AbstractIn India, disposal of vegetable market waste along with municipal solid waste in landfills or dumpsites is creating much nuisance in terms of odor nuisance, leachate production, and greenhouse gas emission into the atmosphere. Therefore, vegetable waste with high biodegradable and nutrient content is composted in a 550-L batch scale rotary drum composter to study the degradation process and its compost properties for its potential reuse as high quality compost. A total 150 kg of working volume was fixed for composting studies with two different ratios, trial A (6:3:1) of C/N 24 and trial B (8:1:1) of C/N 30, respectively. A maximum of 63.5°C and 61.2°C was observed in trials A and B; an average of 55°C for more than 5 days, which helped in the degradation of organic matter and reduction of total and fecal coliform. The temperature dropped suddenly after the thermophilic stage in trial B, and leachate was observed due to insufficient amount of bulking agent. Mesophilic bacteria dominated during the initial stages of composting, and reduced considerably during the thermophilic stage. During the thermophilic stage, the rise in spore-forming organisms, including spore-forming bacteria, fungi, actinomycetes and streptomycetes, increased and these were predominant until the end of the composting process. By examination, it was observed that moisture and leachate production had adverse effects on the compost parameters with higher loss of micronutrients and heavy metals.
1. IntroductionThe handling and utilization of vegetable waste has become a major environmental issue. In India, the amount of vegetable waste generated has increased significantly in the last few decades due to the rapid population growth and economic development in the country [1]. Around 150 million tons of vegetables and fruits are produced annually in India, out of which 50 million tons of waste is being produced and is becoming a source of nuisance in municipal landfills, causing major environmental pollution problems [2]. Hence, management of these organic fractions is very crucial for the preservation of the environment and valorization of the byproducts formed during the process, irrespective of the methodology used. Among the major waste management strategies practiced, composting is gaining much interest for organic waste disposal with more economic and environmental profits, leading to a stabilized end product. The final product can be used to improve and maintain soil quality and fertility [3]. Consistent application of vegetable waste compost as a soil conditioner increases soil organic matter content and soil carbon-to-nitrogen (C/N) ratio to greater levels than those of unamended soil [4, 5]. Since composting is an exothermal process, biological oxidation of organic matter is carried out by a dynamic and quick succession of populations of aerobic microorganisms. Bulk treatment of these organic wastes can be done successfully with the composting process. Ruggieri et al. [6] composted organic fractions of municipal solid waste and studied this extensively at the industrial level. Chanakya et al. [7] has reported the production of large amounts of leachate fraction during the composting of food waste. It has been found that food waste decomposes rapidly to produce organic acids, thereby leading to leachate. In contrast to food waste, vegetable waste also contains high amount of moisture and organic content. A possible way to dispose of this waste and to recycle the nutrients, is composting. There are many studies on the composting of vegetable waste using sawdust and tree leaves with an in-vessel system [8, 9]. An efficient and promising technique using the in-vessel system is the application of a rotary drum composter. The composting time is drastically reduced to 2–3 weeks with pathogen-free end product [10]. However, there are not many literatures available on the composting of vegetable waste using cattle manure and sawdust in higher proportions by using high scale (550 L) batch drum composter. Hence with higher proportions of vegetable waste, there are chances for leachate production for it contains higher biodegradable organic matter. In order to study the composting process at higher proportions of vegetable waste, composting was carried out in two different trials and reported the possible effects of leachate during the process. Moreover, the role of bulking agent during vegetable waste composting was discussed in detail. Compost properties, such as physicochemical, biological, and microbial dynamics, during different intervals of composting period, have been extensively studied and reported.
2. Materials and Methods2.1. Rotary Drum Composter DesignCompost dynamics were studied using a rotary drum composter of 550 L capacity [9]. The drum is made up of a 4-mm thick metal sheet, and the inner side of the drum is covered by anti-corrosive coating. The main unit of the composter, i.e., the drum is 0.92 m in length and 0.9 m in diameter. The drum is mounted on four rubber rollers attached to a metal stand, and the drum is rotated manually. In addition to this, two adjacent holes are made at the bottom of the drum to drain excess water. Aerobic conditions are maintained by opening up both half side doors of the drum after a certain period of rotation, which ensures proper mixing and aeration.
