AbstractThis study examined the effects of ozonation and UV applied in series (O3+UV) or simultaneously (UVO) under four different ozone dosages from 4 to 7 mg/min to understand the surface alterations on polyethylene microplastics in aquatic environments via the photochemical oxidation process. The plastic samples were analyzed by Fourier transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and contact angle measurements. FTIR spectroscopy showed that the levels of carbonyl (ketone and esters) and vinyl groups increased gradually with increasing ozone dose injected; the highest was observed at 6 mg/min of ozone. On the other hand, the levels at 7 mg/min of ozone were slightly lower than those at 4 to 6 mg/min. This could be related to the deeper penetration into the crystalline bulk polymeric chain. The contact angle changed from 125.90° to the lowest value of 120.04° and 123.8° for O3+UV and UVO, respectively. Furthermore, XPS showed that C-O was only presented in the 7 mg/min sample, whereas C-O, OH, C=O, and C-C=O remained for 4 to 6 mg/min. Overall, O3+UV can oxidize the surface of the polyethylene microplastic particles more effectively than those of UVO, irrespective of the ozone dosages.
1. IntroductionMicroplastics (MPs) in marine and freshwater environments have become a significant concern in pollution control and management due to their ubiquitousness. These MP particles can be involved in the natural ongoing processes, ultimately interrupting the natural balance of an ecosystem and potentially causing serious damage in terms of abnormalities in the reproduction patterns, growth rate, and food uptake of various species, including microorganisms [1, 2], plants [3, 4], and animals [5, 6]. Their adverse effects on an ecosystem have persuaded environmental scientists to examine the ongoing natural processes that alter the physicochemical properties of these tiny particles. Thus far, several efforts have been made to investigate the alterations in the surface characteristics of MPs as a result of the biological [7, 8], chemical, and photooxidation processes [9–11]. Despite these attempts, the data related to their alteration behaviors due to ongoing chemical processes in natural water environments is limited, warranting further research. In response to this, the use of ultra-violet (UV) irradiation and ozonation could be a potential candidate to utilize the reactive oxygen species (ROS) generated in natural chemical processes for the surficial alterations of MPs. These oxidation processes can alter the surface characteristics by introducing carbon-oxygen functionalities in a variety of polymers, such as polypropylene (PP), polyethylene terephthalate (PET), and polyethylene (PE). In addition, these processes are economical and eco-friendly [12–14].
The surficial oxidation of polymers under UV and ozonation mainly occurs due to the presence of ROS, which are generated by ozonolysis [15]. These ROS are responsible for the formation of carbon radicals through the abstraction of hydrogen atoms from the polymeric chain [16], eventually combining with the generated ROS to form OH groups. This process ultimately converts these groups to carbonyl groups, such as ketones and esters. Several studies have investigated the surficial changes in polymers due to UV and ozonation. For example, Walzak et al. [17] reported the surficial oxidation of PP and PET films after a UVO treatment. Murakami et al. [18] conducted a feasibility study of polystyrene (PS) oxidation under a UVO treatment, where they observed a comparable increase in functional groups on the surface of PS. Similarly, Yanagisawa et al. [13] reported the effects of different liquid solutions used as media on changing the surface properties of the polymers (i.e., PS) during UVO treatment. They observed the formation of carbonyl and hydroxyl groups using distilled water, amide, and amino groups in an ammonia solution as a medium. The studies above assessed the feasibility of UVO to investigate the surficial changes on polymers that co-exist in a natural environment.
This study examined the alterations on the surface of microplastics during their stay in an aquatic environment. A UVO treatment was used as a potential source of chemical species that are possibly generated in the environment and are responsible for altering the surface of microplastics. This study is the first to report the surface alterations relevant to the formation of functional groups on PE MP particles treated in a submerged UV and ozonation system under two different regimes: serial implementation of ozone followed by UV irradiations (O3+UV) and simultaneous application of UV and ozone (UVO) under four different ozone dosages (i.e., 4, 5, 6, and 7 mg/min) for 180 min. The surface changes were analyzed by Fourier transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and contact angle measurements.
2. Materials and Methods2.1. Experimental ProcedureThe UV and ozonation system used in this study (presented in Fig. S1) consisted of an ozone generation system together with a 24 W UV source lamp (Korex, Korea) with dimensions of 43.6 cm (length) and 1.5 cm (diameter) having a radiation intensity of 72 μm/cm2 at a wavelength of 254 nm. For the ozonation of PE MP particles, ozone was generated through dielectric barrier discharge (DBD) plasma [19] from the dry oxygen (Purity; 99.5–99.9%) fed at a pressure of 1 MPa into the ozone generator (Ozonetech, Korea). The ozone dosage was then adjusted to 4, 5, 6, or 7 mg/min, relevant to the readings of the ozone gas analyzer (H1 Series Ozone Analyzers, INUSA™, USA). The UV source provided photons in the oxidation of PE MP particles. For the treatment, a suspension of 1% of PE MP particles ([CH2CH2]n, the medium density of 0.94 g/mL at 25°C; melting point of 109–111°C, Aldrich Chemical Co., USA) in 1 L of distilled water was prepared (pH of 6.7 at 25°C). The suspension was then loaded into a 1 L plexiglass cylindrical reactor (Scott Duran, Germany), in which the reaction between the aqueous ozone and PE MP particles took place.
