AbstractThe high concentration of nitrogen and phosphorus in wastewater incorporated with the ability to use carbon dioxide as the carbon source make the microalgae become more attractive in wastewater treatment process. This study evaluates the optimal conditions for the digestion of settelable solids from the recirculating aquaculture system to produce the biomass of the green microalga Scenedesmus sp. After solids separation, aerobic digestion of settleable solids under disperse condition produced nitrate as the final product of consequently ammonification and nitrification processes. With the optimal digestion procedure, nitrate concentration during aerobic digestion in 2000 mL vessel increased from 9.63±0.65 mg N/L to 58.66±0.06 mg N/L in 10 days. Thereafter, cultivation of Scenedesmus sp. was performed in 1000 mL Duran bottle with air bubbling. The highest Scenedesmus sp. specific growth rate of 0.321±0.01/d was obtained in treatment using liquid fraction after aerobic digestion as the whole culture medium for Scenedesmus sp. cultivation. With this study, digestion of 8,800±128.12 mg dry weight/L of settleable solids from fish pond finally produced 1,235±21 mg dry weight/L of Scenedesmus sp. biomass.
1. IntroductionRecirculating aquaculture system (RAS) is known as a promising technology for the future of seafood security. However, successful commercial operation of the RAS is still rarely achieved because of high investment and operating cost. One of the challenge for RAS development is the water treatment process especially suspended solid and nutrients e.g. nitrogen and phosphorus removal. Without water exchange and discharge, nutrients and suspended solid are accumulated in the water. This will cause a negative effect on the water quality and fish productivity. During fish culture in closed system tank, settleable solids retrieved from uneaten feed, fish feces, dead fishes, microorganisms and organic decomposition in the pond are accumulated in the water. Solids removal is one of the most critical processes in aquaculture systems. Settleable solids produced from tilapia (Oreochromis spp.) tank are in the range of 520–650 kg per ton fish production with high nitrogen and phosphorus content of 72.4 kg-N and 23–29 kg-P per ton fish production, respectively [1].
Microalgae have been used in wastewater treatment as for BOD treatment, nitrogen, phosphorus and heavy metal removal, inhibition of coliform bacteria, and CO2 fixation [2]. The high concentration of N and P in wastewaters incorporated with the ability to use CO2 as the carbon source make the microalgae become more attractive in wastewater treatment process. Moreover, microalgal biomass produced after treatment could be used for methane production, composition, production of liquid fuels, production of animal feed and production of fine chemicals. Bruton et al. [3] and Mata [4] reported that the green microalga Scenedesmus sp. could be cultured in several types of cultivation systems from open ponds to the sophisticate photobioreactor. Biomass of Scenedesmus sp. contains high carbohydrate, protein and lipids which are high value biochemical products. Moreover, cell harvesting could be done by various techniques such as filtration, sedimentation, centrifugation and flocculation. This is the advantage over other small microalgal species such as Chlorella which require high cost centrifugation process for cell harvesting.
This study evaluates the optimal conditions for Scenedesmus sp cultivation using nutrients derived from indoor recirculating aquaculture system (RAS). Water and settleable solids from the RAS are used as the major source of nutrients for algal cultivation and the optimization of a serial aerobic digestion and microalgal assimilation is conducted under laboratory conditions.
2. Materials and Methods2.1. Microalgal Strains
Scenedesmus sp. was obtained from the culture collection of Center of Excellence for Marine Biotechnology, Chulalongkorn University. Stock culture of Scenedesmus sp. was incubated in BG11 medium containing (g/L): K2HPO4·3H2O, 0.04; MgSO4·7H2O, 0.075; CaCl2·2H2O, 0.036; ferric ammonium citrate, 0.006; EDTA, 0.001; NaNO3, 1.5; Na2CO3, 0.02; trace metal mix A5, 1.0 mL (consist of (g/L) H3BO3, 2.86; MnCl2·4H2O, 1.81; ZnSO4·7H2O, 0.222; NaMoO4·2H2O, 0.39; CuSO4·5H2O, 0.079; and CoCl2 ·6H2O, 0.05 [5]) under continuous light (5000 lux.) and continuous aeration at 25±1°C temperature.
