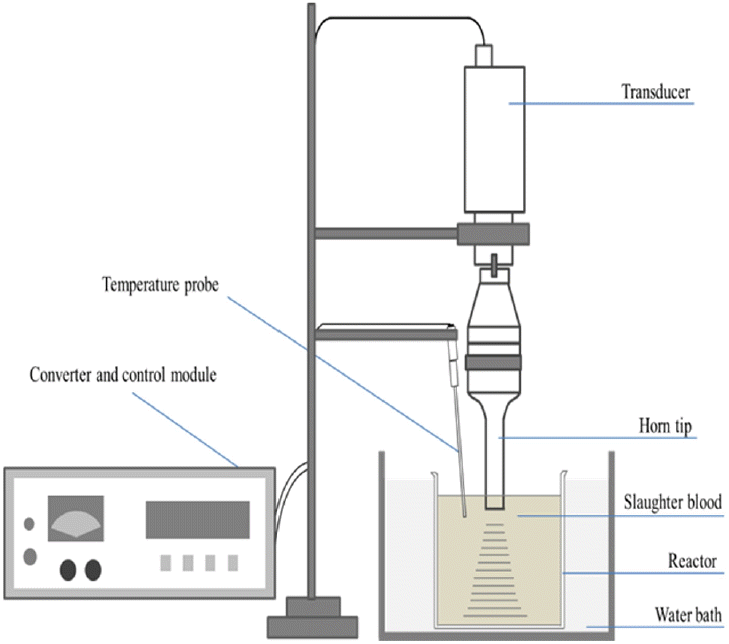
The apparatus used in the experiment was comprised of a grinder and an ultrasonic treatment system. Because blood begins to clot as soon as it is discharged out of the body, a cutter-type lab-scale grinder with a grinding speed range of 3,000 to 15,000 rpm was used to homogenize the clotted blood. The ultrasonic apparatus for blood solubilization was comprised of an ultrasonic wave generator and a reactor, of which the generator consisted of a converter, control module, transducer and horn tip of probe type (Fig. 1). The horn is a probe generating frequencies of 20, 24 and 28 kHz in the low frequency range, which effectively destroys cells, and the maximum power for these frequencies were 2,000 W, 1,000 W, 300 W, respectively. The reactor consisted of a 2 L beaker, water bath for regulation of internal temperature, temperature sensor, and an agitator which could operate at a maximum speed of 1,000 rpm.
The experimental conditions for the ultrasonic treatment are summarized in Table 1. The experiments were carried out in a batch-type method based on an experimental volume of 1L. In order to determine the optimal frequency of the three frequencies of 20, 24 and 28 kHz, treatment was performed at fixed ultrasonic density and ultrasonic treatment time. Then, the frequency at which the highest efficiency was observed was fixed in order to determine the other optimum treatment conditions. The upper limit of the ultrasonic density was set at 2 W/mL, taking into consideration the actual scale of the process and operation costs that were expected, and it was varied from 0.1 W/mL to 2.0 W/mL to examine the pretreatment characteristics. Also, the pretreatment characteristics were observed by varying the ultrasonic treatment time from 0 – 60 min. The experiments were carried out with a target solubilization rate of over 95%.
2.3. Physicochemical Analysis
For the analysis of the characteristics of waste livestock blood, the moisture, organic and inorganic contents were analyzed using the standard method [12], while total lipid was measured using the Sulfo-Phospho-Vanillin analysis method. The blood proteins were analyzed to determine the total protein, albumin, fibrinogen, platelets and hemoglobin counts, using an automatic hematology analyzer (COULTER AcT; Beckman Coulter Inc., USA).