The effects of O3+UV and UVO on the surficial properties of PE MP particles were investigated by performing the procedure in two different regimes (i.e., O3+UV and UVO) with the other experimental conditions kept constant. The experiment for the O3+UV regime was conducted so that the particle suspension was treated under ozonation for 90 min followed by irradiation of the UV source (24 W lamp) in a submerged orientation for a further 90 min. In comparison, oxidation of the PE MP particles in the UVO system was carried out by the simultaneous application of the UV source and ozone in 1 L of the PE MP particles suspension for 180 min. Subsequently, the UV and ozone-treated particles under the given experimental conditions were filtered through a 0.45 μm cellulose acetate membrane filter (ADVANTEC®, Tokyo Roshi Kaisha Ltd., Japan) and dried overnight in an oven (Vision Scientific Co., Ltd., Korea) at 50ºC. The dried particles were stored in a desiccator for the FTIR, XPS, and contact angle analyses. Each set of experiments was performed in triplicate, and the data are expressed with the arithmetic means value with the standard deviation.
2.2. Fourier Transform Infrared (FTIR) SpectroscopyThe FTIR spectra of PE MP particles oxidized under O3+UV and UVO for four different ozone dosages were obtained by making a pellet by grinding a mixture of 100 mg of potassium bromide (KBr, FTIR grade, Merck, Germany) and 5 mg of these particles in an agate mortar followed by pelletizing with an Altas™ manual hydraulic press (SPECAC Inc., USA). The surface modification of the obtained pellets was analyzed by FTIR (VERTEX 80V, Bruker, Germany) spectroscopy over the wavenumber range, 4,000 cm−1 to 400 cm−1, with a resolution of 4 cm−1. The generated FTIR spectra for each pellet representing each experimental condition were corrected using the baseline method [20] and then normalized to the absorbance at 1,472 cm−1 (C=C, double bond stretching) because it was relatively unchanged compared to the virgin PE peak, despite being oxidized. The remaining peaks were changed after oxidation. The keto-carbonyl (KCBI), ester carbonyl (ECBI), ether (EI), and internal double bond/vinyl group indices (VBI) were determined with the absorbance at 1,716 cm−1, 1,720 cm−1, 1,130 cm−1, and 908 cm−1, respectively.
In addition, the FTIR spectra of the oxidized PE MP particles were used to estimate the extent of the changes in the crystalline phase, as suggested by Zerbi et al. [21]. Briefly, a comparison of the peak height at 730 cm−1 (corresponding to the crystalline content) was compared with that at 719 cm−1 (amorphous content) to estimate the crystalline phase content, as calculated by Eq. (1):
where I730 and I719 are the respective absorbance intensity of peaks at 730 cm−1 and 719 cm−1, respectively, in the normalized FTIR spectra; 1.233 is the theoretical intensity ratio,
, at a settling angle of 42º.
2.3. Contact Angle MeasurementThe contact angles of the oxidized PE MP particles in the O3+UV and UVO system under four different ozone dosages were measured by the sessile drop method using an optical contact angle goniometer (Surface Electro-Optics, Korea). For this, 0.1 g of oxidized MP particles was pre-flattened on a glass slide with dimensions of 76 × 26 × 1 mm (Superior Marienfield, Germany) and then compressed using another glass slide. Subsequently, a 5 μL deionized water droplet was deposited onto the pre-flattened MP. A digital image of the water droplet was captured when the droplet reached a steady state on the substrate. The same procedure was repeated three times for each sample to ensure reproducibility.
2.4. X-ray Photoelectron Spectroscopy (XPS)The XPS (K-alpha Thermo Scientific, USA) of MP samples were analyzed using a monochromatic AI-Kα source. From this, a low-resolution spectrum was obtained over the binding energy range from 0 to 1,200 eV with a resolution of 1.00 eV, after which the elemental composition and oxygen to carbon (O/C) ratio were determined. The high-resolution spectra for the C1s region (280–293 eV) and O1s (526–538 eV) were obtained with a resolution of 0.10 eV. They were deconvoluted into subpeaks to determine the types of carbon-oxygen functionalities that appeared on the PE surface using Origin software (OriginPro, version 9.0, USA).