2.2. Collection and Characterization of Fish Tank Settleable SolidsThe RAS for tilapia culture at the Center of Excellence for Marine Biotechnology, Chulalongkorn University consisted of 3900 L fish tank with 5 kg/m3 fish density and approximately 230 mg/L of total suspended solids. This moderate density RAS was operated under nitrification biofloc protocol along with regularly removal of excess solids. The settleable solids with small amount of water was collected from sediment settling tank and kept frozen. Prior to use, frozen settleable solids was defrosted to room temperature and diluted with water at 20% V/V (solids/water). The final solids solids concentration was approximately 8800 mg dry weight/L. The pH, total nitrogen (TN), ammonia (NH3-N), nitrate (NO3−), nitrite (NO2−), total phosphorus (TP), phosphate (PO4−3), total solids (TS) of settleable solids samples were analyzed using standard methods for water and wastewater analysis [6, 7].
2.3. Evaluation of the Suitable Nitrogen Form in BG11 Medium for Scenedesmus CultivationTwo forms of nitrogen, ammonium and nitrate, can be used as nitrogen source for algal growth. This experiment examined the favorable nitrogen for Scenedesmus sp. culture using BG11 medium. Noted that, unlike nitrate, high concentration of ammonium is toxic to algae, the concentration of ammonium-nitrogen in modified BG11 medium was therefore kept not higher than 100 mg-N/L. With this experiment, growth of Scenedesmus was compared using modified BG11 medium containing 100 mg-N/L nitrate and 100 mg-N/L ammonium and the original BG11 medium containing 245 mg-N/L was assigned as the control. Ten milliliters of water was collected daily to observed microalgae growth and analyzed for nutrients concentration. Cells density was count with haemacytometer and converted to dry weight using the equation 1 in section 2.6.
2.4. Comparison of Disperse and Semi-Disperse Digestion of Settleable Solids from Tilapia TankThe aerobic digestion with a combination of ammonification and nitrification processes was set up in 2000 mL Duran bottles containing 1600 mL of water and 400 mL of settleable solids from fish tank. With this experiment, two types of aerobic digestion were performed. The first condition was under well-mixed aeration as air bubbles was provided at the bottom of the bottle and settleable solids was completely dispersed. The second condition was done with aeration at the middle of the bottle. This provided incomplete mixing and approximately half of settelable solids was settled at the bottom. Aeration was supplied with the aeration rate of 75.96±4.83 mL/s. Water samples were collected daily to analyze nitrogen and phosphorus concentrations.
2.5. Optimal Process for Settleable Solids Aerobic Digestion to Provide Nutrients for Scenedesmus sp. CultivationAerobic digestion of settleable solids from fish tank released significant amount of nitrate and phosphate which is the nutrients source for microalgae. Further use of liquid fraction after digestion as the culture medium for Scenedesmus sp. culture was then evaluated. At the beginning, 8,800±128 mg of settleable solids from fish tank was digested by disperse air bubbling for 10 days in 2000 mL Duran bottle. The digested settleable solids at day 10 was collected for Scenedesmus sp. culture experiment during day 10–20. As illustrated in Fig. 1, the experiment consisted of 6 conditions as following: 1: Scenedesmus sp. cultured in BG-11 medium as control (C), 2: dispersed and aerated settleable solids without Scenedesmus sp. (negative control: (A1)), 3: dispersed settleable solids mixed with Scenedesmus sp. (A2), 4: 50% diluted settleable solids mixed with Scenedesmus sp. (A3), 5: bottom deposited settleable solids (reduced aeration rate) mixed with Scenedesmus sp.(A4), and 6: only liquid fraction after solids digestion, removed solids and mixed with Scenedesmus sp. (A5). For control and treatments with algal culture, stock culture of Scenedesmus sp. was added into the bottle to the final density of 22±7 (×104) cells/mL. With these procedures, the treatments A2-A4 contained both solids and algal cells while A5 was the algal culture without solids. All treatments were incubated under continuous aeration at 75.96 ± 4.83 mL/s with continuous fluorescent light at approximately 5000 lux. The experiment was conducted with three replicates.
