1. Introduction
The presence of heavy metals in the environment is of major concern for human life and various ecosystems due to its toxicity, bio-accumulating tendency and persistency in the terrestrial, atmospheric and aquatic ecosystems. Lead, cadmium and mercury are examples of heavy metals that have been classified as priority pollutants by the US Environmental Protection Agency and are called “the big three”. These pollutants are in the limelight due to their major impact on the environment [1]. Mercury is regarded as one of the most harmful metals found in the environment. The main sources of the mercury in the aquatic environment are industries such as chloralkali, paint, fertilizers, battery manufacturing and pharmaceutical preparations [2].
Mercury and its compounds are cumulative toxins and even in small quantities are known to be hazardous to human health [3]. WHO guideline value for mercury in drinking water is 0.001 mg/L [4]. Mercury can affect the central nervous system and in particular, the brain. High concentration of Hg(II) causes impairment of pulmonary function and kidney. Methyl mercury is a neurological poison affecting primarily the brain tissues. Mercury toxicity also affects the reproduction capacity in fish species. It can impact aquatic plants in a variety of ways across a wide range of concentrations, including survival and growth. Consequently, removal of mercury in water and wastewater is of paramount importance [5]. The mercury poisoning intensifies the neurological and renal disturbances as it can easily pass the blood – brain barrier. Hence, it is essential to remove mercury and its compounds from wastewater before its transport into the environment [6].
Several methods for removal of metal ions from wastewaters have been developed, but most have demerits such as continuous input of chemicals, high cost, high energy requirement and even incomplete metal removal at low concentrations [7–9]. Pure activated carbon is widely used as adsorbent for the removal of heavy metal ions from industrial wastewater. However, it has been found to be very expensive and it requires chelating agents to enhance its performance, thus increasing treatment cost. Therefore, the need of alternative low-cost adsorbents has prompted the search for new and cheaper sorption processes for aqueous effluent treatment, as these materials could reduce significantly the wastewater-treatment cost [10].
Biosorption is a relatively new technique that emerged in the last few decades and gained a considerable amount of attention since it has shown to be effective and a promising tool in the removal of contaminants from effluents in an eco-friendly way [11, 12]. Biosorption refers to the passive or physicochemical attachment of chemical species to the functional groups present in the biomass. Biosorbents are generally inexpensive because they are either naturally abundant or found as waste material from certain processes. It has been shown that different kinds of inexpensive plant-derived biomasses can be used as biosorbents to sequester toxic contaminants from waters and wastewaters [13].
Biosorbents might be classified according to the following sources: bacteria, algae, fungi, plant-derived, and animal-derived. Biosorption process also offers several advantages (other than the ones mentioned earlier) over conventional treatment methods including cost effectiveness, efficiency, minimization of chemical sludge, and regeneration of biosorbent with possibility of metal recovery [14]. Biomaterials have been used as biosorbents either in raw or chemically modified forms. Chemical modification generally improves the biosorption capacity and stability of biosorbents. Biomaterials have also been immobilized in different matrices for similar purposes.
Tridax procumbens (Asteraceae) is a medicinal herb found in agricultural fields in Tamil Nadu, India throughout the seasons. The extract of the green leaves is traditionally used as medicine in healing of cuts and wounds. The present work investigates the potential use of biocarbon of Tridax procumbens (Asteraceae) for biosorption of Hg(II) ions from synthetic wastewater. The factors that influence biosorption capacity such as pH, contact time, metal ion concentration and biocarbon dosage were evaluated. Essential biosorption isotherms such as Langmuir and Freundlich models were applied to the experimental data.
2. Materials and Methods
2.1. Metal Solution
A stock mercury ion solution (1,000 mg/L) was prepared by dissolving analytical grade mercuric chloride (HgCl2) in double distilled water. The desired pH values of the working solutions were adjusted using 0.1 N NaOH and 0.1 N HCl. The mercury concentration was determined using atomic absorption spectrophotometer (Shimadzu AAS 6200).
