1. Introduction
Zeolites are microporous alumino-silicates that are widely used in various applications, such as catalysis, separation, and adsorption. There are more than 200 types of natural and synthetic zeolites, with 60 types of basic frameworks. A vast database of information on zeolites has been amassed from research which has been conducted over the course of approximately 50 years. In recent years, the number and quality of studies on zeolites has continued to expand. The ion-exchange capacity, shape selectivity, porous structure, solid acid sites, etc. of zeolites have been exploited in a plethora of industrial applications [1, 2]. Metal-containing zeolites are particularly attractive [3], and iron-containing zeolites have specifically been applied to the Fenton reaction [4], NOx reduction [5, 6], as heterogeneous catalysts, in petrochemistry [7, 8], and as materials for gas-separation membranes [9]. However, compared to fields, such as petrochemistry, separation, and Significant Code Review (SCR) reactions, the use of zeolites has not become widespread in the environmental engineering field. The majority of zeolites used in the environmental engineering field are employed for the removal of ion species, due to their outstanding ion-exchange capacity [10].
Fine iron particles are used for the reductive degradation of organic compounds present in ground water, soil, and air; for removing heavy metals, and as coagulants to capture inorganic pollutants, because of their high reactivity [11]. Consequently, iron-loaded zeolites have been synthesized and applied to the removal of ammonium and phosphates as alternatives to coagulants. The methods for the preparation of iron-loaded zeolites can be classified into three groups: iron incorporation, ion exchange, and ion exchange following calcination [12, 13]. The differences between the iron-incorporated and iron-exchanged zeolites have been studied, and different pretreated zeolites have been evaluated [8, 14, 15]. In this study, the optimal iron content for the zeolites is initially determined based on the evaluation of the synthesized iron-incorporated zeolites. This optimal iron content is then extrapolated to the preparation of the ion-exchanged and calcined zeolites [6, 12, 13]. The characteristics of the zeolites prepared by different methods and their ammonium and phosphate removal properties are also investigated.
Although nutrient control has been attempted by many researchers, there has been little research on the removal of ammonium and phosphate using synthetic zeolites, except for a use of zeolite synthesized from fly ash (ZFA), in which fly ash from a thermoelectric power plant was transferred to ZFA by hydrothermal synthesis with the injection of NaOH [16]. Various attempts at ammonium and phosphate removal using ZFA have been made since 2000. Since the initial attempt at phosphate removal using raw fly ash as a coagulant, this process has gradually progressed to the removal of ammonium and phosphate using salt-treated or acid-treated ZFA, synthesized using NaOH [15, 17]. However, research on the removal of ammonium and phosphate using synthetic zeolites is still in its early stages, because synthetic zeolite and ZFA are completely different, and their mechanisms of removal, as well as the regeneration of the latter, have yet to be elucidated [15–18].
Herein, the optimal molar ratio of iron-containing zeolites is determined, and a comparative analysis of the ammonium and phosphate-removal efficiency of zeolite type-A (Z-A), relative to iron-incorporated-Z-A (IIZ), iron-exchanged-Z-A (IEZ), and iron-calcined-Z-A (ICZ), prepared by different methods, is presented.
2. Materials and Methods
2.1. Preparation of Zeolites
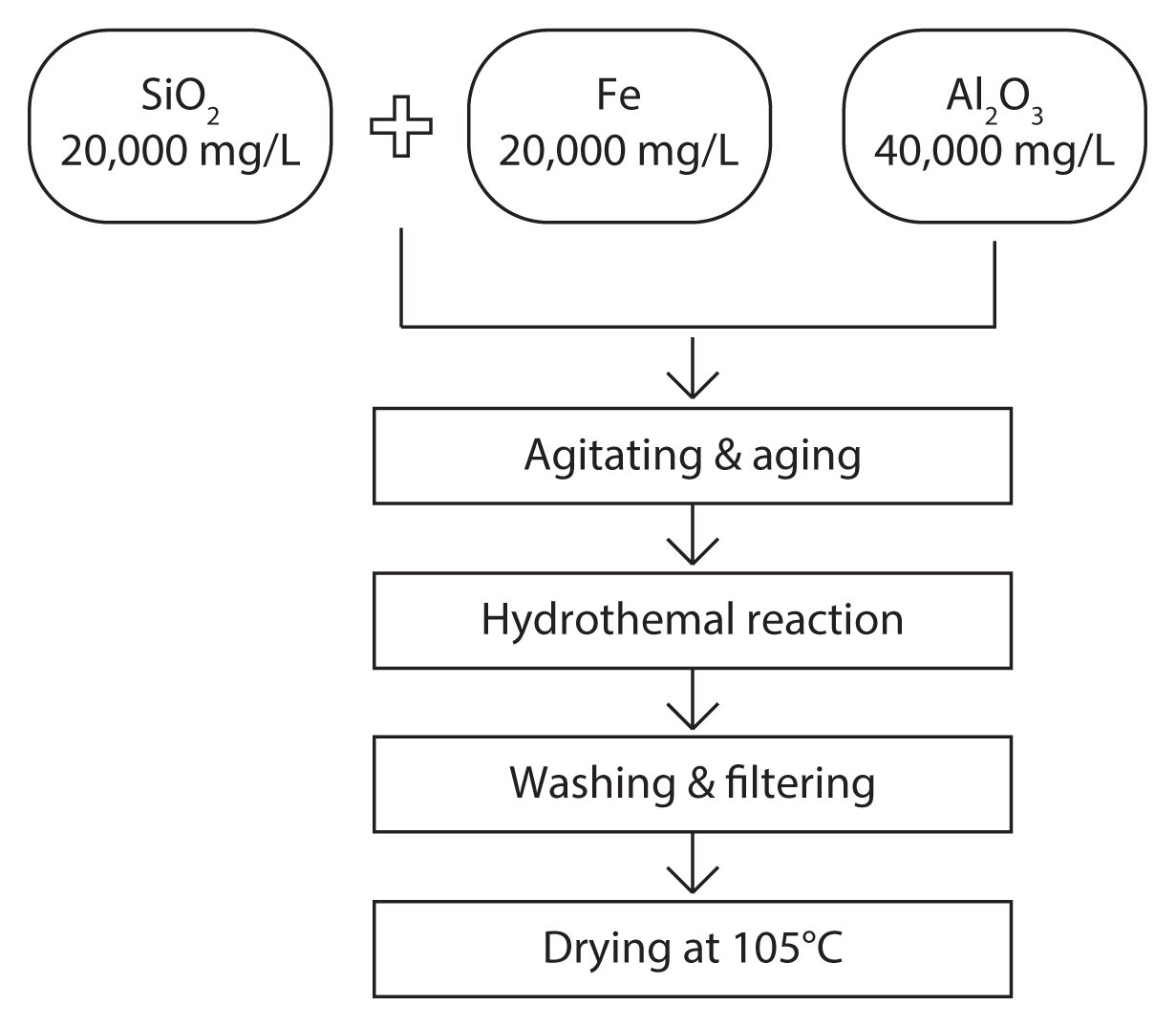