2.6. Microalgal Growth MeasurementCell density was counted by a haemocytometer under microscope. To calculate dry weight of the algae, the linear relationship between dry weight and cell density of the microalga was expressed as Eq. (1) and the specific growth rate during exponential growth phase (μ) was calculated using the Eq. (2)
Where Nt = number of cells at the end of log phase, No = number of cells at the beginning of log phase, Tt = final time of log phase, To = beginning time of log phase.
3. Results and Discussion3.1. Characteristics of Fish Pond Settleable Solids and BG11 Medium
Table 1 shows that, after the dilution of settleable solids at 200 mL solids per liter of water, total solids concentration in this experiment was approximately 8 g/L. Nitrogen and phosphorus were mostly present in the insoluble organic particle as total nitrogen and total phosphorus were far higher than those of inorganic forms. Ammonia, nitrite and nitrate in fish pond settleable solids sample were found in low concentrations (0.28±0.01 mg N/L, 0.05±0.01 mg N/L, and 6.09±0.23 mg N/L, respectively). With this solid sample, aerobic decomposition of organic compounds could produce high amount of ammonia into the water as a result from ammonification process. This high amount of ammonia nitrogen was further transformed to nitrate via nitrification process which could be used as nitrogen source for microalgal growth.
3.2. Suitable Form of Nitrogen in BG11 Medium on Growth of the Microalga Scenedesmus spIt was found that modified BG11 medium with 100 mg-N/L nitrate provided similar growth of Scenedesmus as the control. On the other hand, 100 mg-N/L ammonium in modified BG11 was not suitable for this alga (Fig. 2). The highest density of Scenedesmus were 1965.31±62.78 mg/L, 1563.22±17.41 mg/L and 465.67±6.66 mg/L for BG11 (Control), modified BG11-Nitrate (100 mg N/L) and modified BG11-Ammonium (100 mg N/L), respectively. The data obtained in this experiment was slightly different from Park et al. [8] which used lower concentration of nitrogen (Bristol medium with 45 mg/L of NO3-N or NH4-N) for Scenedesmus cultivation. In general, ammonia at low concentrations can be the nitrogen source for microalgae [9–11] but high ammonium concentration could inhibit growth of Scenedesmus cells. The results from this experiment illustrated that the medium for Scenedesmus growth preferred nitrogen in nitrate form and high concentraion of ammonium must be avoided. Therefore, further nitrogen conversion from ammonium to nitrate by the serial processes ammonification-nitrification is essential and it was implied in the following experiments.
3.3. Comparison of Disperse and Semi-Disperse Aerobic Digestion of Settleable Solids from Tilapia Tank
Fig. 3 illustrate that disperse aeration induced ammonification-nitrification processes as nitrate nitrogen increased from 6.09±0.22 mg N/L to 28.56±0.19 mg N/L. On the other hand, semi-disperse aeration was not enough to provide oxygen for nitrification process hence nitrate concentration was rather constant. Increase of phosphate in the reactor was found as phosphate concentration increased from 19.63±0.47 mg P/L to 27.55±0.31 mg P/L in 7 days (Fig. 3(b)). Higher phosphate release was found in semi-dispersed condition due to low oxygen at the bottom (16 cm depth in Fig. 3(c)). With phosphate, release of phosphate from heterotrophic bacteria during sludge digestion was reported in several studies. The polyphosphate-accumulating organisms (PAOs) under low oxygen condition obtain energy from the hydrolysis of accumulated polyphosphate to uptake volatile fatty acids (VFA) as poly-hydroxyalkanoates (PHA) and poly-hydroxybutyrates (PHB) and release soluble ortho-phosphate into the water [12]. Data in Table 2 shows that, after 7 days of digestion, disperse aerobic digestion could convert particulate nitrogen into dissolved form (nitrate) at higher proportion than semi-disperse condition. Dispersed condition was therefore essential for the completion of the serial ammonification-nitrification processes.