3. Results and Discussion3.1. FT-IR Analysis of the PE MP ParticlesFor the raw PE MPs, the characteristic spectral peaks were observed at 719 cm−1 (C=C-H, stretching), 1,377 cm−1 (-O-H, stretching), 1,472 cm−1 (C=CH2, double bond stretching), 2,851 cm−1 (-CHO, stretching), 2,920 cm−1 (-CH3, stretching), and 3,447 cm−1 (O-H, stretching). On the other hand, the FTIR spectra from the treated PE MP particles under two different regimes (O3+UV and UVO) for the four different ozone dosages revealed a variety of new functional groups together with the main characteristic peaks possibly formed during the oxidation process under the given experimental conditions: 1,716 cm−1 (RC=O-R′), 1,720 cm−1 (RCOOR′), 1,130 cm−1 (R-O-R′), and 908 cm−1 (-CH2=CH2). The peak intensities appeared to increase with increasing ozone dosages (i.e., 6 mg/min versus 4 mg/min) irrespective of the regimes. Moreover, the ozone dose of 7 mg/min made a more prominent contribution to increasing the carbon-oxygen functionalities on the surface of the PE particles. The appearance of new functionalities on the surface of PE MPs compared to raw PE MPs particles was commonly observed, but the variations in their extent under the two regimes were different. That is, O3+UV appeared to be more effective under the given experimental conditions (ozone doses from 4 to 7 mg/min) compared to those of UVO. This means that ROS, including HO2−, ●O2−, ●OH and ●O2, formed due to the decomposition of ozone in water according to Eq. (2)–(5) [21–23].
These ROS oxidize the PE MP particles by attacking the polymeric chain structure [25], which leads to the formation of peroxyl radicals (Eq. (6)), and the generation of hydroperoxides (Eq. (7)) together with ketones and alcohols (Eq. (8)) [26]. Subsequently, an immediate exposure of ozonated PE MP particles to a UV source is likely to facilitate this ongoing oxidation process to attack the generated oxidized species, specifically promoting the generation of the surface functionalities, including C-O, carbonyl, and carboxyl groups, ultimately increasing the carbonyl and vinyl absorbance.
The UVO regime showed a significant difference in increasing the absorption peaks, referring to the enhanced surficial modification to that of the control (ozone-treated without UV irradiation) [27]. Conversely, the extent of surface oxidation of the PE MP particles was lower than that produced in the O3+UV system. This discrepancy was attributed to the type of reactions in the two regimes because ozone generated from UVO might be scavenged more promptly by UV irradiation. Hence, the presence of a UV source generating excessive chemical reactive species, as presented in Eq. (2)–(5), may play a further role in rapidly reproducing ozone, but which is instantly destroyed [17]. In the long run, this limited the contribution of ROS for the PE MP particles to be oxidized around the surficial area. Moreover, the distance of the particles apart from the UV source during the UVO treatment might be another contributing factor because the particles near the UV sources might be oxidized more readily than the particles residing far from the lamp. This might explain the difference in overall changes in the functionalities of the surface of the PE MP particles.
The extent of the variation in the degree of carbon-oxygen functional groups on the surface of the MP particles treated for a reaction time of 180 min at four different ozone dosages under two different regimes was examined by comparing the keto-carbonyl (KCBI), ester carbonyl (ECBI), ether (EI), and vinyl bond indices (VBI), as presented in Fig. 1. The KCBI and ECBI level showed the lowest levels for 4 mg/min of ozone, which increased gradually with increasing ozone dose up to 6 mg/min irrespective of the regimes. In contrast, the extent of the increase in EI and VBI, as reported elsewhere [23], were similar regardless of the regimes and ozone concentration. These functional groups referred to as the intermediates might have been generated to similar levels of amounts during the oxidation process, and eventually converted to carbonyl and carboxyl moieties, such as ketones, esters, and carboxylic acids.
The changes in the crystalline phase with respect to the control were also analyzed comparatively for the O3+UV and UVO modes. From Fig. 2, an increasing trend for both modes was observed according to the ozone doses, but their degrees of crystallinity differed according to the mode. Those of O3+UV were slightly higher than those of UVO, showing 0.2~1% more at all ozone dosages. The addition of 7 mg/min possibly resulted in more destruction of the crystalline forms than at lower dosages. Such higher crystallinity suggests that O3+UV had followed a Norrish Type II reaction, which was principally demonstrated by FTIR spectroscopy forming more carbonyl and vinyl groups, along with an increase in the crystalline phase fraction. UVO could make a lesser contribution to the change in crystalline properties. As described before, the ozone dosage for O3+UV could be more effective in the surficial oxidation of PE MP particles than UVO despite UV being a major determining parameter.