Quantitative analysis of hemoglobin and the gel permeation chromatography (GPC) were conducted in order to examine the degree of solubilization of protein in the waste livestock blood that underwent grinding and ultrasonic pretreatment. The concentration of hemoglobin was determined using the cyanmethemoglobin method. Of the blood proteins, hemoglobin was reacted with potassium ferricynide (K4[Fe(CN)6] · 3H2O) to obtain methemoglobin, which was then reacted with potassium cyanide to produce cyanmethemoglobin. The concentration of the final product was then measured using a UV-Vis Spectrophotometer (Cary 4000; Varian Australla Pty Ltd., Australia) at 540 nm with the hemoglobin standard reagent. Then, the solubilization rate (%) of the pretreatment was then calculated using the hemoglobin concentration obtained through the above analysis.
Where, SR is solubilization rate, Ht is hemoglobin in plasma of treated blood, Hu is hemoglobin in plasma untreated blood and Hw is hemoglobin in whole blood.
The GPC used in the experiment was the 1100 HPLC manufactured by Agilent. The experiments were carried out using the Phenomenex BioSep-SEC-S 2000 (300 × 7.8 mm, 5 micron) column with 20 mM phosphate buffer as the mobile phase. Albumin and insulin were used as the standard reagents to check the molecular weight distribution of protein. The pathogens contained in the waste livestock blood were analyzed as follows: first, 10 mL of sample was added to 90 mL of 0.85% NaCl solution that had been sterilized for mixing and the sample mixture was then gradually dispensed in 9 mL of 0.85% NaCl solution for serial dilution; and then, the general bacteria and total coliforms were analyzed based on a dry rehydratable film method using 3M™ Petrifilm, whereas salmonella was analyzed based on the standard plate culture method. In order to determine any possible morphological changes in the blood, the external shape and appearance of the RBCs were examined before and after the pretreatment using a phase contrast microscope (TS100F; NIKON, Japan), and the particle size distribution of waste livestock blood was examined using a particle size analyzer (1090LD; CILAS, France).
3. Results and Discussion
3.1. Characterization of Waste Livestock Blood
The various parameters of waste livestock blood, such as moisture, organic content and inorganic content, were confirmed according to the standard method for the examination of water and wastewater [12] and it is shown in Table 2. Moisture, organic and inorganic content were determined to be 79.14%, 19.40% and 1.46%, respectively, while the total protein content was found to be 18.22%. This demonstrated that the majority of the organic content was comprised of proteins. The proteins in waste livestock blood were mainly albumin, globulin, fibrinogen and hemoglobin, with hemoglobin being predominant protein in RBCs accounting for 14.02% of the composition. This means that a cell destruction process is an absolute requirement for hemolysis that is the process in which hemoglobin is liberated from RBCs, and is mixed with the plasma, for effective recycling of proteins in waste livestock blood.