Iron-loaded Z-A was synthesized by three methods; the resulting zeolites were evaluated in the removal of nutrients, such as NH4+ and PO43− from an aqueous medium. Firstly, experiments on an optimal molar ratio of SiO2:Al2O3:Fe for preparing of Z-A incorporated with iron were carried out, in which iron species are placed into the framework of Z-A. The second is the exchange method in which iron species are exchanged with sodium ions in Z-A [12], and the third is calcination followed by an exchange protocol, in which iron-exchanged Z-A is calcined at 500°C for 5 hr [13]. Initially, the optimal iron content for incorporation into Z-A, and the most favorable synthetic conditions, were determined based on the results obtained from the synthesis of IIZ. After determining the optimal iron content, IEZ and ICZ were also prepared for ammonium- and phosphate-removal experiments. The preparation procedure for IIZ is shown in Fig. 1. The experimental conditions for the synthesis of IIZ are shown in Table 1.
Among the three methods used for the preparation of iron-containing zeolites, iron-incorporation was carried out first, to generate IIZ, in order to determine the optimal iron content. In this method, iron species are added to an alumino-silicate gel prior to crystallization of the zeolite, as described by Lee [19] and Kanthasamy and Larsen [13]. Sodium silicate (Na2SiO3; Sigma-Aldrich, St. Louis, MO, USA; SiO2, 44%–47%), sodium aluminate (NaAlO2; Sigma-Aldrich; Al2O3, 51%–55%), and ferric chloride hexahydrate (FeCl3·6H2O; Daejung Chemicals, Siheung, Korea; 98%) were used for the synthesis of IIZ. The molar ratio of SiO2:Al2O3 in Z-A was 1:1. Next, 44.44 g of Na2SiO3 and 75.6 g of NaAlO2 were dissolved in 1 L of distilled water to produce 20,000 mg/L SiO2 and 40,000 mg/L Al2O3 solutions (solutions A and B), respectively. The molarity of SiO2 and Al2O3 in solutions A and B were 0.33 and 0.39 mol/L, respectively. The SiO2:Al2O3 ratio of 1 was modified by mixing 30 mL of solution A and 25.5 mL of solution B with 20 mL of additional distilled water in a 100-mL beaker. After 1 hr of agitation, the alumino-silicate gel was formed and was placed on a shaking incubator and agitated for 24 hr. The agitated gel was transferred to an autoclave for hydrothermal synthesis at 120°C for 48 hr, followed by several washings with distilled water, filtration through a GF/C glass fiber filter (0.45 μm pore size), and drying at 105°C in a drying oven for 24 hr. IEZ was synthesized under two conditions: using SiO2/(Al2O3+Fe) = 1 (condition 1 of Table 1) and using SiO2/Al2O3 = 1 followed by addition of iron species (condition 2 of Table 1) to determine which conditions were more suitable for zeolite crystallization. FeCl3·6H2O was dissolved in water to prepare a solution of 4,000 mg Fe/L (solution C). The iron-exchange method described by Lee [19] and Kanthasamy and Larsen [13] was used. Lee [19] mixed 50 g of zeolite with 500 mL of 0.35 M Fe solution and allowed it to react for 24 hr, whereas Kanthasamy and Larsen [13] placed 100 mg of zeolite into 3 mL of 1.6 M Fe solution and allowed it to react for 24 hr. The methods were examined for reproducibility, but both methods altered the zeolite to the gel phase with a deterioration of its crystallinity. This may have been caused by the strong acidity of the Fe solution and extended contact time. Lee [19] and Kanthasamy and Larsen [13] did not use zeolite, but used more iron-oxide-like materials. Therefore, this paper investigates a new method for the preparation of IEZ, termed the rapid adsorption method, in which zeolite is placed into an iron solution of a predetermined concentration, mixed for a few minutes, and then filtered directly. Based on the results obtained from the synthesis of IIZ, the optimal synthetic molar ratio was determined as Fe/Al2O3 = 0.05, whereas the iron content was 0.76 wt%, as determined from XPS analysis. Consequently, 20 g of Z-A and 152 mg of Fe 0.76 wt% were required.
The third method for synthesizing iron-loaded zeolites was adapted from Asedegbega-Nieto et al. [12] and involves the complete oxidation of the iron species in the zeolite. Unlike the method presented by Esther, the zeolite source used in the third method was the one synthesized by the new method (the rapid adsorption method) in IEZ synthesis. Assuming iron to be the main reactant and zeolite to be a support, IEZ was calcined so that all of the iron species were converted to iron oxides. The zeolite was not affected by calcination because of its thermal resistance, and thus, only iron species were oxidized.