2.2. Preparation of Biocarbon
Tridax procumbens (Asteraceae) is an important medicinal plant widely distributed in agricultural fields. The plant leaves were collected and air dried for 48 hr. The dried leaves were grounded in ball mills and the screened homogeneous powder was used for the preparation of biocarbon. The activated biocarbon was prepared by treating the leaves powder with the concentrated sulphuric acid (Sp. gr.1.84) in a weight ratio of 1 : 1.8 (biomaterial : acid). The resulting black product was kept in an air-free oven, maintained at 160 ± 5°C for 6 hr followed by washing with distilled water until free of excess acid, then dried at 105 ± 5°C. The particle size of activated carbon between 100 and 120 μm was used [15].
2.3. Batch Biosorption Process
Biosorption studies were conducted in desired number of 250 mL Erlenmeyer flask containing a 100 mL of metal ion solution. The effect of pH, contact time, amount of biocarbon dose and influence of temperature (30 to 60°C) were evaluated by using the initial metal ion concentration of 100 mg/L. For optimization of the amount of biosorbent; 0.5, 1.0, 1.5, 2.0, 2.5, 3.0 and 3.5 g of biocarbon was treated with the test solution (pH 4.0). For the comparative evaluation, metal ion concentration of 25 and 50 mg/L were carried out at the optimum pH of 5.5. All the experiments, expect influence of temperature on biosorption of metal ion was carried out at 28 ± 2°C. In the batch experiments, flasks were agitated at 250 rpm constantly up to 160 min to establish the equilibrium condition and samples were collected at the end of the pre-determined time (20 min) intervals and the amount of metal ions content was analyzed using cold vapour AAS technique. The pH of the test solution was monitored by using a Hanna pH Instruments (Italy).
2.4 Data Analysis
Biosorption values were determined as the difference between the initial metal concentration and that in the supernatant. The amount of metal ion adsorbed per unit mass of the biosorbent was evaluated by using the following equation:
where qe = amount of metal ion adsorbed (mg/g), V = volume of metal ion solution (mL), w = mass of biocarbon (g), Co = initial metal ion concentration (mg/L) and Ce = metal ion concentration at equilibrium (mg/L). The percent of metal ion removal was evaluated from the equation:
3. Results and Discussion
3.1. Characteristics of Biocarbon
The characteristics of the biocarbon reveal that, total carbon content was high which has a more carbonaceous matter and has less ash content (Table 1). Bulk density and particle size of biocarbon are essential parameters which should be known before its application in wastewater treatment systems. It is observed that, the surface is relatively high and is responsible for potential removal of metal ions from synthetic wastewater system. Further, the values of methylene blue, ion exchange capacity and phenol number are also significantly good. The result indicates the biocarbon with good activity for adsorption.
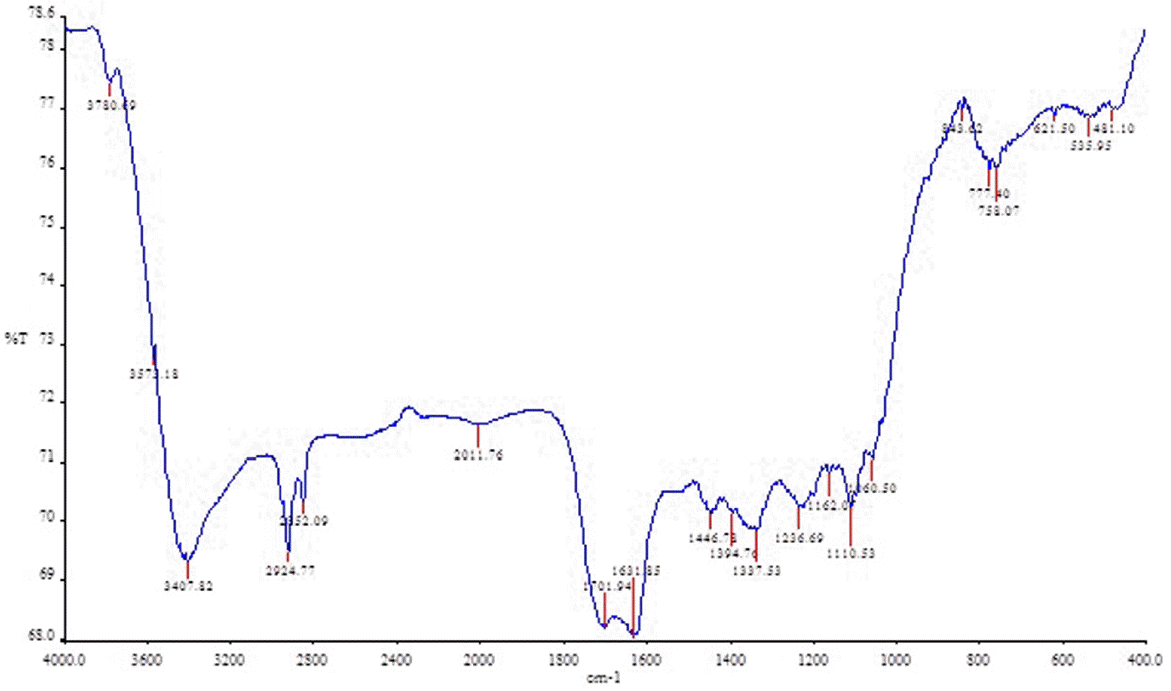
3.2. FT-IR Analysis
The Fourier-transform infrared spectroscopy (FT-IR) technique is an important tool to identify the characteristic functional groups present on the biocarbon surface (Fig. 1). The FT-IR spectrum was recorded on a Spectrum One (Perkin Elmer, USA). The important peaks of infra-red analysis are shown in Table 2. The FT-IR analyses support that the main mechanism of biosorption of mercury was chelating with different functional groups such as –OH, –C=O, –C=C, and –C–H. The presence of polar groups on the biocarbon surface is likely to be responsible for the metal ion removal from the bulk solution.
3.3. Surface Morphology
In addition, scanning electron microscopy (SEM) was used to visualize the surface morphology of the biocarbon (Fig. 2). The sample was scanned by using JSM 6701F (JEOL, Japan). The SEM micrograph of biocarbon indicates the regular morphological structure of the particles and also presents some leaf like structures and large numbers of nano grains which makes possible for the adsorption of heavy metal ions on different parts of the biocarbon.