3.2. Pretreatment by Grinding
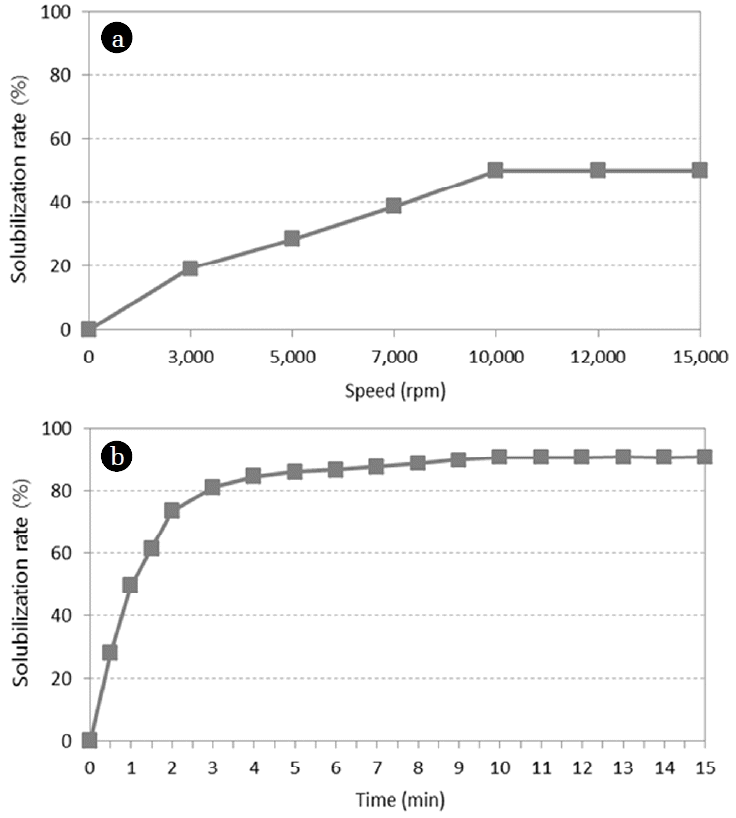
The grinding speed and time were set as variables to determine the optimum grinding treatment conditions. The first experiment was carried out with the grinding speed varied at 6 levels between 3,000 rpm and 15,000 rpm to understand the differences in the blood solubilization characteristics caused by changes in grinding speed. The experiment used 1 L of blood in batch type reactor, which was the same volume condition as the ultrasonic treatment. And then the grinding time was set discretionally at 1 min to determine optimal speed. The concentration of hemoglobin in the plasma of the untreated waste livestock blood was 0.08%, and the concentration rose with an increase in the grinding speed. At a grinding speed of 10,000 rpm, the concentration of hemoglobin was found to be 7.25% on average, with the solubilization rate being 50.12%, and the solubilization rate stopped increasing at speeds over 10,000 rpm. Based on this result, the optimum grinding speed was deemed to be 10,000 rpm.
After the grinding speed fixed at the optimum level of 10,000 rpm, the optimum grinding time was determined by varying the time from 30 s to 900 s at an interval of 30 s. The results showed that the solubilization rate increased rapidly to 80% up to 180 s but the increase became more gradual after 180 s and stopped after 600 s, at which point a solubilization rate of 90% was observed. Thus, the optimum grinding time could be interpreted as being anywhere between 180 s and 600 s. For the purpose of this study, the maximum grinding time of 600 s was selected as the optimum grinding time so as to minimize the required ultrasonic treatment time and to reduce the power consumption relatively. The hemoglobin solubilization rate at a grinding time of 600 s was 90%. In other words, homogenization of clotted blood prepares the blood for ultrasonic treatment and also leads to the additional solubilization of protein.
3.3. Solubilization of Protein at Different Ultrasonic Frequencies
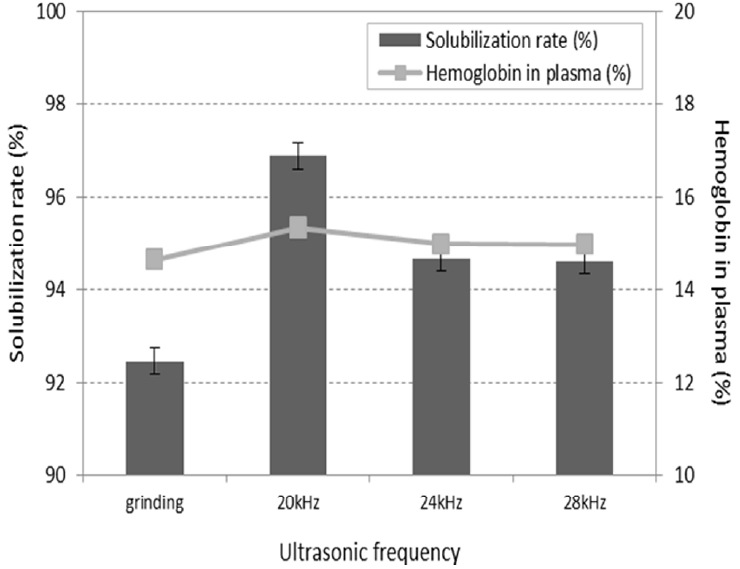
In the past, the treatment of sewage sludge was studied using ultrasonic technologies [13, 14]. Moreover ultrasonic treatment was used in environment such as water treatment, soil washing, air pollution and waste treatment; removing the cell wall, destroying the microorganism, washing the surface and degrading the pollutant [15, 16]. Because of this reason, ultrasonic treatment was used in pretreatment of waste livestock blood and the results are represented in Figures and Table below. The samples were pretreated under the optimum grinding conditions prior to the homogenization of waste livestock blood and the first solubilization process. Because