3.4. Optimal Process of Settleable Solids Digestion as Nutrients Supplement for Scenedesmus sp. CultivationAfter solids digestion and used as culture medium for Scenedesmus culture, it was found that solids remained in algal cultures strongly affected growth of Scenedesmus. This was probably due to shading effect of solids which reduce significant amount of light penetration in the culture resulting in low photosynthesis. Further release of nitrate (Fig. 4(a)) was found in several conditions with remaining solids in the culture bottles such as A1, A2 and A3. However, growth of Scenedesmus sp. with these treatments were remarkably low due to low light penetration. Non-dispersed solid digestion (A4) had low nitrate concentration especially during day 10–20 because settled solids cause anoxic denitrification condition which removed nitrate.
On the other hand, liquid fraction after aerobic digestion (A5) provided the best growth of Scenedesmus sp. (specific growth rate 0.321/d) in which the culture reached the maximum density of 1,235±21.00 mg/L in 8 days (Fig. 4(c)). This was even higher than control (BG11) which is the standard algal medium used in the laboratory and also higher than previous experiment of Whangchenchom et al. [13]. Changes of nutrients concentration during the experiment were showed in Fig. 3(a), (b). The overall nitrate removal from a combination of aerobic digestion and algal culture was 59.01% and overall phosphate removal was 49.95%.
4. ConclusionsResults from this study illustrated that aerobic digestion of settle-able solids from fish (Tilapia) tank promoted ammonification and nitrification processes so organic nitrogen was finally converted into nitrate. Phosphorus conversion to phosphate was also found during digestion. The nitrate and phosphate produced from aerobic aeration was further used as the nutrients source for the microalga Scenedesmus sp. cultivation. The aerobic digestion under disperse condition which enhance complete ammonification and nitrification processes is essential for nitrogen conversion. Finally, supernatant after digestion could be further used as the whole culture medium for Scenedesmus sp. cultivation. With this study, the highest specific growth rate of 0.321/d was obtained in treatment using supernatant after aerobic digestion as the whole culture medium for Scenedesmus sp. and the digestion of 8,800±128 mg dry weight/L of suspended solids finally produced 1,235±21 mg dry weight/L of Scenedesmus sp. biomass. The results from this study could therefore be used for further development of solids treatment and the production of valuable algal biomass using wastes from the recirculating aquaculture systems.
AcknowledgementsThe authors would like to thank TAIST-Tokyo Tech for supporting staffs and partial funding. The equipments and facilities were provided by the Center of Excellence for Marine Biotechnology, Department of Marine Science, Faculty of Science, Chulalonghorn University, Thailand. Partial funding for this study was provided by the Ratchadapisek Sompoch Endowment Fund (2014), Chulalongkorn University (CU-57-023-FW).
References2. Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM. Microalgae and wastewater treatment. Saudi J Biol Sci. 2012;19:257–275.
3. Bruton T, Lyons H, Lerat T, Stanley M, Rasmuussen MB. A review of the potential of marine algae as a source of biofuel Ireland. Glasnevin: Sustainable Energy; 2009.
4. Mata TM, Martins AA, Cactano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14:217–232.
5. Zhou W, Min M, Li Y, et al. A hetero-photoautotrophic two-stage cultivation process to improve wastewater nutrient removal and enhance algal lipid accumulation. Bioresour Technol. 2012;110:448–455.
6. Strickland JDH, Parsons TR. A practical handbook of seawater analysis. 2nd edCanada: Department of Fisheries and the Environment and the Environment; 1972.
7. APHA. Standard method for the examination of water and waste water. 21st edAmerican. Washington DC: Public Health Association; 2005.
8. Park JBK, Craggs RJ, Shilton AN. Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol. 2011;102:35–42.
9. Przytocka-Jusiak M, Duszota M, Matusiak K, Mycielski R. Intensive culture of Chlorella vulgaris/AA as the second stage of biological purification of nitrogen industry wastewaters. Water Res. 1984;18:1–7.