2.2. Feedstock MaterialVegetable waste (uncooked), cattle (Buffalo) manure, and sawdust was used for the preparation of different waste mixtures. Vegetable waste was collected from different student hostels of the Indian Institute of Technology Guwahati campus, India. Cattle manure was obtained from a dairy farm near the campus, and sawdust was purchased from a nearby saw mill. The compost was prepared with two different proportions using vegetable waste, cattle manure, and sawdust of two different ratios, trial A (6:3:1) of C/N 24 and trial B (8:1:1) of C/N 30, respectively, with a total volume of 15 kg. Initial characterization of the raw materials is detailed in Table 1. Prior to composting, the maximum particle size of the composting material was restricted to 1 cm, in order to provide better aeration and moisture control by means of mechanical shredder equipped with 3.5 kW motor.
2.3. Sampling and Analysis of Compost PropertiesTriplicates of the homogenized mixture were collected every fourth day from the rotary drum for analysis. Oxygen uptake rate (OUR) and CO2 evolution were carried out, as described in Kalamdhad et al. [9]. Moisture content of the compost was analyzed at 105°C for 24 hr. Sub-samples were air dried immediately, ground to pass a 0.2 mm sieve, and stored for further analysis. Each sub-sample was analyzed for the following parameters: pH and electrical conductivity (EC) (1:10 w/v wastewater extract), total nitrogen (T-N) using the Kjeldahl method, ammoniacal nitrogen (NH4-N) using KCl extraction [11], and total and available phosphorus (acid digest) using the stannous chloride method [12]. Nutrients (Na, K, Ca) were analyzed by using Flame photometry, and total metals (Zn, Cu, Mn, Mg, Fe, Pb, Ni, Cd, and Cr) were determined by atomic absorption spectrometer (SpectrAA 55B; Varian Inc., Palo Alto, CA, USA), after the digestion of 0.2 g sample with 10 mL of HS2O4 and HClO4 (5:1) mixture in the block digestion system (Pelican Equipments, Chennai, India) for 2 hr at 300°C.
2.4. Sample Preparation for Microbial AnalysisMicrobial count was analyzed by adding 10 g of waste or compost into 90 mL of sterile distilled water containing 0.85% (w/v) sterile sodium chloride solution in 250 mL Erlenmeyer flasks. The solution was mixed mechanically at 150 rpm for 2 hr at 25°C. Finally, the waste suspensions were diluted serially and 0.1 mL of each suspension used for microbial counts on appropriate media [13].
2.5. Culture Media and ConditionsNutrient agar medium was used for the total count of prokaryotes. Cycloheximide (0.2 g/L) was added to inhibit fungal growth. The final pH of the medium was 7.3 ± 0.1 at 25°C. Finally, prepared plates were incubated in an inverted position for 24–48 hr at 25°C and 50°C for thermophilic spore-forming bacteria. Sabouraud 4% dextrose agar supplemented with 5 g peptone from casein, 5 g peptone from meat, and 40 g D-(+)-glucose per liter was used for the total count of fungus. The final pH of the medium was 5.6 ± 0.2 at 25°C. The number of viable fungus was determined by plating appropriate diluted suspensions. Finally, prepared plates were incubated for 3–4 days at 25°C. Actinomycete isolation agar supplemented with 2 g sodium caseinate, 0.1 g L-asparagine, 4 g sodium propionate, 0.5 g K2HPO4, 0.1 g MgSO4, 0.001 g FeSO4, and 5 mL glycerol per liter was used for the enumeration of actinomycetes. Cycloheximide (0.2 g/L) was added to inhibit fungal growth. The final pH of the medium was 8.1 ± 0.2 at 25°C. Finally, prepared plates were incubated in an inverted position for 4–6 days at 25°C. ISP medium No. 4, supplemented with 10 g starch soluble, 1 g K2PO4, 1 g MgSO4·7H2O, 1 g NaCl, 2 g (NH4)2SO4, 2 g CaCO3 per liter, was used for enumeration of streptomycetes. Fungal growth was inhibited by adding 0.2 g cycloheximide. The final pH of the medium was 7.2 ± 0.2 at 25°C. Finally, prepared plates were incubated in an inverted position, for 4–6 days at 25°C. Total coliforms (TC) and fecal coliforms (FC) were analyzed by inoculation of culture tube media with Lauryl tryptose broth and EC medium, respectively, using the most probable number (MPN) method [12]. Fecal streptococci and enterococci were identified by using Azide dextrose broth and Pfizer selective enterococcus agar. Appropriate diluted suspensions were inoculated onto plates and kept for 24–48 hr incubation at 35°C [14].