the intensity of cavitation varies depending on the ultrasonic frequency, ultrasonic frequency was expected to influence the solubilization rate. Thus, in order to determine the optimum frequency with the strongest influence on the solubilization rate, ultrasonic treatment was performed at an ultrasonic density of 2 W/mL for 1 h. The results of pretreating the waste livestock blood at varying ultrasonic frequencies, in terms of the solubilization rate of hemoglobin in RBCs, are shown in Fig. 3. The hemoglobin concentration was maximal at a frequency of 20 kHz, with the determined solubilization rate being 96.87%. In addition, the solubilization rate was 94.72% and 94.61% at 24 kHz and 28 kHz, respectively. This showed that increasing ultrasonic frequency resulted in a reduction in the solubilization rate. Solubilization rate was already over 90% using only grinding. However, it could not destroy microorganism and degrade the pollutant. Destroying the microorganism and degrading the pollutant are important to resource waste livestock blood because they are harmful to the animals and plants and make the odor. Because of this reason, the optimum frequency was determined to be 20 kHz. This is consistent with the previous studies, suggesting that the most effective frequency for cell destruction is 20 kHz [5].
3.4. Solubilization of Protein at Different Ultrasonic Density and Ultrasonic Treatment Time
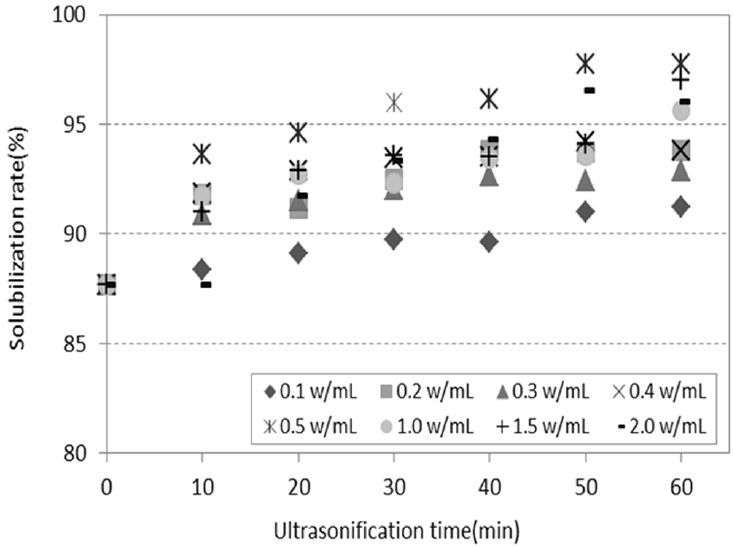
As mentioned earlier, different ultrasonic frequencies, such as 20 kHz, 24 kHz and 28 kHz, were performed to confirm solubilization of protein in study and the experiment according to changes of various ultrasonic density and ultrasonic treatment time was executed below. The frequency set at 20 kHz, which resulted in the highest solubilization rate. Further experiments were carried out to determine the optimum pretreatment conditions with respect to the ultrasonic density and ultrasonic treatment time, and the results are shown in Fig. 4. The maximum solubilization rate resulting from irradiation at an ultrasonic density range of 0.1 to 0.4 W/mL was about 91 to 93%. It was not significantly higher than the solubilization rate of the grinding treatment (87.68%), while an ultrasonic density exceeding 0.5 W/mL resulted in a maximum solubilization rate of 96 to 97%. This showed that there were little to no differences in the solubilization rate which resulted through varying ultrasonic density. It is generally reported that an increase in ultrasonic density leads to enhanced ultrasonic treatment efficiency [11]. However the experiment in this study showed that no significant differences in efficiency were caused by varying density, while confirming that the solubilization rate increased with longer ultrasonic treatment time. This is thought to be due to the fact that hemolysis occurs easily by physical force, and thus, is not significantly affected by ultrasonic density [17].