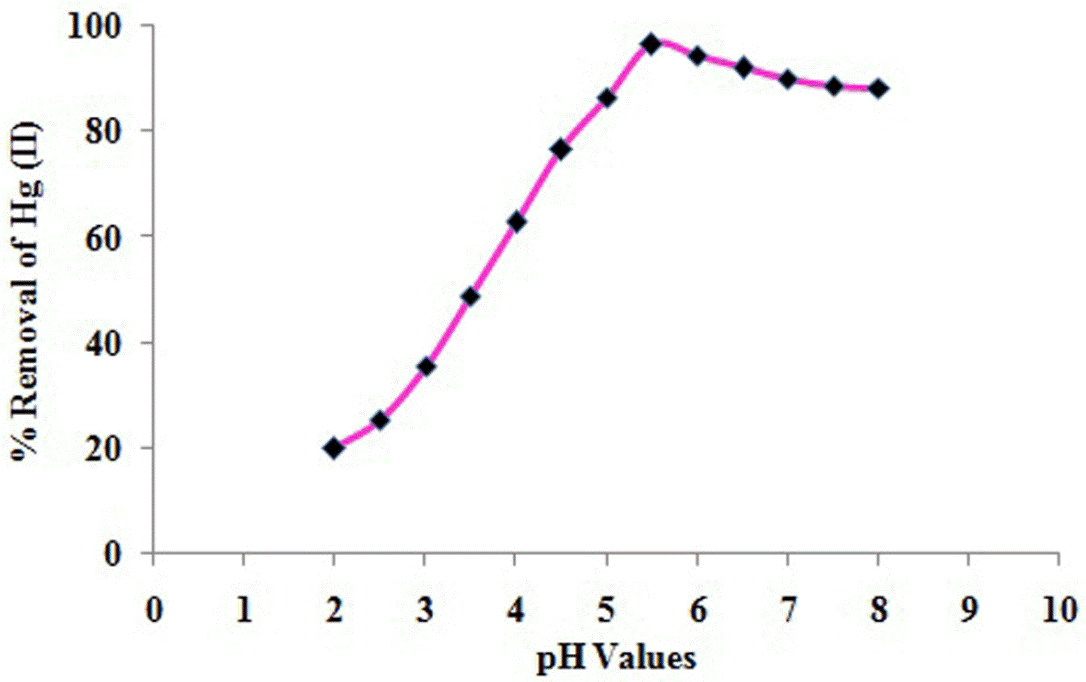
3.4. Effect of pH
The pH of the solution is one of the important factors governing the biosorption of metal ions. The removal of Hg(II) ions was performed with the initial concentration of 100 mg/L and the optimized biocarbon dose of 2.5 g. Fig. 3 shows the effect of pH change in the range 2 to 8 with the interval of 0.5 on the biosorption of Hg(II) on the biocarbon. The initial pH of the solution has a significant influence on the amount of metal ion sorbed. It was observed that removal Hg(II) ions in the aqueous solution is low at low pH values and increased with increasing pH of the solution. The optimum pH for the maximum removal (96.5 %) of Hg(II) was 5.5. Further increase of pH leads to decrease in Hg(II) removal efficiency. The influence of pH can be related with electrostatic repulsion where its decrease will be associated with reduction of positive charge density on the sorption sites thus resulting in an enhancement of metal biosorption [16].
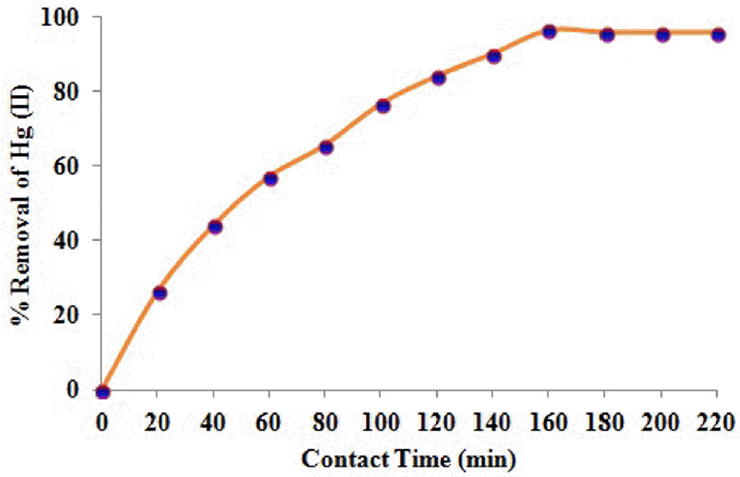
3.5. Effect of Contact Time
The effect of contact time on Hg(II) ions removal was investigated at regular time intervals of 20 minutes at an initial concentration of 100 mg/L and the profiles of metal ion biosorption trends and experimental conditions was shown in Fig. 4. The biosorption was progressed steadily and reached to the equilibrium at 160 min. After the equilibrium is reached, the biosorption of metal ions did not change significantly. The maximum biosorption of metal ion may be due to the presence of large number of accessible binding sites and the affinity of the functional groups on the biocarbon, as well as the mode of interaction between the metal ion and the biocarbon [17].