3.2. Contact Angle MeasurementThe contact angle (CA) was measured for the virgin PE MP particles and oxidized PE MP particles after treatment in two different regimes and at four different ozone concentrations, as listed in Table 1. The CA appeared to decrease by approximately 5º for O3+UV and 1º for UVO compared to the virgin PE MP particles, suggesting that the surface of the PE particles for the former tended to be more hydrophilic. For O3+UV implementation, there was a more abrupt decrease in contact angle with increasing ozone dosage from 4 to 7 mg/min with the lowest drop to 124.42º and the highest drop to 120.04º, respectively. Moreover, the reported results were comparable to those of virgin PE MP particles (i.e., 125.90º). In contrast, the UVO showed an overall slight decrease in CA, even though it was observed at the highest drop to 123.80º at 7 mg/min. This contrasted with the FTIR spectra showing that the absorbances for carbonyl and double bonds at 7 mg/min were lower than at 4 to 6 mg/min. In response, it would have expected a smaller drop of the CA. On the other hand, at the highest drop, the surface was more hydrophilic due to the difference in the target measuring points of CA around the uppermost surface layers [28], compared to the FTIR spectra, which measures to certain depths (not the surface) [29].
3.3. X-ray Photoelectron Spectroscopy (XPS) AnalysisThe extent of surface oxidation of PE MP particles after O3+UV and UVO under four different ozone dosages was compared by XPS in low and high-resolution scan modes. First, the low-resolution scan of the O3+UV and UVO treated PE particles was compared with their elemental composition and O/C ratio, revealing possible surface oxidation regardless of the type of regime and ozone dosage (Table S1). Carbon (C) and oxygen (O) were detected along with relatively small quantities of sodium (Na), nitrogen (N), and silicon (Si), which might have originated from the plastic additives used in the manufacturing process. The O/C ratio of the treated PE MP particles under O3+UV increased with increasing ozone dosage from 4 to 7 mg/min (Fig. 3), indicating that the surface oxidation of PE is related to the increased hydrophilicity. For the O3+UV regime, a slight increase in the O/C ratio was first observed under an ozone dosage of 4 to 5 mg/min, which jumped to 6 mg/min, ultimately reaching the highest level 0.043 at 7 mg/min. Nevertheless, the slope of O/C between 5 to 6 mg/min was higher at approximately +0.71 than between 6 to 7 mg/min (+0.05). This suggests that oxygen uptake by MP particles was slow at lower ozone dosages but accelerated at higher ozone dosages reaching a maximum and decreasing at higher doses, which might eventually penetrate deep into the bulk of the polymeric chain [23]. For UVO, the oxygen uptake gradient between 4 to 5 mg/min was +0.89, but was −0.38 between 5 to 6 mg/min, finally increasing to +0.24 between 6 to 7 mg/min. This suggests that under the UVO treatment, surface oxidation appeared to be greater at lower ozone dosages (4 and 5 mg/min) than at higher ozone dosages, meaning that the lower ozone dosage was more favorable to the formation of carbon-oxygen functionalities, as reported elsewhere [17].
For a detailed analysis of the changes that occurred on the surface of PE, high-resolution scans of C1s for both treatment modes, O3+UV and UVO, were compared under four different ozone doses. The peaks were deconvoluted into sub-peaks at 285 eV (C-C/C-H), 286.5 eV (C-OH), and 288 eV (C=O), as shown in Fig. 4 and Fig. 5. Fig. 4 shows that the O3+UV treatment of the PE MP particles showed different variations for carbon-oxygen functionalities depending on the four different ozone dosages. In other words, the particles treated with 4 mg/min ozone showed C-OH and C=O (Fig. 4(a)), which are considered ethers/esters and carbonyl groups, respectively [17]. The extent of their appearance increased with increasing ozone dosage of 5 mg/min (Fig. 4(b)). A larger amount of ROS and intermediates appeared by ozonation, after which they were exposed to the UV sources. This resulted in the attack of the polymeric chain to produce the high concentrations of those functionalized groups. The appearance of a new functional group of C-C=O was detected simultaneously, while C-OH and C=O groups were also observed at an ozone dosage of 6 mg/min (Fig. 4(c)). On the other hand, increasing the ozone dose to 7 mg/min resulted in mainly C-OH groups (Fig. 4(d)), with a concomitant decrease in the intensity of C-C peaks. Hence, at higher ozone dosage, the oxidized functionalities with a double bond could be decomposed further with carbons themselves to be more oxidized, which might start ROS penetrating the bulk of the polymeric chain. This was consistent with the investigations with the FTIR spectra and contact angles measurements, where a somewhat lower level of crystallinity and higher hydrophilicity were observed compared to those of lower dosages.