The highest solubilization rate was observed during treatment at an ultrasonic density of 0.5 W/mL, at which point there were little to no differences in the solubilization effect caused by increasing the ultrasonic treatment time. However it would be more efficient to perform the treatment at a minimum ultrasonic density of 0.5 W/mL rather than increasing the ultrasonic treatment time, because of its operation costs. The target solubilization rate was obtained when treatment was conducted for 30 min. In summary, it would be most effective to perform the treatment at an ultrasonic density of 0.5 W/mL for 30 min, in order to attain a target solubilization rate of 95%.
In this study, the most important consideration was the change in the solubilization rate caused by varying the ultrasonic treatment conditions, since the purpose of this study was to recycle the proteins in waste livestock blood into resources. Therefore, the concentration of hemoglobin was examined before and after pretreatment under the optimum conditions, as derived through the aforementioned experiments; the hemoglobin concentration in the plasma of the untreated waste livestock blood was 0.03%, whereas the post-treatment concentration was determined to be 15.35%. The post-pretreatment solubilization rate was 97.92%, which confirmed the hemolysis of the majority of hemoglobin present in the RBCs. In other words, proteins in waste livestock blood can be solubilized without the separate chemical treatment, and thus, recycling of proteins in waste livestock blood can be regarded as a highly valuable process.
3.5. GPC Analysis of Waste Livestock Blood with Ultrasonic Treatment
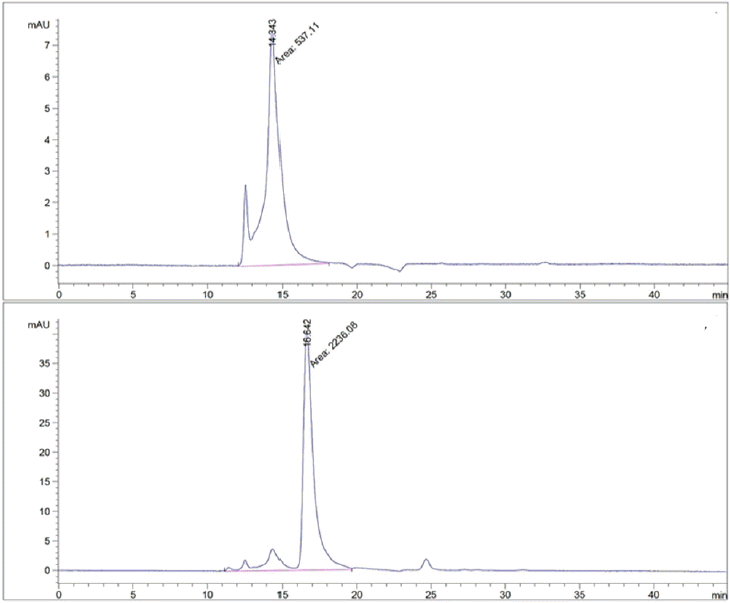
GPC was performed to confirm the solubilization distribution according to the molecular weight of proteins in waste livestock blood (Fig. 6). Prior to the GPC of waste livestock blood before and after the pretreatment, the molecular weights of albumin and insulin were confirmed to be about 66,000 Da and 5,000 Da when the retention time was 15 min and 25 min, respectively. An analysis of the waste livestock blood showed that the relative amount of protein was 537.11 before pretreatment. It increased by 4-fold to 2236.08 after the pretreatment. This increase in the total protein amount is thought to be caused by hemolysis, through which the hemoglobin was released into the plasma. The albumin peak was observed within 15 min of ultrasonic treatment in blood, but some of the peaks were observed after 15 min which was thought to be due to depolymerization. A protein peak which was not observed prior to pretreatment was found at around 25 min after pretreatment because it suggested that the proteins were solubilized and were then converted into peptides. This showed that ultrasonic treatment can convert waste livestock blood proteins into low molecular compounds, which can result in higher efficiency during additional treatments such as enzymatic hydrolysis.