3. Results and Discussion3.1. Physicochemical AnalysisTemperature is an important factor determined by the microbial diversity and their metabolic activities during the composting process. An increase in temperature was observed during early stages of composting, i.e., 18 hr in both the trials A and B. However, the rise in temperature preceded in the same manner in both the trials, but was observed to be greater in trial A than trial B. A maximum of 63.5°C was observed in trial A and 61.2°C in trial B; an average of 55°C was maintained till the end of the 5th day, and thereafter it started to decrease. A temperature from 52°C to 60°C is considered to maintain the greatest thermophilic activity in composting systems [15]. Since the organic content is very high in vegetable waste, the microbial activity started at the early hours of the composting, leading to rise in temperature. Furthermore it also resulted in gradual release of CO2 and a high amount of moisture.
Sawdust was used as a bulking agent to maintain moisture content and provide better aeration during the composting process as suggested by Tsai et al. [16] and many other researchers [17, 18]. But it was not found successful in the present study with the usage of vegetable waste mixture at 150 kg working volume. However, it was successful in the same range for different substrate like water hyacinth with the same proportions studied by Singh and Kalamdhad [19]. Leachate production started soon after the thermophilic phase in both the trials, but was found very high in trial B compared to trial A, which might be due to insufficient bulking agent. The leachate produced during the process was collected through the holes that were made at the bottom of the drum. The collected leachate was then quantitatively measured every day. It was observed that trial B leached out 20 L and trial A leached 4 L in total at the end of 20 days. The major proportion of leachate was observed during the thermophilic stage of composting, and gradually decreased as the process proceeded. Initial moisture content of the raw material was maintained at around 77% and 84% in trials A and B, respectively. Not much appreciable amount of moisture content reduction was observed at the end of 20 days, compared to studies conducted in drum composting [8, 20]. During leachate production, a sudden drop of temperature was observed during the active thermophilic phase, thereby reducing the loss of moisture during the process. It was observed with a direct relation between the active thermophilic phase and leachate production during composting. With rise in temperature, reduction in moisture content was observed; however, after the production of leachate, the moisture content was found to be almost constant; and finally, it was in the range of 67% in trial A and 76% in trial B. Moreover, the leachate production had great influence on the compost parameters. Most compost has a pH value in the range of 6 to 8 and it generally increased during the composting process [21]. Similar results were observed in the present study with the initial pH 6.6 and 6.45 increasing to 7.4 and 6.9 in trials A and B, respectively.
The EC of trial A was observed to increase from 3.5 to 7.4 dS/m, which could be due to the release of mineral salts and ammonium ions through the decomposition of organic matter, as reported by Nair and Okamitsu [22]; whereas in trial B, there was a gradual reduction from 6.2 to 4.7 dS/m, which might be due to the large amount of leachate production. While the metabolic process is in progress, the volatilization of ammonia and precipitation of mineral salts occur through which the EC of the material will be increased, and also leads to the stabilization of pH to alkaline conditions. Due to leachate production and inconsistent moisture content during composting, there was not much loss of organic carbon during the process. Decrease in total organic carbon from 26% and 44% to 22% and 42% in trials A and B, respectively, was observed as given in Table 2. This minimal reduction of carbon is contradictory, as compared to the traditional compost process that leads to great loss in carbon content. But, such reduction correlates with the reports by Andersen et al. [12] in which he stated that carbon loss is greatly dependent on leachate production.
From the results, it was observed that leachate production did not have much effect on the T-N dynamics in both the trials, as the values increased from 1.05% and 1.45% to 1.75% and 1.85% finally in trial A and trial B, respectively. Ammoniacal nitrogen also showed considerable decrease from 0.70% and 0.83% to 0.50% and 0.62% in trials A and B, respectively. During the mineralization of organic matter, the phosphorous present in it is released by the action of microorganisms. It was observed with a good increase in the total and available phosphorous in both the trials. Table 3 illustrates the total concentration of micronutrients (K, Na, and Ca) and heavy metals (Ni, Co, Cr, Cu, Fe, Mn, Mg, Pb, and Zn), during the composting process. These nutrients are used as mineral fertilizers in the compost. Trial A showed a considerable amount of increase in the total amount at the end of the composting period; whereas, in trial B, there was a drastic reduction in all the nutrients, which is considered due to the large amount of leachate production as most of the nutrients might have washed away.