The UVO treatment showed very different intensities for the type of carbon-oxygen versus C-C bonded functionalities compared to O3+UV, as shown in Fig. 5. Therefore, ozone may produce a different pathway with different byproducts. Under the implementation of ozone first, the ozone is naturally decomposed in water, but the concomitant exposure to UV can result in faster ozone decomposition and regeneration through a photodecomposition phenomenon [17]. Hence, the ozone contribution in altering the surface properties of PE MP particles is unlikely to decline. In addition, the distance of the particles from the UV source could be another reason. The particles closer to the UV source might be oxidized more readily with the combined effect of ROS generated from ozone and photons than those further away, leading to the generation of a smaller number of C-OH groups. In other words, PE MP particles float on the surface of the water due to the low density, so particles near the UV source might be effectively oxidized compared to the particles further from the UV source. In the case of O3+UV, the particles can be oxidized by ozone and later by UV. Hence, there is no significant interference with UV, which quickly decomposes ozone when occurring in UVO. Therefore, distance had less significance in the oxidation process. With UVO, however, it was implemented simultaneously, so particles that are near the UV sources are oxidized by both UV and ozone (e.g., ROS). In contrast, particles further away from the UV source are not effectively oxidized.
Nevertheless, the effect of the ozone dosage can be determined for UVO. From Fig. 5, the presence of C-OH is commonly observed under the given experimental conditions. In contrast, the degree of the appearance of C-OH groups decreases with increasing ozone dosage. This means that a significantly lower ozone concentration under the UVO treatment can oxidize the surface of PE MP particles, which is in good agreement with the results discussed above. The carbonyl functionalities (C=O) also appeared in the case of 5 mg/min (Fig. 5(b)), meaning that it was expected to be the maximum oxidation that occurred in this experimental regime. On the other hand, the treatment time provided in this study might not have been sufficient to allow the molecules to fully penetrate the bulk polymer and initiate the chain scission of the PE MP particles.
The high-resolution scans of the O1s region were deconvoluted into three sub-peaks: 531 eV (OH), 533 eV (C-O/C=O), and 534.5 eV (O-C=O), as described in Fig. 6. The presence of OH groups and C-O/C=O were commonly observed under both O3+UV and UVO regimes under all applied ozone dosages. On the other hand, the degrees of their appearance for both regimes differed according to the experimental conditions. The carbon-oxygen for the O3+UV was readily converted to O-C=O when the ozone dosage was increased from 4 to 5 mg/min (Fig. 6(a) and (b)), while the extent of C-O/C=O groups declined. The degrees of all these oxidized functionalities were highest at an ozone dosage of 6 mg/min (Fig. 6(c)) and lower at 7 mg/min. Therefore, 6 mg/min of ozone might be the optimal ozone dosage for the maximal alteration of the surficial properties of PE MP particles. In comparison, the UVO showed that adding 4 mg/min (Fig. 6(e)) produced the maximal levels of the carbon-oxygen functionality in terms of OH and C-O/C=O groups, but an increase from 5 to 7 mg/min could result in their disappearance.
As a consequence, the generation of functional groups on the surface of PE MP particles was verified by XPS. PE MP particles were oxidized under the application of two different regimes (i.e., O3+UV and UVO), but the extent of the oxidation of these carbon-oxygen functionalities was different. Furthermore, surface changes that appeared due to the O3+UV and UVO treatment systems might have occurred only in the amorphous region. In contrast, it could not further penetrate the bulk of the polymer sufficiently to alter the polymeric chain for a prolonged duration.
4. ConclusionsThe study examined the effects of UV and ozone on the surficial chemistry of PE MP particles. The particles were treated in suspension for two different regimes (O3+UV and UVO) at four different ozone dosages (4, 5, 6, or 7 mg/min) for the oxidation of 180 min. The possible surficial changes were monitored by FT-IR spectroscopy and XPS. The contact angle was also measured to determine the change in hydrophilicity.
From the FTIR spectra, O3+UV was more likely to be effective with regard to the generation of a variety of functional groups with the highest appearance for 6 mg/min ozone, which could be regarded as the optimized dosage. The concurrent application of UVO showed similar byproducts, but the extent of the surficial oxidation was slightly lower than those of O3+UV.
The contact angles showed the highest decline at an ozone dosage of 7 mg/min showing a CA of 120.04º, whereas the lowest drop to 124.42º at 4 mg/min under the O3+UV regime. In contrast, for the UVO treatment, there was a slight decrease in CA with the lowest drop to 124.77º at 4 mg/min and the highest drop to 123.80º at 7 mg/min compared to the untreated sample (i.e., 125.90º).
XPS revealed C-O and OH groups on the surface of the treated PE MP particles regardless of the types of regimes and ozone concentration. Furthermore, the greatest extent of oxidation on the surface of the PE MP particles was evident at an ozone dosage of 6 mg/min under O3+UV, while increasing to 7 mg/min could reduce the intensity of C-C groups, which could be attributed to the penetration of ROS into the bulk of the polymer.