2.2. Ammonium and Phosphate Removal
Z-A is generally known to exhibit a high removal efficiency for ammonium. The ammonium-removal capacities of Z-A, IIZ, IEZ, and ICZ were compared. IIZ with a molar ratio of Fe/Al2O3 = 0.05 was used under condition 2. The initial concentration of NH4+ was 15 mg/L, prepared by diluting a 250 g/L standard solution. Each zeolite, in samples of 0.1 g, 0.5 g, and 1 g, were injected into 500 mL of the ammonium solution. Sampling was performed at 1, 10, 20, 40, 80, and 120 min, respectively. Collected samples were filtered using a GF/C glass fiber filter (0.45 μm pore size) and the ammonium concentration in the solution was analyzed in order to determine the ammonium-exchange capacity. The experiments were also performed after acid treatment at pH 4 after 3 hr of contact time, as determined from the experiments under condition 2. The acid treatment of zeolite after experiments was carried out to determine the regeneration of zeolite incorporated with iron.
Phosphate-removal experiments were conducted without acid treatment by injecting 1 g of each type of zeolite into 500 mL of 5 mg PO43−-P/L solution. Because the zeolites had no capacity for phosphate removal in the absence of acid treatment, as indicated by Zhang et al. [15], the optimal acid-treatment conditions were determined from trials at pH 4 for 12 hr, pH 4 for 3 hr, and pH 3.8 for 3 hr. A 10 N HCl solution was diluted to 1, 0.1, and 0.01 N. The zeolite was first placed into 500 mL of 0.01 N HCl, and its pH was then adjusted to 4 using 1 N HCl. For experiments carried out at pH 4 and pH 3.8, approximately 20 mL and 40 mL, respectively, of 1 N HCl solution were consumed. Under the strongly acidic conditions of pH 2, 3, and below, the zeolite structure collapsed, making the zeolite unavailable for further experiments. Based on the experiments, acid treatment at pH 4 for 3 hr was determine to be optimal for the construction of a product with a maximal zeolite efficiency, and thus, these conditions were adopted for the ensuing experiments, in which the zeolites and loadings were varied. Regeneration of the zeolite was also carried out over three cycles. Zeolite can be regenerated owing to the heterogeneous nature of the phosphate-removal reaction.