3.2. Biological AnalysisCO2 evolution is used to evaluate the compost stability as it measures the carbon derived directly from the compost being tested, and is correlated with aerobic respiration. The composting process is believed to continue as long as the total amount of biodegradable organic material is stabilized, and the percentage of readily available biochemical oxygen demand (BOD) is believed to be an important aspect of compost quality [23, 24]. Higher CO2 evolution was observed at the initial stages of the composting period in both the trials A and B; but the values were inconsistent during the composting period, as it correlates with the minimal of carbon during the process; finally, it was in the range of 2.1 and 2.8 mg/g VS/day in trials A and B, respectively. The lower emission of CO2 during the final stage of compost denotes that the material is in the maturation phase as suggested by Kalamdhad et al. [9].
OUR determines the biological activity of the compost and it is widely accepted. It relates the compost stability to the amount of readily biodegradable organic matter present in the sample, through its carbonaceous oxygen demand [20]. At the end of 20 days, it was observed in the range of 2.4 and 2.7 mg/g VS/day in trials A and B, respectively. Soluble BOD and soluble chemical oxygen demand (COD) can be measured by the respiration of BOD, through aerobic conditions maintained in the compost. Soluble BOD and COD decreased as a result of the destruction of biological organic matter, resulting in decreased emission of CO2 [20]. Similar observations were found in the present study, indicating the reduction of soluble COD from 1,400 and 1,560 mg/L to 550 and 610 mg/L in trials A and B, respectively. Consequently, the soluble BOD reduced from 718 and 940 mg/L to 295 and 370 mg/L in trials A and B, respectively.
3.3. Microbial DynamicsBacterial populations are the natural microflora colonizing the first substrate, preferentially degrading the liable organic matter. Fig. 1 compares the changes in the cell density of mesophilic bacteria over time during the composting process in trials A and B. During the mesophilic stage, there was a predominance of mesophilic population in both the trials in the range of 6.5 × 109 and 6.8 × 1010 CFU/g, respectively. These mesophilic bacteria are heavily involved in the degradation of simple organics. During this stage, metabolic activities by the mesophilic population will result in the release of CO2 and temperature [22]; thereby heating up the material in the reactor. Due to the high organic content in vegetable waste, the microbial activity started at the early stage of mesophilic phase, resulting in the rise of temperature. The temperature rise had major effect on the growth of mesophilic bacteria, which is evident during the fourth day of the composting period in both the trials. A major drop in the population of mesophilic bacteria was observed, and was in the range of 4.25 × 108 and 4.45 × 108 CFU/g in trials A and B, respectively. At the same time, there was enormous growth of spore-forming bacteria from 3.9 × 106 and 4.4 × 106 CFU/g to 5.6 × 107 and 4.75 × 107 CFU/g in trials A and B, respectively, from initial day to fourth day of the process. Most of these thermophilic bacteria are spore-forming organisms, which can survive from 10°C to 70°C in the composting environment [25].
The decline in mesophilic bacteria due to higher temperature in the compost correlates with the report of Ryckeboer et al. [13]; furthermore it was reported that the bacterial and fungal populations are clearly influenced by temperature especially in the thermophilic stage. Soon after the decline in the thermophilic stage, a rise in mesophilic bacteria was observed in the range of 3.0 × 107 and 4.5 × 105 CFU/g in trial A and trial B, respectively. A higher microbial population was observed at the end of 20 days, which might be due to the considerable amount of moisture content. Whereas, studies by Bhatia et al. [8] with vegetable waste composting, using the in-vessel system, reported much reduction in the microbial population at the end of the composting period, but in which in no leachate was reported. Hence, in the present study due to high moisture content, the microbial population prevailed in large amount even at the end of the composting period and it correlates with the report by Saidi et al. [21]. Therefore, leachate production adversely affects the microbial population during the compost process, which in turn results in poor degradation process.