In conclusion, the serial implementation of ozone resulted in more oxidation of PE MP particles. On the other hand, more study is needed to obtain evidence related to the chain scission or bulk penetration.
AcknowledgmentThis research was a part of the project titled “Development of a water treatment system to remove harmful substances from ecological disturbances emitted from quarantine stations such as imported fishery products (No. 20180341)” supported by the Korea Institute of Marine Science and Technology Promotion and funded by the Ministry of Oceans and Fisheries (MOF) and partially supported by Inha University.
NotesAuthor Contributions R.Z. (M.S. student) performed conceptualization, methodology, formal analysis and investigation, and wrote the original draft preparation. S.Y.P. (Ph.D. student) wrote review and edited the manuscript. C.G.K (Professor) supervised and approved all the experimental results and modified the manuscript. References1. Sun X, Chen B, Li Q, et al. Toxicities of polystyrene nano-and microplastics toward marine bacterium Halomonas alkaliphila. Sci Total Environ. 2018;642:1378–1385.
2. Nomura T, Tani S, Yamamoto M, et al. Cytotoxicity and colloidal behavior of polystyrene latex nanoparticles toward filamentous fungi in isotonic solutions. Chemosphere. 2016;149:84–90.
3. Bhattacharya P, Lin S, Turner JP, Ke PC. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem C. 2010;114:16556–16561.
4. Kalčíková G, Gotvajn AŽ, Kladnik A, Jemec A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ Pollut. 2017;230:1108–1115.
5. Fossi MC, Panti C, Guerranti C, et al. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar Pollut Bull. 2012;64:2374–2379.
6. Ziková A, Lorenz C, Hoffmann F, et al. Endocrine disruption by environmental gestagens in amphibians-A short review supported by new in vitro data using gonads of Xenopus laevis. Chemosphere. 2017;181:74–82.
7. Park SY, Kim CG. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere. 2019;222:527–533.
8. Yang J, Yang Y, Wu WM, Zhao J, Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol. 2014;48:13776–13784.
9. La Mantia FP, Ascione L, Mistretta MC, Rapisarda M, Rizzarelli P. Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers. 2020;12:753.
10. Liu P, Qian L, Wang H, et al. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ Sci Technol. 2019;53:3579–3588.
11. Ariza-Tarazona MC, Villarreal-Chiu JF, Barbieri V, Siligardi C, Cedillo-González EI. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram Int. 2019;45:9618–9624.
12. Strobel M, Walzak MJ, Hill JM, Lin A, Karbashewski E, Lyons CS. A comparison of gas-phase methods of modifying polymer surfaces. J Adhes Sci Technol. 1995;9:365–383.
13. Yanagisawa K, Murakami TN, Tokuoka Y, Ochiai A, Takahashi M, Kawashima N. Immobilization and enzymatic activity of glucose oxidase on polystyrene surface modified with ozone aeration and UV irradiation in distilled water and/or aqueous ammonia solution. Colloids Surfaces B Biointerfaces. 2006;48:67–71.
14. Santana HS, Silva JL, Aghel B, Ortega-Casanova J. Review on microfluidic device applications for fluids separation and water treatment processes. SN Appl Sci. 2020;2:395.
15. Hidetoshi K, Tekayuki K, Takaomi K. Surface modification of polymers by thermal ozone treatments. Adv Technol Mater Mater Process J. 2007;9:91–98.
16. Yousif E, Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus. 2013;2:1–32.
17. Walzak MJ, Flynn S, Foerch R, et al. UV and ozone treatment of polypropylene and poly (ethylene terephthalate). J Adhes Sci Technol. 1995;9:1229–1248.
18. Murakami TN, Fukushima Y, Hirano Y, Tokuoka Y, Takahashi M, Kawashima N. Surface modification of polystyrene and poly (methyl methacrylate) by active oxygen treatment. Colloids Surfaces B Biointerfaces. 2003;29:171–179.
19. Liu CZ, Wu JQ, Ren LQ, et al. Comparative study on the effect of RF and DBD plasma treatment on PTFE surface modification. Mater Chem Phys. 2004;85:340–346.
20. Stark NM, Matuana LM. Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym Degrad Stab. 2004;86:1–9.
21. Zerbi G, Gallino G, Del Fanti N, Baini L. Structural depth profiling in polyethylene films by multiple internal reflection infra- red spectroscopy. Polymer (Guildf). 1989;30:2324–2327.
22. Chtourou H, Riedl B, Kokta BV. Surface modification of polyethylene pulp fiber by ozone treatment. An analytical and thermal characterization. Polym Degrad Stab. 1994;43:149–156.
23. Peeling J, Clark DT. Surface ozonation and photooxidation of polyethylene film. J Polym Sci Polym Chem Ed. 1983;21:2047–2055.