The respective concentrations of ammonium and phosphate were measured using a Hach spectrophotometer (DR/4000; Hach, Loveland, CO, USA). The concentration of ammonium was measured using the Nessler method (method 8038), which has a detection limit of 0 to 3.03 mg NH4+/L. In the case of phosphate, the Molybdovanadate method (method 8114) was used with detection range 0 to 45 mg PO43−-P/L.
3. Results and Discussion
3.1. SEM and XPS Images of Zeolite with Iron Content
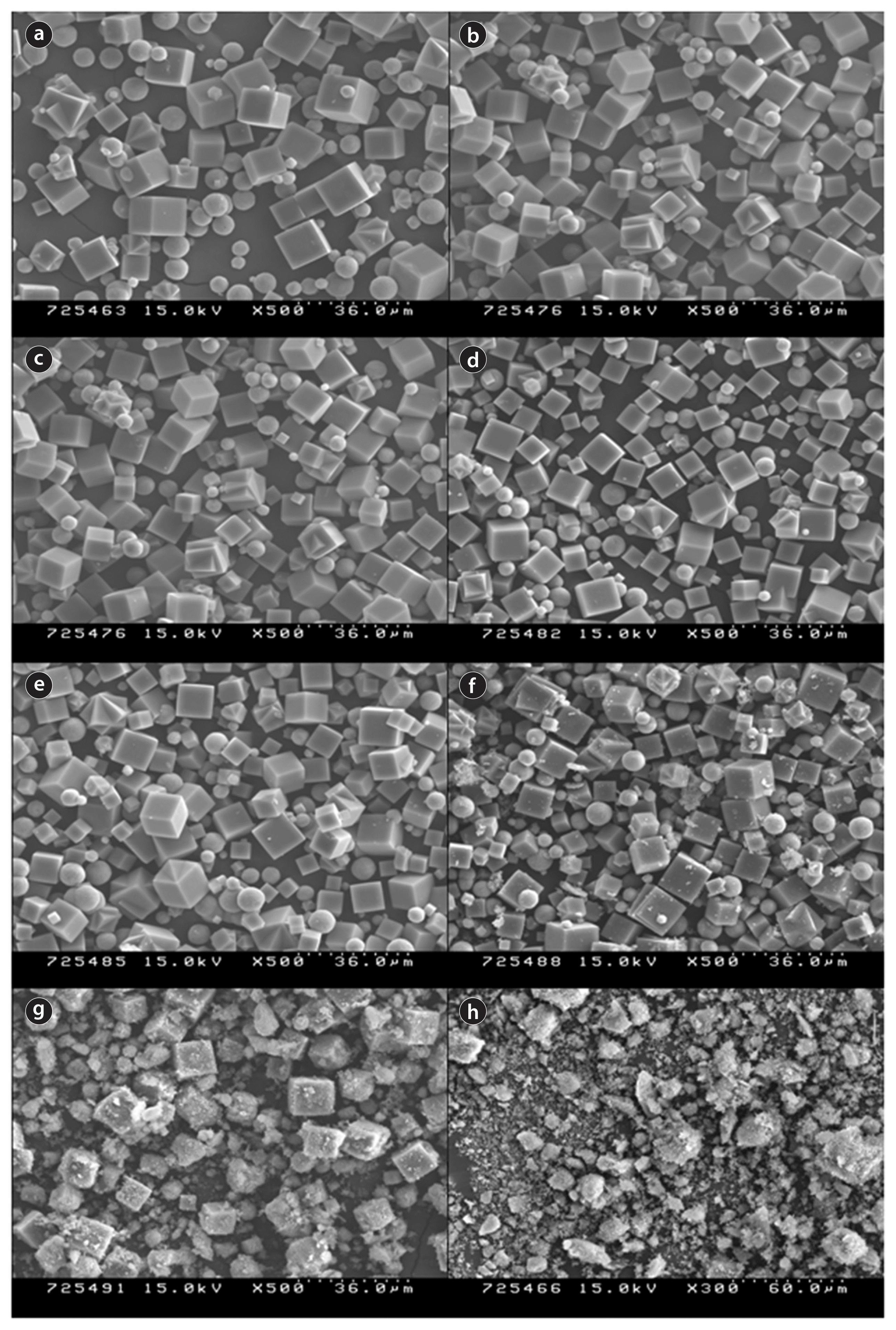
The SEM images of IIZ synthesized under conditions 1 and 2 of Table 1, along with the iron content, are shown in Figs. 2 and 3, respectively. The first image in Fig. 2 corresponds to Z-A, and the others correspond to iron contents of 0.001, 0.003, 0.005, 0.01, 0.03, 0.05, and 0.3 in the SiO2/(Al2O3+Fe) molar ratio of 1, and in the iron contents added to SiO2/Al2O3 molar ratio of 1. Except for some un-crystallized particles, though rough, most of the zeolite appears to be well-crystallized with a regular hexahedral shape. Based on the images in Fig. 2(b) to (h), it is clear that as the iron content increases, the number of crystallized regular hexahedrons decreases, although the number of globular particles, which seem to be un-crystallized zeolite, increases. The increasing residue that was apparent on the zeolite surface with increasing iron content appeared to be iron-oxide particles. At an iron content of 0.3, no zeolite crystals were apparent, and the majority of the sample consisted of iron oxides and amorphous particles. Fig. 3 shows a pattern similar to that of Fig. 2. Therefore, the ideal iron content was determined to be below 0.05, because no crystals were observed at an iron content of above 0.3.