Moreover, fungi and actinomycetes can survive at high temperatures as spores, and started to increase soon after the decline of thermophilic phase. The results agreed with the reports by Ryckeboer et al. [13] for low population of these species during the initial stages of composting due to domination of the bacteria, and high proliferation soon after the thermophilic phase. The reason for the rise in population may be due to the loss of readily available carbon source during the initial phase, and hence the left out lingo-cellulolytic material had been degraded by these populations. Xiao et al. [5] also suggested that temperature higher than 50°C favors the growth of actinomycetes. There was a tremendous growth in fungi, actinomycetes, and streptomycetes during the late thermophilic stage as reported by Tchobanoglous et al. [26]. The fungi population increased from 2.8 × 106 and 3.2 × 106 CFU/g to 4.1 × 107 and 3.0 × 106 CFU/g at the end of fourth day in trials A and B (Fig. 1).
Actinomycetes, which are considered for the degradation of cellulose and lignin, were found in the range of 2.8 × 104 and 3.85 × 106 CFU/g in trials A and B at the end of 20 days. Streptomycetes population was observed in the range of 4.15 × 106 and 3.6 × 105 CFU/g in trials A and B, respectively (Fig. 2). Ishii et al. [27] have reported that at the end of the composting period, there were nil fungi and fewer amounts of actinomycetes. However, these reports were disagreed with by Ryckeboer et al. [13] and it was explained that the differences can be possible by high moisture content and low C/N ratio. Furthermore, it might also be due to the slow growth rate of fungi and actinomycetes. The present study results agree with the above statement for high moisture content and also for high populations of fungi, actinomycetes, and streptomycetes at the end of the composting period. Mustin [28] indicated that the microorganisms of compost can create the conditions of their own destruction at any moment, which are optimal for the microorganisms engaged in composting.
3.4. PathogensThere is always concern about the hygienic quality of the final compost. The recommended FC and fecal streptococci densities for compost hygienization are 5 × 102 and 5 × 103 MPN/g, respectively. Moreover the presence of coliform bacteria is often used as an indicator of overall sanitary quality of the compost [20]. Salmonella species were regarded as a problem of the hygienic quality of MSW compost and is destroyed, when the temperature of the compost reaches 55°C. It is also reported that Escherichia coli and Salmonella sp. are killed in 20 min at 60°C. In addition, the thermal death points of most disease-causing organisms, including Salmonella typhosa, Mycobacterium tuberculosis, Mycobacterium diphtheriae, and Brucella abortus or Brucella suis, are well within the temperature range achieved in composting, if exposed to these temperatures for at least a few minutes. Finally, organisms are exposed to high temperatures for many hours and/or days during composting, which is more sufficient in eliminating pathogens [22, 29]. The present study also achieved a maximum temperature of around 66°C that was able to reduce the TC from 11 × 108 and 24 × 108 MPN/g to 4.6 × 103 and 9.3 × 103 MPN/g in trials A and B, respectively. Similarly, the average number of FC considerably decreased from 4.3 × 105 and 3.5 × 105 MPN/g to 3.5 × 102 and 4.6 × 102 MPN/g in trials A and B, respectively. In addition, streptococci and enterococci population were reduced to considerable amount at the end of 20 days in trial A as compared to trial B, as given in Table 2.
4. ConclusionsThe present study revealed that leachate production and high moisture content during the composting process had a huge effect on the microbial population, and also adversely affected the composting parameters. Even though the process was normal during the early stages of composting, soon after the thermophilic phase, a huge amount of leachate production was observed due to insufficient bulking agent, i.e., sawdust for vegetable waste composting. The reason for the leachate production might be due to saturation of sawdust with huge amount of moisture content during the metabolic process by active microorganisms, and once the saturation point has exceeded, it started to leach out.
It was also observed that higher diversity of microbial population prevailed during composting; however, because of leachate production, inconsistent degradation pathway was observed during the process. Finally, the authors would like to conclude that the presence of water is necessary for microbial activity and transport of soluble substances for organic matter destruction. However, excess moisture had a major effect on OUR and heat release in the process. Therefore, leachate production should be avoided during vegetable waste composting, due to its major effect on the process. It is also suggested that the application of proper bulking agent should be of major concern during vegetable waste composting.
AcknowledgmentsThe authors gratefully acknowledge the financial support of the Ministry of Drinking Water and Sanitation, Government of India (Grant No. W. 11035/07/2011-CRSP (R&D) 12/12/2012).
References1. Kumar JS, Subbiah KV, Prasada Rao PV. Management of municipal solid waste by vermicompost: a case study of Eluru. Int. J. Environ. Sci. 2010;1:82–90.