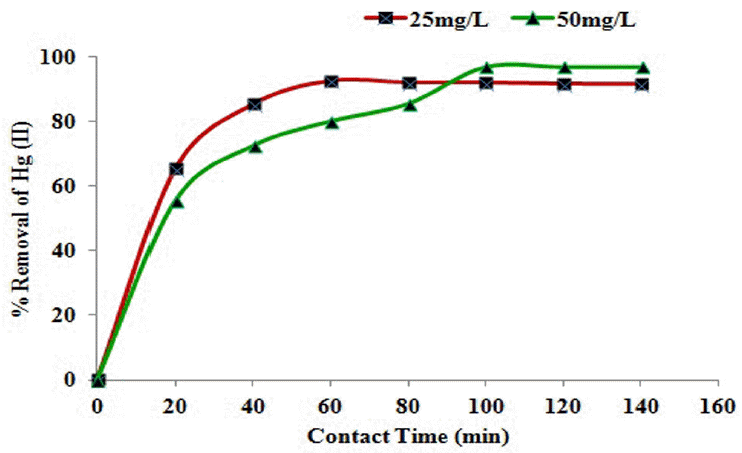
3.6 Effect of Metal Ion Concentration
The initial mercury (II) ions concentration of the solution is an important factor which influences the aqueous phase equilibrium between adsorbed species and biosorbent. Generally, biosorption decreases with increasing metal ions strength of the aqueous solution. This is explained by the fact that at low concentrations, most of the metal ions are sorbed by specific active sites and at higher concentrations the biosorption of metal ions decreases significantly. The phenomenon can further be attributed to the saturation of sorption sites on the biocarbon [18]. A result demonstrating the effect of initial concentration on removal of Hg(II) was shown in Fig. 5. The biosorption of metal ions increases from 92.5% to 97.0% as the concentration increases from 25 to 50 mg/L. This observation is attributed to the increase in the driving force which overcomes all mass transfer resistance of metal ion between the aqueous and solid phases [19]. The established equilibrium time is 60 and 100 min respectively. Similar results were also reported by researchers who indicated that increasing initial mercury concentrations in solution resulted in increasing its biosorption efficiency until the saturation of adsorbing sites are reached [20–22].
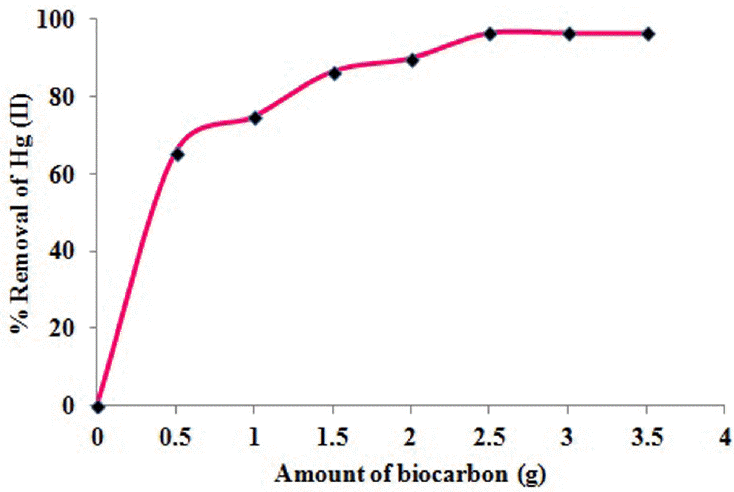
3.7. Effect of Biocarbon Dosage
The effect of biocarbon dosage on the biosorption of Hg(II) ions was performed with the initial metal ion concentration of 100 mg/L at the pH of 5.5 as shown in Fig. 6. The biosorption efficiency of the biosorbent gradually increases as the amount of biocarbon is varied from 0.5 to 3.5 g. It was observed that, the maximum (96.5%) removal of Hg(II) was established at 160 min at the biocarbon dose of 2.5 g. This can be attributed to the fact that, an increase in biosorbent dosage increases the surface area of biosorbent leading to an increase in the number of metal binding site on the biosorbent surface. This factor also reveals the presences of large number of vacant binding sites, which are available for biosorption during the initial stages, but thereafter the attraction to the remaining vacant sites will be difficult due to the repulsive forces between the Hg(II) ions on the biosorbent and the liquid phases [8, 23].