24. Kefeli AA, Razumovskii SD, Zaikov GY. Interaction of polyethylene with ozone. Polym Sci USSR. 1971;13:904–911.
25. Staehelin J, Hoigne J. Decomposition of ozone in water: rate of initiation by hydroxide ions and hydrogen peroxide. Environ Sci Technol. 1982;16:676–681.
26. Yamauchi J, Yamaoka A, Ikemoto K, Matsui T. Reaction mechanism for ozone oxidation of polyethylene as studied by ESR and IR spectroscopies. Bull Chem Soc Jpn. 1991;64:1173–1177.
27. Zafar R, Park SY, Kim CG. Surface modification of polyethylene microplastic particles during the aqueous-phase ozonation process. Environ Eng Res. 2021;26:200412.
Fig. 1Four different intensities relevant to carbonyl and vinyl species formed during O3+UV and UVO treatment at varying ozone dosages of (a) 4, (b) 5, (c) 6, and (d) 7 mg/min in comparison to the control (PE MP particles ozonated in the same manner except for the application of the UV source). 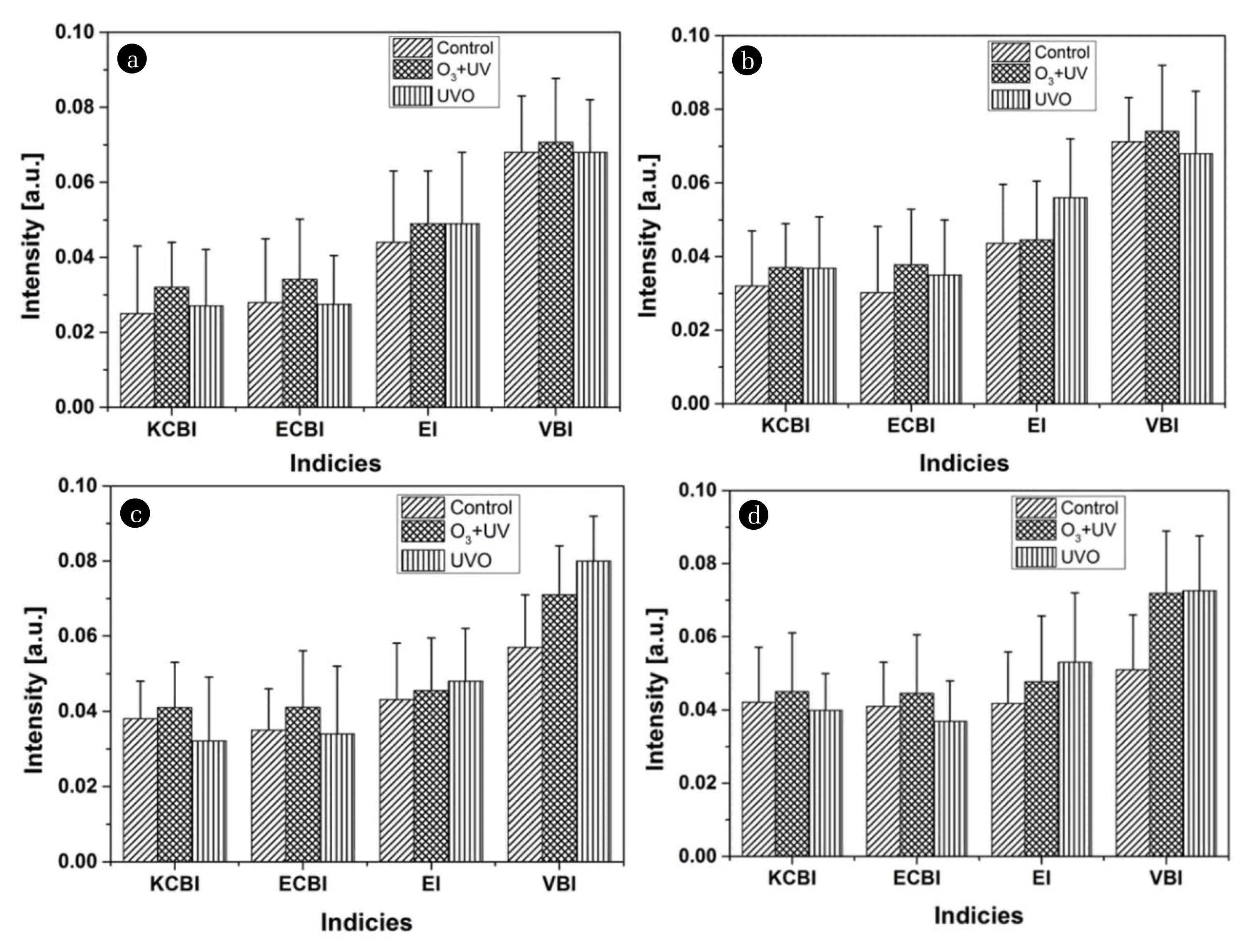
Fig. 2Changes in the crystallinity of PE MP particles treated with O3+UV and UVO at ozone dosage of 4, 5, 6, and 7 mg/min. 