2. Ghatwai M. Perishable production [Internet]. New Delhi: The Indian Express; c2014. [cited 2013 Oct 8]. Available from: http://archive.indianexpress.com/news/perishable-production/1165189/
3. Larney FJ, Hao X. A review of composting as a management alternative for beef cattle feedlot manure in southern Alberta, Canada. Bioresour. Technol. 2007;98:3221–3227.
4. Montemurro F, Maiorana M, Convertini G, Ferri D. Compost organic amendments in fodder crops: effects on yield, nitrogen utilization and soil characteristics. Compost Sci. Util. 2006;14:114–123.
5. Xiao Y, Zeng GM, Yang ZH, et al. Changes in the actinomycetal communities during continuous thermophilic composting as revealed by denaturing gradient gel electrophoresis and quantitative PCR. Bioresour. Technol. 2011;102:1383–1388.
6. Ruggieri L, Gea T, Mompeo M, Sayara T, Sanchez A. Performance of different systems for the composting of the source-selected organic fraction of municipal solid waste. Biosyst. Eng. 2008;101:78–86.
7. Chanakya HN, Ramachandra TV, Guruprasad M, Devi V. Micro-treatment options for components of organic fraction of MSW in residential areas. Environ. Monit. Assess. 2007;135:129–139.
8. Bhatia A, Ali M, Sahoo J, et al. Microbial diversity during rotary drum and windrow pile composting. J. Basic Microbiol. 2012;52:5–15.
9. Kalamdhad AS, Pasha M, Kazmi AA. Stability evaluation of compost by respiration techniques in a rotary drum composter. Resour. Conserv. Recycl. 2008;52:829–834.
10. Kalamdhad AS, Kazmi AA. Mixed organic waste composting using rotary drum composter. Int. J. Environ. Waste Manag. 2008;2:24–36.
11. Tiquia SM, Tam NF. Fate of nitrogen during composting of chicken litter. Environ. Pollut. 2000;110:535–541.
12. Andersen JK, Boldrin A, Samuelsson J, Christensen TH, Scheutz C. Quantification of greenhouse gas emissions from windrow composting of garden waste. J. Environ. Qual. 2010;39:713–724.
13. Ryckeboer J, Mergaert J, Coosemans J, Deprins K, Swings J. Microbiological aspects of biowaste during composting in a monitored compost bin. J. Appl. Microbiol. 2003;94:127–137.
14. Eaton AD, Clesceri LS, Rice EW, Greenberg AE. Standard methods for the examination of water and wastewater 2. 1st edWashington: American Public Health Association; 2005.
15. Mohee R, Mudhoo A. Analysis of the physical parameters of an in-vessel composting matrix. Powder Technol. 2005;155:92–99.
16. Tsai SH, Liu CP, Yanga SS. Microbial conversion of food wastes for biofertilizer production with thermophilic lipolytic microbes. Renew. Energy. 2007;32:904–915.
17. Smith DC, Beharee V, Hughes JC. The effects of composts produced by a simple composting procedure on the yields of Swiss chard (Beta vulgaris L. var. flavescens) and common bean (Phaseolus vulgaris L. var. nanus). Sci. Hortic. (Amsterdam). 2001;91:393–406.
18. Smith DR, Cawthon DL, Sloan JJ, Freeman TM. In-vessel, mechanical rotating drum composting of institutional food residuals. Compost Sci. Util. 2006;14:155–161.
19. Singh J, Kalamdhad AS. Concentration and speciation of heavy metals during water hyacinth composting. Bioresour. Technol. 2012;124:169–179.
20. Kuhlman LR. Windrow composting of agricultural and municipal wastes. Resour. Conserv. Recycl. 1989;4:151–160.
21. Saidi N, Kouki S, M’hiri F, et al. Microbiological parameters and maturity degree during composting of Posidonia oceanica residues mixed with vegetable wastes in semi-arid pe-do-climatic condition. J. Environ. Sci (China). 2009;21:1452–1458.
22. Nair J, Okamitsu K. Microbial inoculants for small scale composting of putrescible kitchen wastes. Waste Manag. 2010;30:977–982.
23. Kalamdhad AS, Singh YK, Ali M, Khwairakpam M, Kazmi AA. Rotary drum composting of vegetable waste and tree leaves. Bioresour. Technol. 2009;100:6442–6450.
24. Wang P, Changa CM, Watson ME, Dick WA, Chen Y, Hoitink HA. Maturity indices for composted dairy and pig manures. Soil Biol. Biochem. 2004;36:767–776.