Chemical bonds on the IIZ surface were investigated by XPS. The results for the samples synthesized under condition 1 are shown in Fig. 4. The spectra in the lower part of the figure correspond to Z-A containing no iron species and indicate the presence of Na, Si, Al, O, etc., in Z-A. Results of XPS spectra of IIZ synthesized under condition 1 are shown in Fig. 4. No peak corresponding to iron species is apparent for the iron content ranging 0.001 to 0.005. On the other hand, within iron content ranging 0.01 to 0.3, iron was detected from 0.4 to 2.6 wt% (condition 1 of Table 2).
When the iron content is 0.3, the peak pattern is abnormal, though the iron content is apparently high. Thus, the optimal iron content based on XPS analysis is within the range of 0.01 to 0.1.
The XPS spectra of IIZ prepared under condition 2 are shown in the upper part of Fig. 5. Overall, the pattern obtained for IIZ synthesized under condition 2 is similar to that synthesized under condition 1. However, as shown in Fig. 5, there is a difference in the Fe2p peak of these two species: the Fe2p peak is clearly apparent for the sample synthesized under condition 2, unlike condition 1, where the intensity of the Fe2p peak is very weak for species with iron contents above 0.05, though this peak is apparent when the iron content is 0.03. The results of surface component analysis using XPS indicated that iron species are present in greater volume in IIZ synthesized under condition 2 than in that synthesized under condition 1.
3.2. Ammonium-Removal
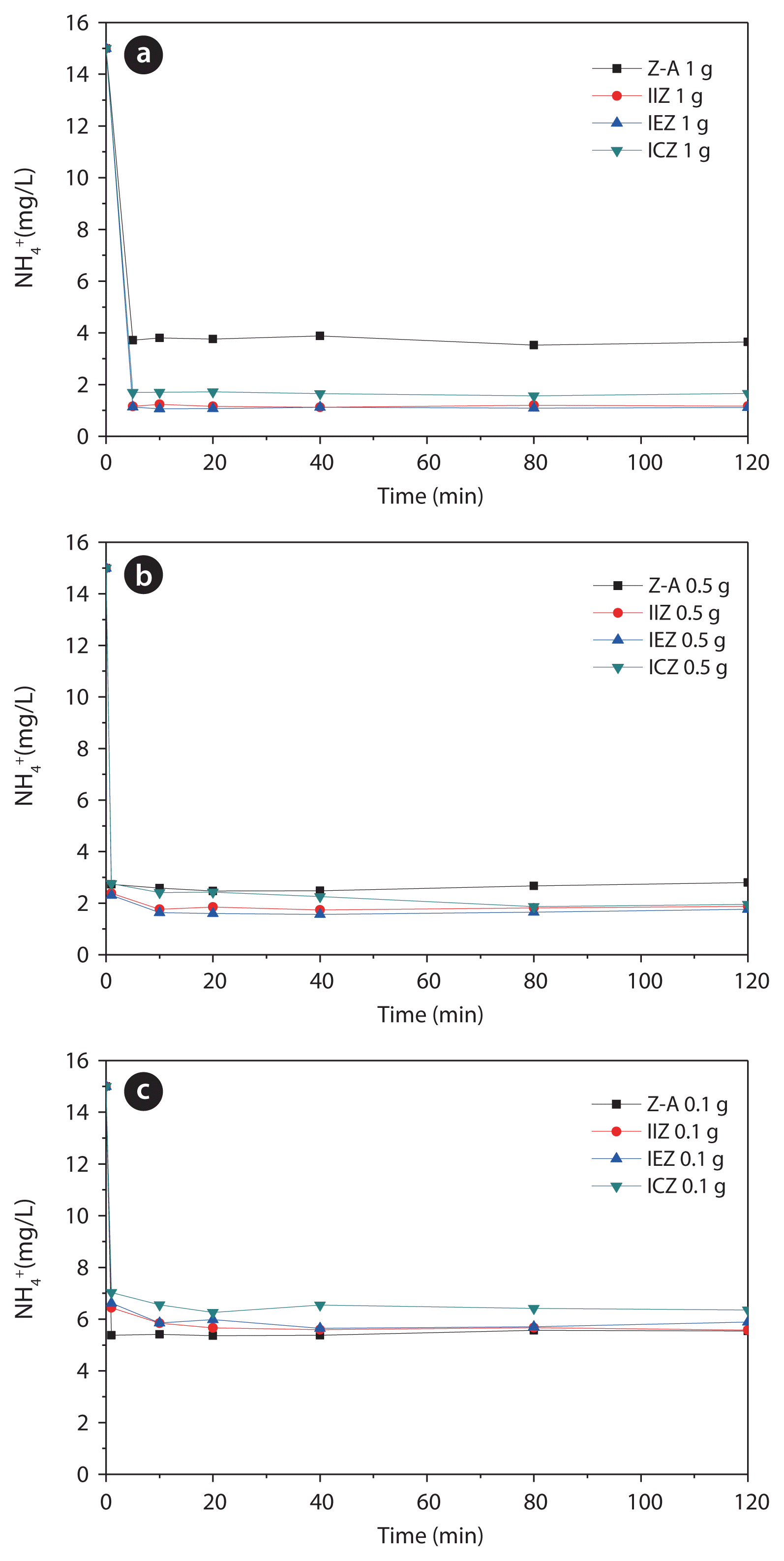
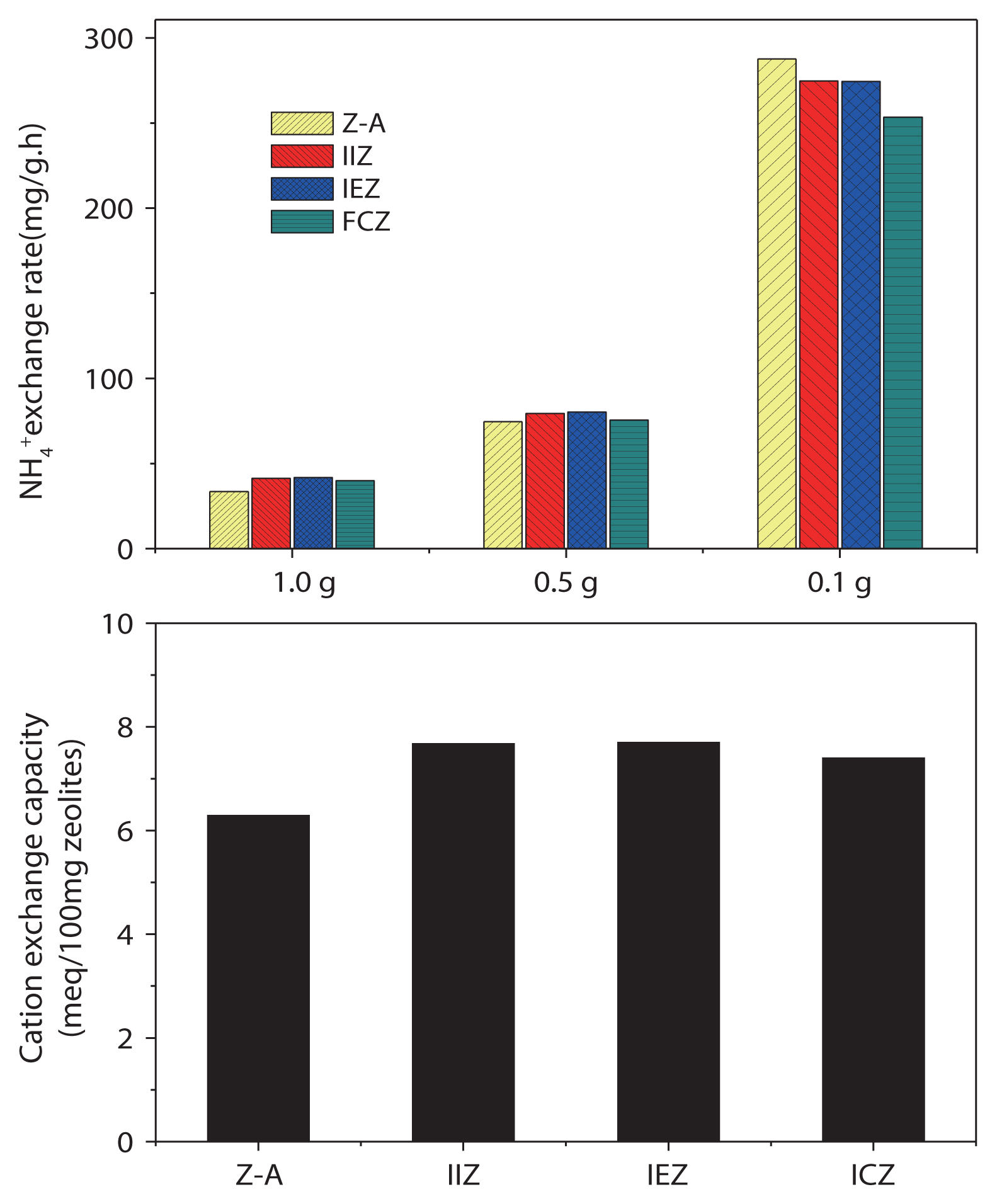
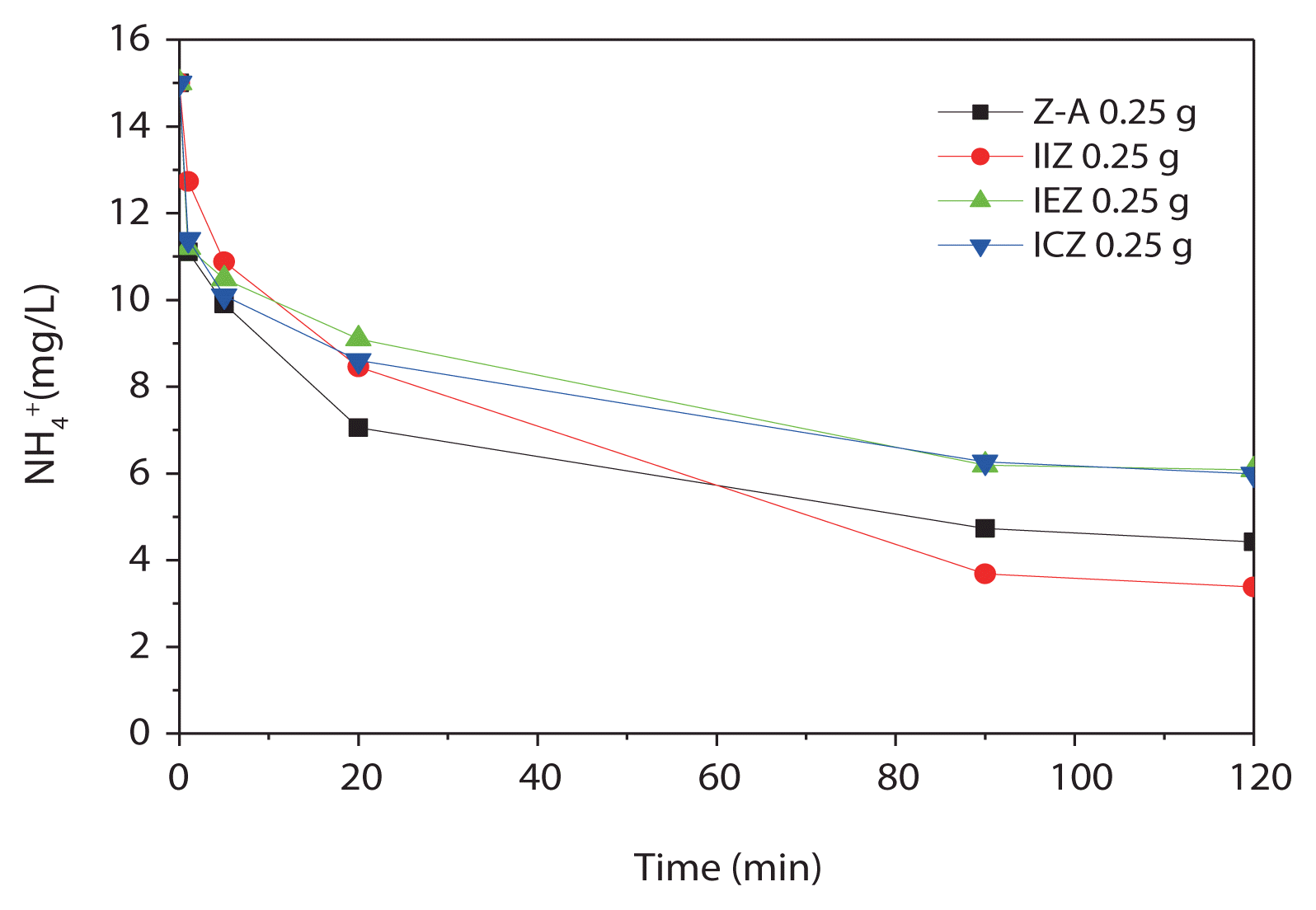
Ammonium-removal experiments were carried out on all zeolites before and after acid treatment, using an initial ammonium concentration of 15 mg/L. The results of the experiments using adsorbent dosages of 1.0, 0.5, and 0.1 g are shown in Fig. 6(a)–(c), respectively, demonstrating the rapid ammonia removal properties of the zeolites. The ammonium-removal rate and cation-exchange capacity of the zeolites are shown in Fig. 7, respectively. The rapid removal rate can be attributed to the concentration gradient between ammonium and the zeolites, as the initial ammonium concentration is fixed at 15 mg/L, while the zeolite dosage is varied. From Fig. 7, it is apparent that the cation-exchange capacities of IIZ and IEZ were better than that those of Z-A and ICZ.