Fig. 3O/C ratio of PE MP particles treated with O3+UV and UVO at varying ozone dosages: 4, 5, 6, and 7 mg/min. 
Fig. 4Deconvoluted peaks of high-resolution spectra of C1s into C-C, C-OH, and C=O for the PE MP particles treated under O3+UV system at varying ozone concentrations (a) 4, (b) 5, (c) 6, and (d) 7 mg/min. 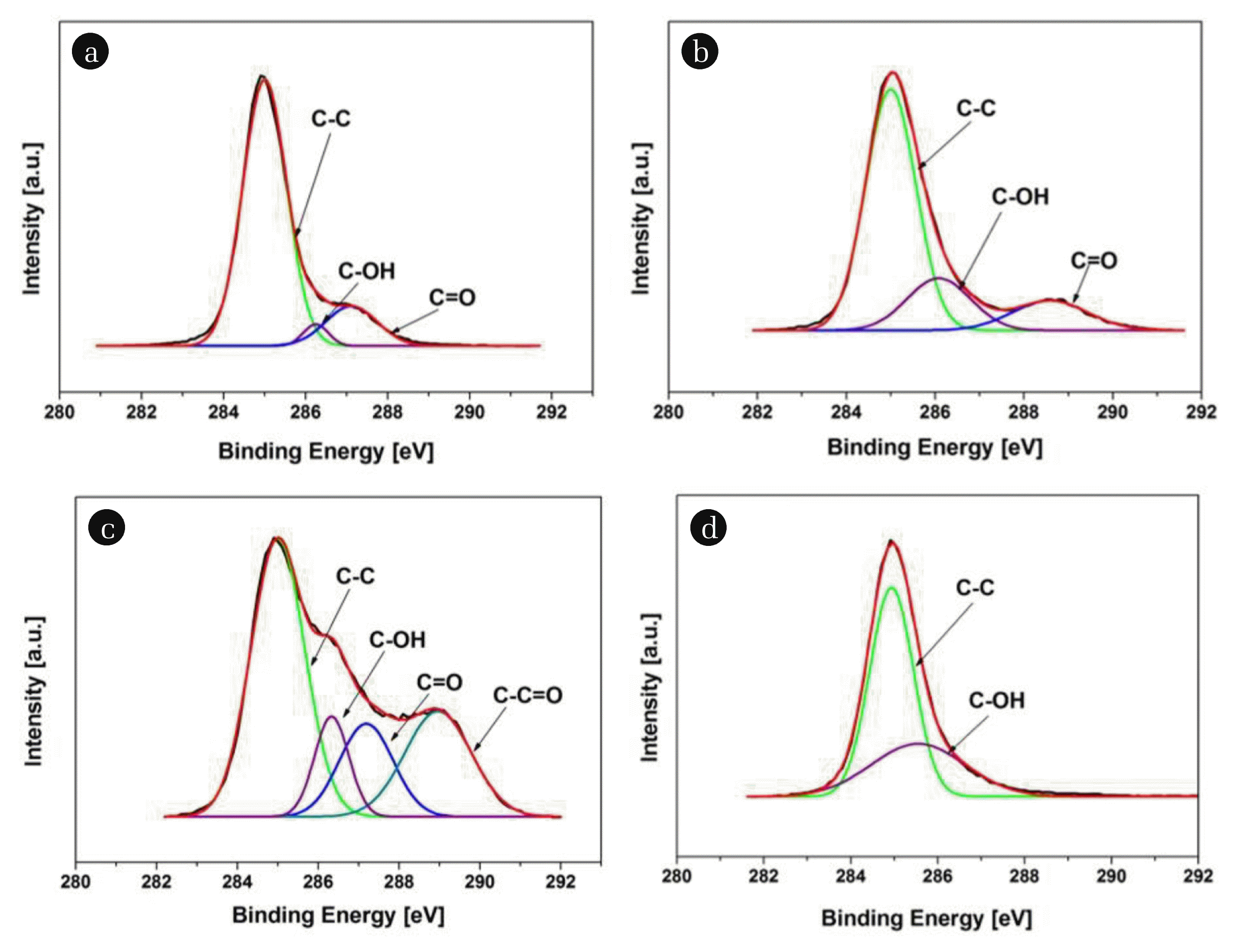
Fig. 5Deconvoluted peaks of high-resolution spectra of C1s into C-C, C-OH, and C=O for the PE MP particles treated under the UVO system for ozone dosages of (a) 4, (b) 5, (c) 6, and (d) 7 mg/min. 
|
|
||||||||||||||||||||||||||||||||||||||||