3.6. Morphological Change of Waste Livestock Blood with Ultrasonic Treatment
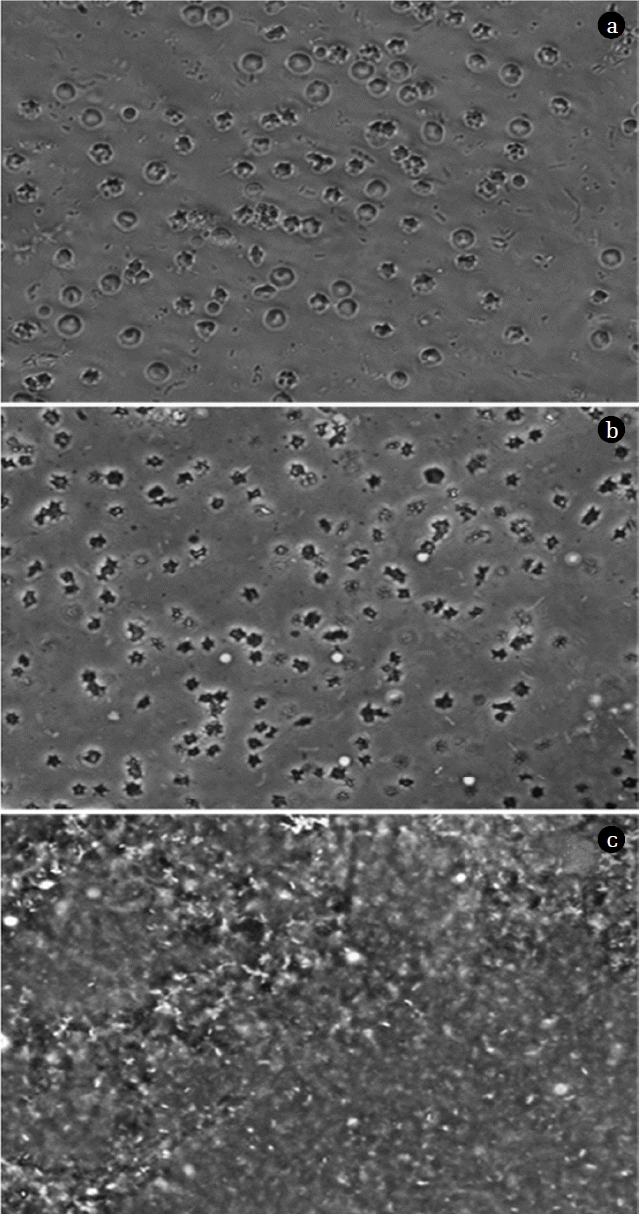
A phase contrast microscope was used to observe the morphological changes in the RBCs of the waste livestock blood before and after pretreatment, and the results can be seen in Fig. 7. An observation at 40× magnification revealed RBCs with well-defined globular shapes present in the untreated waste livestock blood, whereas torn cell walls and empty blood cells were observed following the grinding procedure. This damage to the RBC structure is expected to have resulted in the hemoglobin being released into the plasma. Also, all the RBCs present in the blood were destroyed after the grinding and ultrasonic treatment and had indeterminate shapes. Based on these results, it was confirmed that solubilization started to occur in the grinding process and that the ultrasonic treatment resulted in complete solubilization
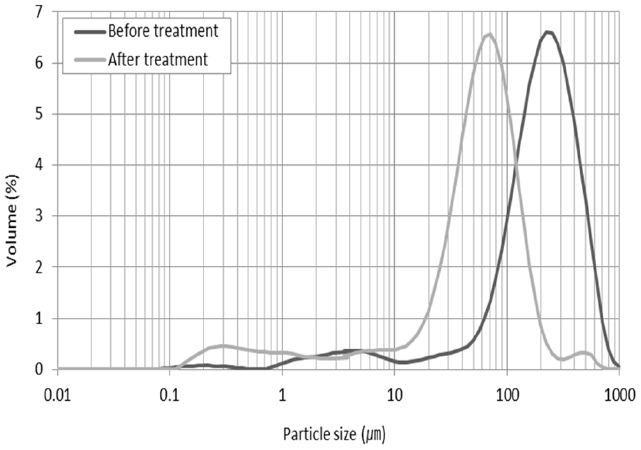
The results of the analysis of particle distribution before and after the pretreatment are shown in Fig. 8. The average particle size in waste livestock blood was determined to be 222.83 μm before pretreatment and 67.83 μm after pretreatment under the optimum pretreatment conditions. This demonstrates that grinding and ultrasonic treatment led to a reduction in the size of the particles in the waste livestock blood. This is thought to be caused by not only the destruction of RBCs, but also by the large amount of particulate solid matter in the waste livestock blood. When performing enzymatic hydrolysis of proteins in the blood, the presence of large-sized particles means that the process will occur at a reduced speed due to the small specific surface area. In contrast, the reduced particle size caused by ultrasonic treatment in this study can be explained as a reduction in size of the proteins, and this is expected to enzymatic hydrolysis.
3.7. Death of Bacteria in Waste Livestock Blood with Ultrasonic Treatment
Waste livestock blood can become contaminated with a variety of bacteria during the slaughtering process. Such microbial contamination can cause unpleasant odors during recycling of waste livestock blood, as well as having a potential for disease transmission. In particular, it can also lead to reduced rate of conversion from protein to amino acids in the enzymatic hydrolysis process, after which the remaining bacteria can cause various adverse effects, such as gas generation. Based on the notion that bacteria can be killed through ultrasonic treatment, eliminating the need for additional treatment, an experiment was carried out to determine the presence of general bacteria, total coliforms and salmonella following ultrasonic treatment.