Freundlich plots were constructed for all of the evaluated zeolites (Fig. 8). Correlation factors of the plots for Z-A, IIZ, IEZ, and ICZ were 0.977, 0.999, 0.984, and 0.940, respectively, each a relatively high correlation. Based on these results, it is concluded that ammonium is removed, not only by the ion-exchange phenomenon, but also via adsorption. If it is assumed that ammonium is removed solely through ion-exchange, there is no explanation for the increased removal capacity of the iron-loaded zeolites for ammonium. However, if the ammonium ions are also removed by adsorption, the change in the pores of the iron-loaded zeolites deserves serious consideration. In other words, sodium ions that act as electric-charge-compensation materials in the original zeolite are replaced with iron species, thereby expanding the cavity of zeolites because of the larger ionic radius of iron, leading to better ammonium removal. Among the iron-loaded zeolites, the ammonium-removal efficiency of ICZ was lower than that of IIZ and IEZ because of the conversion of the majority of the iron species in ICZ to iron oxides during calcination at 500°C.
In order to apply the zeolites to phosphate removal, acid treatment at pH 4 for 3 hr is required. Experiments were performed to determine whether the acid-treated zeolites (prepared for phosphate removal) still retained their ammonium-removal capacity. The dosage of zeolite was 0.25 g, calculated on the basis of the loss of water content. As shown in Fig. 9, all of the zeolites still exhibited ammonium-removal capacity, reaching equilibrium within 2 hr, although the removal rate was not as rapid as that of the untreated zeolites. The ammonium-removal rate and cation-exchange capacity were also evaluated. As discussed, the removal rate and cation-exchange rate may be influenced by the adsorbent dosage. Though absolute comparison was not possible, a relative comparison indicates that the results obtained using the acid-treated zeolites were similar to those obtained using the untreated zeolites.