10. Azov Y, Goldman JC. Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol. 1982;43:735–739.
11. Tam NFY, Wong YS. Effect of ammonia concentrations on growth of Chlorella vulgaris and nitrogen removal from media. Bioresour Technol. 1996;57:45–50.
Fig. 1Diagram of Scenedesmus sp. culture experiment in 2000 mL Duran bottles as described in Section 2.5. Settleable solids was digested for 10 days under disperse condition before used. After digestion, water containing solids was added to treatment A1, A2 and A4. Treatment A3 was with 50% dilution of digested solids. Treatment A4 was with bottom deposit solids while treatment A5 was without solids. 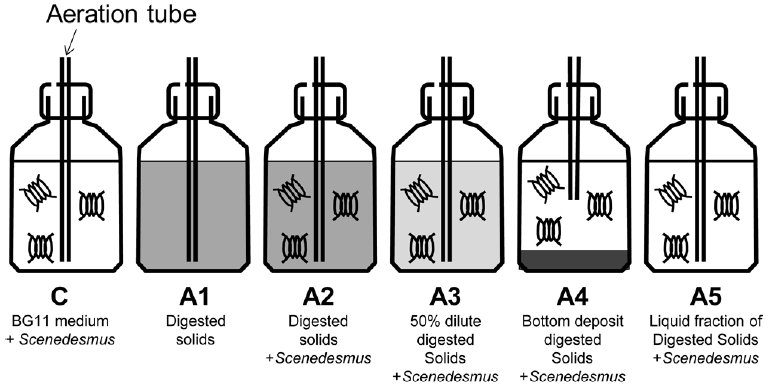
Fig. 2Growth curve of Scenedesmus sp. using BG11(Control), modified BG11-Nitrate (100 mg N/L), modified BG11-Ammonia(100 mg N/L) under continuous light and aeration. 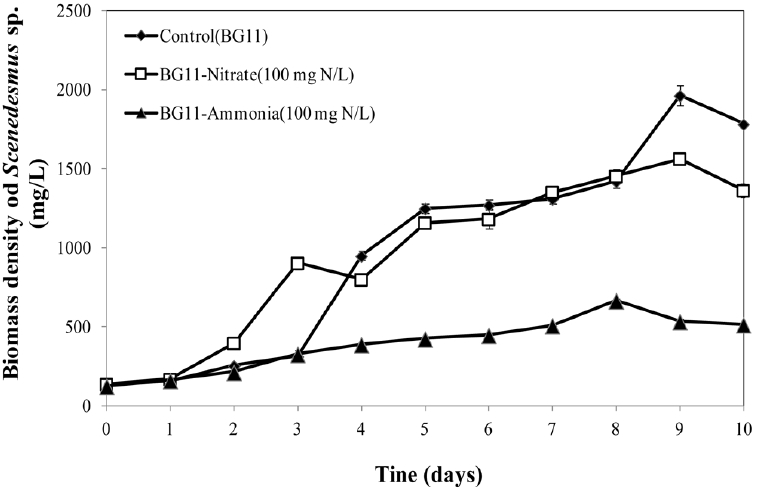