The results of the analysis showed that the concentration of general bacteria was 1.5E+05 CFU/mL in untreated waste livestock blood and 1.3E+02 CFU/mL in treated waste livestock blood, indicating a sterilization rate of 99.93%. The concentration of total coliforms, on the other hand, was found to be 2.0E+03 CFU/mL in the untreated sample, whereas no colonies were observed after treatment, indicating a sterilization rate of 100%. Salmonella was confirmed in this study because it was found worldwide in animal blood and caused illnesses such as food poisoning and typhoid fever to the person. As for salmonella, no colonies were observed in any of the samples, either before or after treatment. The death of bacteria is most strongly influenced by ultrasonic cavitation, in addition to the inactivation of microorganisms caused by increased temperature in the ultrasonic treatment process. Prior studies have reported that pathogenic bacteria cannot be completely killed with the use of ultrasound alone [4]. However, this experiment demonstrated the high efficiency of ultrasonic treatment, which led to the non-detection of total coliforms. Thus, the implementation of an ultrasonic treatment is expected to not only cause solubilization of protein in RBCs, but also to kill most bacteria in waste livestock blood.
4. Conclusions
The purpose of this study was to perform ultrasonic treatment for the solubilization of proteins in the waste livestock blood generated during animal slaughtering, with the ultimate aim of deriving the optimum conditions for the recycling of waste livestock blood. The optimum conditions were determined to be an ultrasonic frequency of 20 kHz, ultrasonic density of 0.5 W/mL, and ultrasonic treatment time of 30 min. Under these conditions, the resulting solubilization rate was 97.72%, and the proteins were converted into low molecular weight compounds, as confirmed by GPC. Pretreatment also resulted in reduced particle size, as shown in the results of particle analysis, and the deaths of general bacteria and total coliforms at a death rate of 99.93% and 100%, respectively. Therefore, these results confirmed the excellence of ultrasonic treatment as a method of pretreating and preparing the proteins in slaughter for recycling into further biological resources.