3.3. Phosphate-Removal
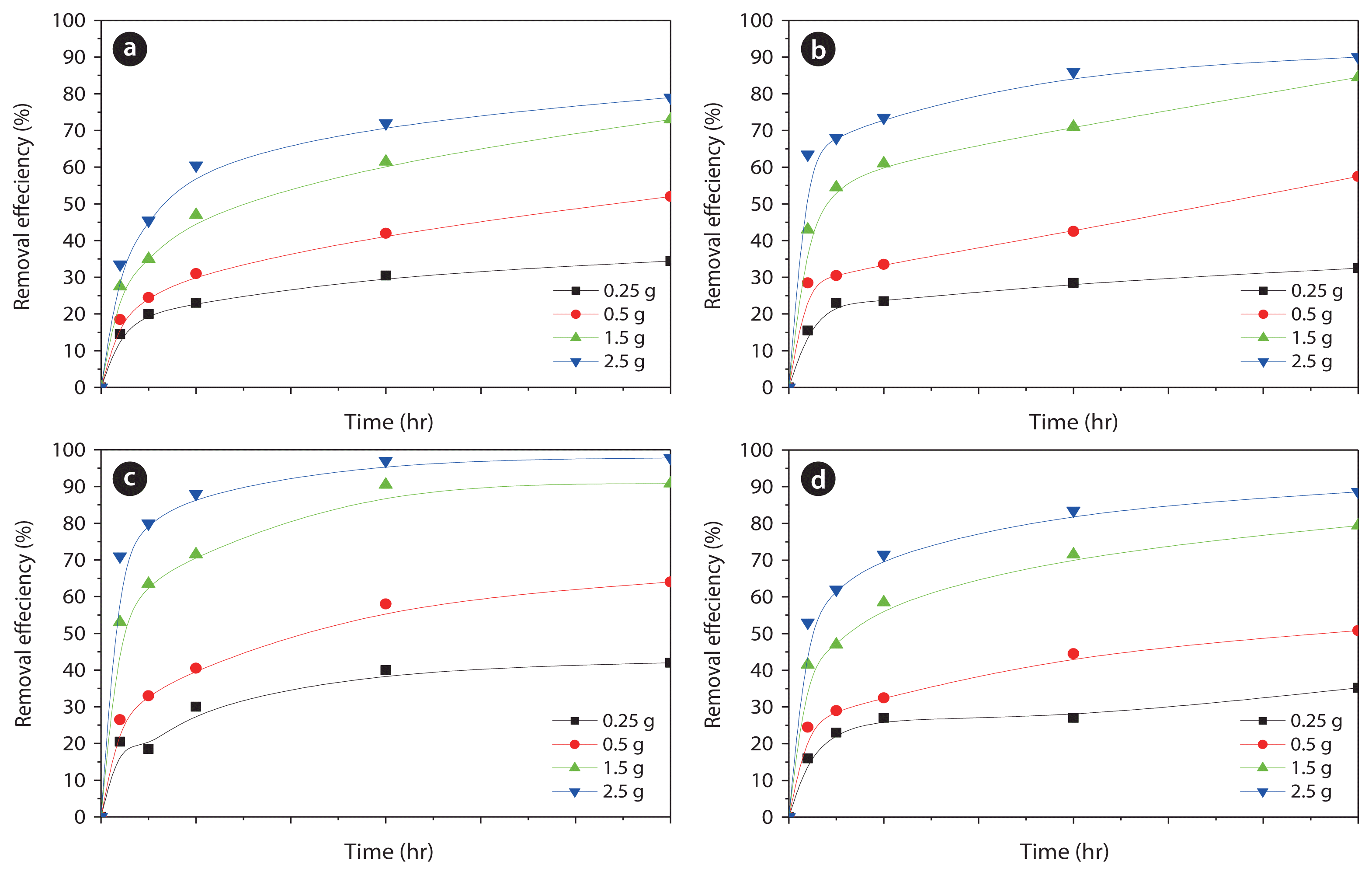
Under the optimized acid-treatment conditions of pH 4 for 3 hr for a given contact time, the effect of varying the zeolite dosage on the phosphate-removal efficiency was investigated. The results are shown in Fig. 10. The reaction reached equilibrium at approximately 3 hr, and IEZ exhibited the most rapid phosphate-removal rate, whereas the worst performance was achieved with ICZ. This is because of the readily reactive active sites in IEZ compared to the highly oxidized state of iron in ICZ, which leads to diminished reactivity.