25. Amner W, McCarthy AJ, Edwards C. Quantitative assessment of factors affecting the recovery of indigenous and released thermophilic bacteria from compost. Appl. Environ. Microbiol. 1988;54:3107–3112.
26. Tchobanoglous G, Theisen H, Vigil SA. Integrated solid waste management: engineering principles and management issues. New York: McGraw-Hill; 1993.
27. Ishii K, Fukui M, Takii S. Microbial succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J. Appl. Microbiol. 2000;89:768–777.
28. Mustin M. Le compost: gestion de la matiere organique. Management of compost as organic matterParis: Francois DUBUSC Edition; 1987.
29. Kalamdhad AS, Kazmi AA. Effects of C/N ratio on mixed organic waste composting in a rotary drum composter. Int. J. Environ. Eng. 2009;4:187–207.
Fig. 1(a) Temperature, (b) total heterotrophic bacteria, (c) spore-forming bacteria, and (d) total fungal variation during composting. 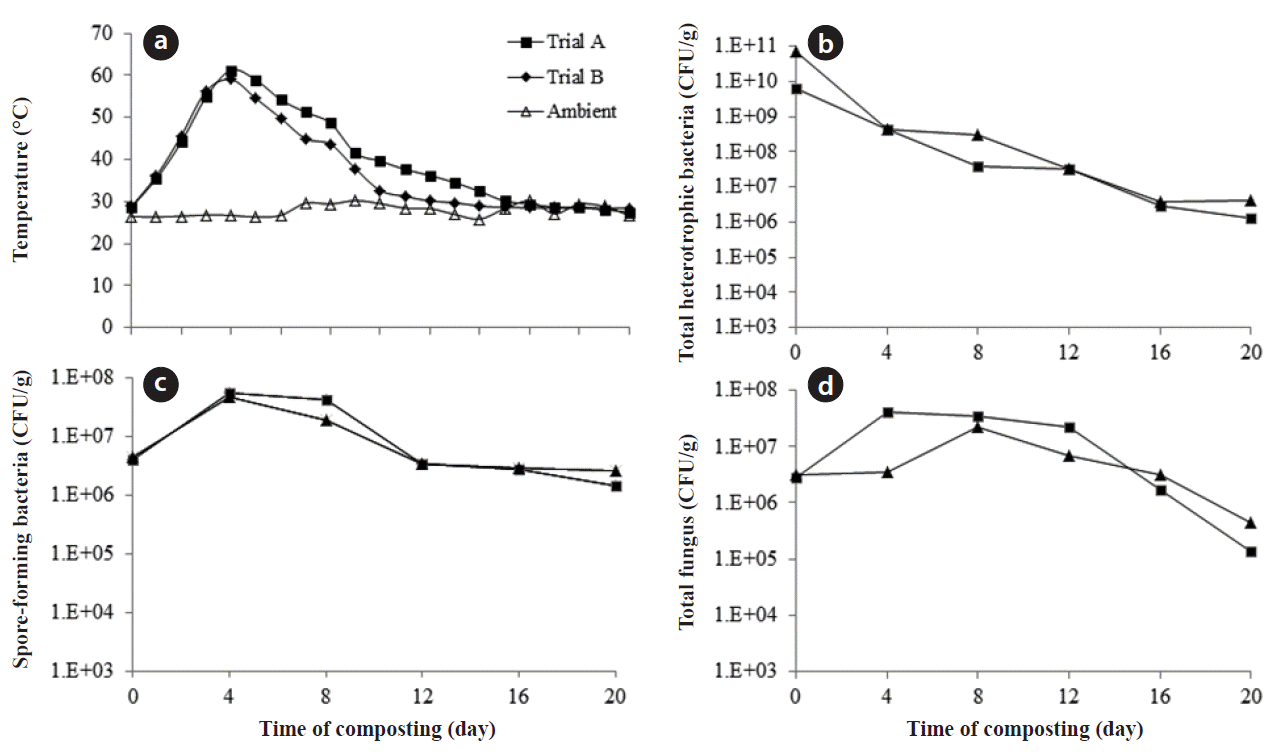
Table 1Initial characterization of waste material Table 2Physicochemical and biological pattern during composting Table 3Variation of nutrients and heavy metals during composting |
|
||||||||||||||||||||||||||||||||||