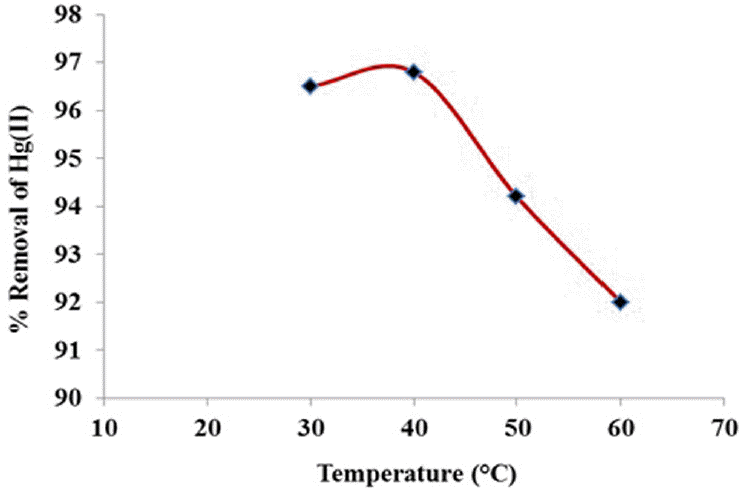
3.8. Effect of Temperature
The removal of Hg(II) ions was evaluated at four temperatures as 30, 40, 50 and 60°C. The initial concentration of metal ions was 100 mg/L and pH was 5.5. The results indicate that, the highest removal was obtained in the temperature 40°C and then decreased with the increase of temperature (Fig. 7). It indicates the process to be endothermic. Hence, rise in temperature beyond certain limits might causes a decrease in sorption capacity of the biocarbon.
3.9. Biosorption Isotherms
The study of application of biosorption isotherm models to the equilibrium data is important to optimize the design of the biosorption system for the removal of the metal ions. The biosorption of a substance from one phase to another leads to a thermodynamically defined distribution of that substance between the phases as the system reaches equilibrium state [24]. The most common biosorption isotherm models of Langmuir and Freundlich have been tested in the present study.
The data obtained from biosorption of mercury (II) was analyzed using Langmuir isotherms. The linear form of the Langmuir isotherm is given by the following equation.
where, qe is the equilibrium metal ion concentration on the biosorbent (mg/g), qmax is the maximum biosorption capacity of biosorbent (mg/g) and represents a practical limiting biosorption capacity when the surface is fully covered with metal ions. Ce is the equilibrium concentration of the metal in solution (mg/L). The plot of Ce versus Ce/qe should give a straight line, its slope equals to 1/qmax and the intercept has the value of 1/(qmax b), where b is the biosorption coefficient (L/mg). From the plot (Fig. 8(a)), qmax and b values of the Hg(II) were calculated and given in the Table 3.
The essential characteristics of Langmuir isotherm can be expressed by a dimensionless constant called equilibrium parameter RL, defined by Amin [25].
where C0 is the initial concentration of metal ion (mg/L). The characteristics of the RL value indicates the nature of biosorption as unfavourable (RL > 1), linear (RL = 1), favourable (0 < RL < 1) and irreversible (RL = 0). The obtained value of RL is 0.0321 at 28°C which shows that the biosorption of Hg(II) ions onto the biocarbon is favorable.
The Freundlich biosorption isotherm model is an empirical equation based on the biosorption on a heterogeneous surface [26]. The linear form of Freundlich biosorption isotherm can be defined by the following equation:
The constant n is an empirical parameter which reflects the intensity of biosorption that varies with the degree of heterogeneity and Kf is a constant related to biosorption capacity. The constants n and Kf were calculated by plotting log Ce against log qe (the slope = 1/n and the intercept = log Kf). The results were illustrated in Fig. 8(b). The values of n between 1 and 10 represent a favorable biosorption. The obtained value of n is 2.34 shows that the biosorption is more favorable.