Fig. 3Inorganic nitrogen (a), inorganic phosphorus (b) and dissolved oxygen (c) profile during disperse and non-disperse aerobic digestion. 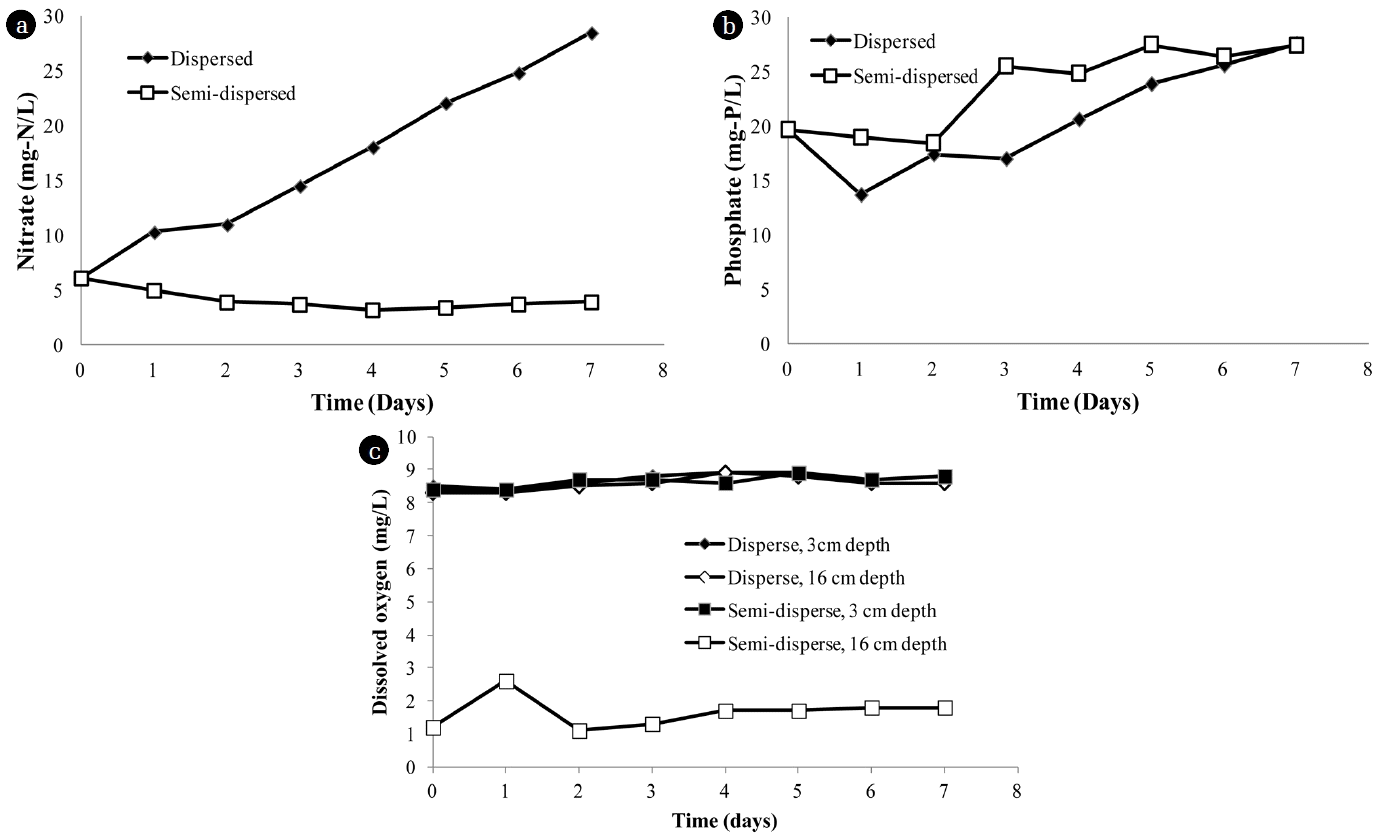
Fig. 4(a) Nitrate, (b) phosphate, and (c) biomass of Scenedesmus sp. cultivation using nutrients from aerobic digestion of settleable solids from fish tank under various digestion procedures i.e. dispersed settleable solids without Scenedesmus sp. (A1), dispersed settleable solids with Scenedesmus sp. (A2), 50% diluted settleable solids then dispersed settleable solids with Scenedesmus sp. (A3), non-dispersed settleable solids with Scenedesmus sp.(A4), liquid fraction after digestion after solids removal with Scenedesmus sp. (A5), and BG-11 algal medium as control (C). 
Table 1Characteristics of Settleable Solids from Recirculating Fish Tank and BG11 Algal Medium
Table 2Nitrogen and Phosphorus in Liquid Phase at Initial and Final Days of Aerobic Digestion under Disperse and Non-Disperse Conditions |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||