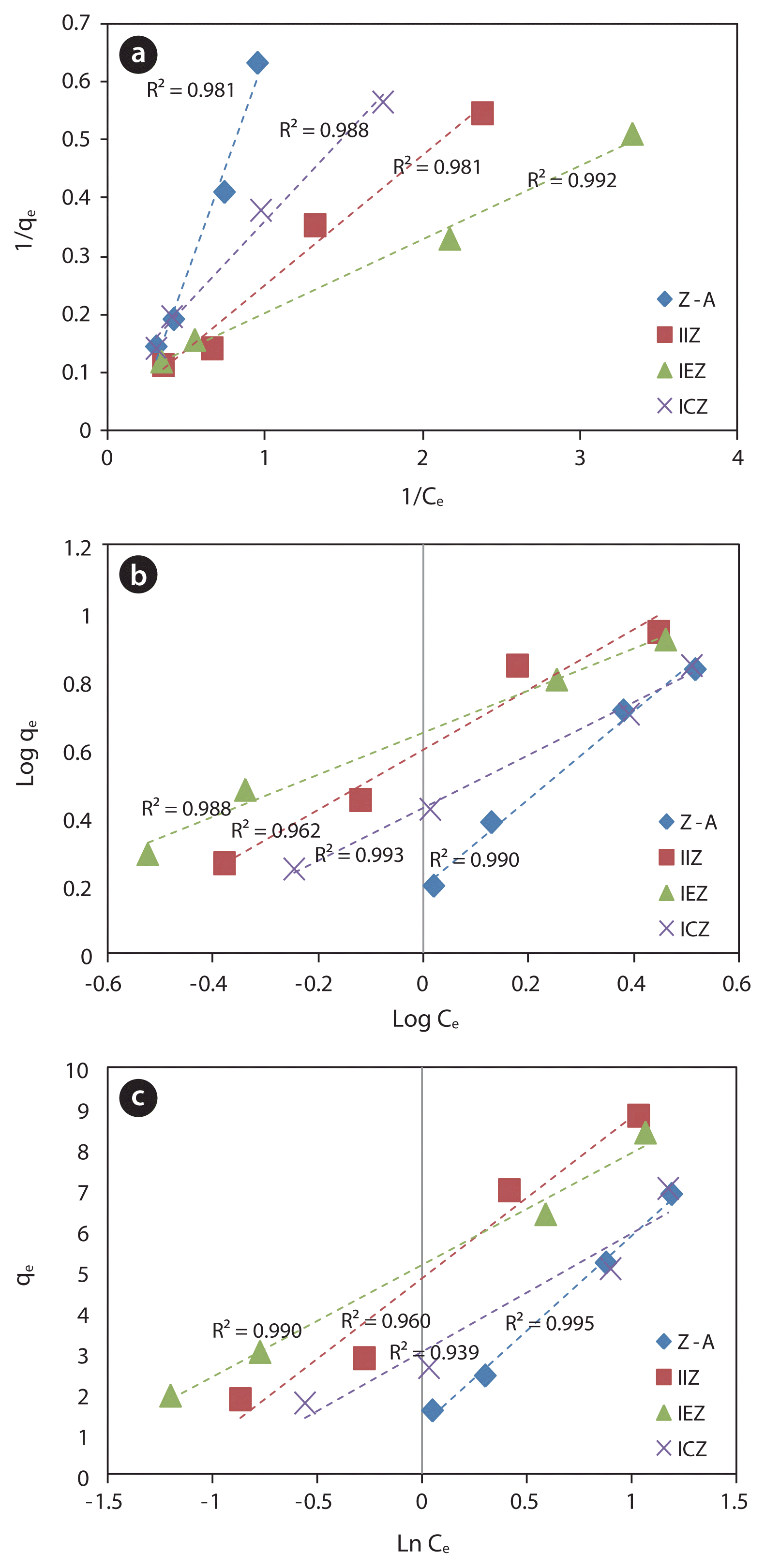
As discussed above, the adsorbent-dosage dependent variation of the removal rate is attributed to the concentration gradient. Three different types of adsorption isotherms are presented in Fig. 11. The correlation factors of the plots for Z-A, IIZ, IEZ, and ICZ were each over 0.981 in the case of the Langmuir plots; 0.990, 0.962, 0.988, and 0.993, respectively, in the case of the Freundlich plots; and 0.990, 0.960, 0.995, and 0.939, respectively, for the Temkin isotherm plots, each of which are relatively high values.
Lu et al. [20] reported the use of fly ash to have a composition which is similar to zeolite, and applied it to the removal of phosphate by precipitation. Zhang et al. [15] and Lu et al. [20] reported that phosphate was immobilized on ZFA, a zeolite synthesized from fly ash. In this study, it was found that the zeolites which were not subjected to acid treatment exhibited no phosphate-removal capacity, and that acid treatment resulted in the detachment of Al from the framework of the zeolite. However, the zeolite structure could be preserved with the appropriate acid treatment. Furthermore, although it was expected that only the iron species in the zeolites would be active for phosphate removal, Z-A that contained no iron was also determined to be effective for phosphate removal. Thus, it is proposed that not only the iron species are active for phosphate removal, but also that adsorption to the internal zeolite structure and chemisorption to Al3+ or NaH+ also play roles in phosphate removal.
A critical disadvantage of using iron salts as coagulants is the production of a large amount of chemical sludge due to precipitation of FePO4 or Fe(OH)3. However, because the iron-loaded zeolites developed herein react in the heterogeneous state to selectively remove phosphate, no sludge was produced during the reaction. Thus, these zeolites are deemed to be effective candidates for application to the phosphate-removal process.
4. Conclusions
In this study, Z-A and iron-loaded zeolites synthesized by three methods (iron incorporation, ion exchange, and ion exchange following calcination) were applied to the removal of ammonium and phosphate ions from aqueous media. The optimal iron content of the zeolites was determined from the results of IIZ synthesis. SEM, and XPS analyses revealed the optimal iron content to be below 0.05, expressed as a molar ratio of SiO2:Al2O3:Fe. Ammonium-removal experiments demonstrated that the iron-loaded zeolites exhibited a higher removal efficiency than Z-A. It is proposed that ammonium is removed, not only by ion exchange, but also by adsorption, due to the high correlation to adsorption plots. The replacement of sodium ions with iron may have expanded the cavity of the zeolite, thus facilitating the increased ammonium adsorption by the iron-loaded zeolites. Phosphate-removal experiments using zeolites and acid-treatment under different conditions indicate that the iron species, but also Al3+, NaH+, are active in phosphate removal, and adsorption may also be responsible for phosphate removal based on the fact that Z-A also exhibited phosphate-removal capacity. In addition, because the reaction of the iron-loaded zeolites with phosphate is heterogeneous, deleterious sludge production did not take place. Among the iron-loaded zeolites, maximal efficiency was exhibited by IEZ because of the presence of readily reactive iron species which were located on the zeolite surface.