3.10. Biosorption Kinetics
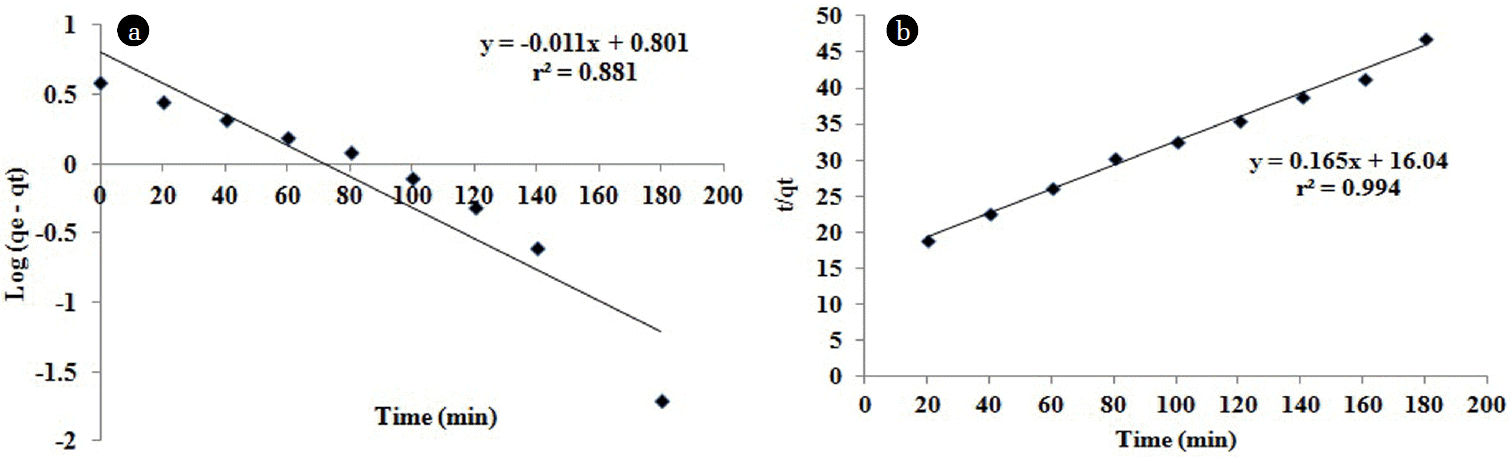
The kinetics of biosorption of Hg(II) ions was studied by using the two important kinetic models of Lagergren pseudo-first-order and pseudo-second-order equations. The general pseudo-first-order equation expressed as
where qe and qt are the amounts of metal ions sorbed onto the biocarbon (mg/g) at equilibrium and at time t (min) respectively. By plotting the log (qe – qt) versus t, the first-order rate constant k1 (1/min) and the equilibrium capacity qe can be obtained from the slope and intercept respectively.
The linear form of the pseudo-second-order chemisorption kinetics rate equation is expressed as
The constants can be determined by plotting t/qt versus t. The second-order rate constant k2 (g/(mg·min)) and qe (mg/g) values can be calculated from the intercept and slope of the plot. The results of the pseudo-first-order and second-order kinetics are shown the Fig. 9(a) and 9(b). The rate constants, metal ion uptake and correlation coefficients have been described in Table 4. It was observed that, the kinetics of the biosorption process followed the pseudo-second order model (r2 = 0.9940) better than the Lagergren model (r2 = 0.8810). The pseudo second order reaction in biosorption is based on the sorption capacity on the solid phase. The correlation coefficient of the pseudo second order reaction was high and this factor suggesting that the biosorption process comply pseudo second order model for the removal of Hg(II) ions.
4. Conclusions
The biocarbon generated from the medicinal herb Tridax procumbens (Asteraceae) can be employed as an eco-friendly biosorbent for the removal of Hg(II) ions from synthetic wastewater. The data from the batch biosorption studies provided essential information in terms of optimum pH, biocarbon dose and the effective equilibrium time for the removal of Hg(II) ions from the synthetic wastewater. The biosorption process mainly depends on pH of solution and optimum pH value was 5.5. The biosorption of Hg(II) ions by the biocarbon was reasonably fast, and the equilibrium reached at 160 min. The optimum amount of biocarbon dose was 2.5 g for the maximum removal (96.5 %) of Hg(II) ions. The analytical results are well fitted both the isotherm models. The results of the biosorption kinetics follows pseudo-second-order model. The investigation also indicated that, the biomaterial can be used to develop high capacity biosorbents for the removal of Hg(II) ions and other potential toxic heavy metals from industrial wastewater.