AbstractMost of the photocatalytic studies for pollutant degradation are based on optimizing a single parameter that results in a non-linear relationship between the overall parameters and the photo-degradation reactions. To address this critical problem, herein, we report the use of Response Surface Methodology based on the Box-Behnken Design (BBD) for modeling the photocatalysis degradation of Malachite Green (MG) dye using nano TiO2 as photocatalyst. The catalyst characterizations are carried out using XRD, SEM, and TEM, indicating that the TiO2 prepared by sol-gel synthesis possesses Anatase phase with particles in the nano regime and porous surface morphology. The optimum operating conditions for degradation of MG was identified by the interactive effects of variable factors such as initial dye concentration 10–30 ppm (x1), catalyst dosage 1–3 mg (x2), contact time 20–60 min (x3) using the Box-Behnken method. Furthermore, the degradation reactions are also evaluated by Artificial Neural Networks (ANN). Their predicted results have been validated by the experimental studies and found to be acceptable. Their optimal results to achieve 90% degradation efficiency at TiO2 nanoparticle dosage (3 mg), reaction time (60 min), and initial dye concentration (20 ppm) have been validated by the experimental studies and found to be acceptable.
1. IntroductionContaminants like heavy metal ions, surfactants, fertilizers, pesticides, and various toxic organic dyes by agriculture, industries, and human activities bring undesirable changes into air, water, and soil leads to environmental pollution [1]. In developing countries, growing population and industrial development demand water supply, but their activities produce a large amount of wastewater, disposed to natural water bodies without treatment causing water pollution. In recent years organic pollutants like dyes, released through wastewater from various industries into natural water bodies (to the environment), became a serious health problem. These dyes are toxic to human beings and aquatic life [2]. Malachite Green (MG) is used as a colorant in various textile industries. It is also used as a food additive, food coloring agent, medical disinfectant, and so on; it is highly carcinogenic and genotoxic. Nowadays, MG dye became a controversial pollutant due to its risk to the immune system [3]. Among various oxidation methods used, photocatalytic oxidation using semiconductor photocatalysts was found to be efficient for environmental applications like water purification and water disinfection [4]. Removal of pollutants along with mineralization can be done by photocatalysis and is more advantageous than other conventional methods [5], such as adsorption, precipitation, co-precipitation, oxidation, reduction, aerobic, anaerobic, and biological treatment methods, because of their inherent limitations like less efficiency, time consumption and the formation of secondary sludge, the disposal of the same is uneconomical.
A review of various reports suggests that the nanomaterials are efficient photocatalysts because of their smaller size and the larger surface area or because of quantum confinement effects of charge carriers for the pollutant degradation [6]. Over the last few decades, the researcher’s attention is to use nano metal oxide semiconductors for photocatalysis. Among various metal oxide semiconductor photocatalysts, ZnO and TiO2 have proven to be the most suitable photocatalysts. TiO2 has been popularized as a photocatalyst because of its long-term stability, high oxidative properties, and environmental friendliness. However, other semiconductor oxides and sulphide catalysts such as Fe2O3, ZrO2, Cr2O3, ZnS, CdS have also used to degrade several contaminants in the presence of UV/Visible light [7]. Photocatalysis has the appeal of being “green” since it only uses light photons to eliminate toxic organic chemicals [8, 9].
Increasing literature in the field of chemistry explaining the use of Artificial Neural Networks (ANN) has evolved for a various range of applications such as Environmental Engineering, Textile Dye Degradation, Photocatalysis, Simulation and Modelling methods, Wastewater Quality Monitoring, Prediction of results (Water Quality Index), Design of reactors and many more. Because ANNs are sets of essential functions, they can deliver better empirical models of complex nonlinear processes that are advantageous for a wide variety of purposes. Pirdashti M. et al. reviewed a variety of ANN applications in chemical engineering. It dealt with the critical aspects of the ANN topology, modelling strategy, the methods of developing and training the data [10]. A systematic classification arrangement is also presented, which reveals, categorizes, and concludes the present works connected to ANN methodologies as well as applications. Theory of neural computing and its applications in the area of chemical science are reported elsewhere [11].
However, an attempt has been made to prent a quick review of the few related articles in this section. In large industrial reactor systems, corrupted data is inevitable. In such cases, different techniques can also be implemented to restructure the lost data. In this regard and in contrast to neural network modelling, Piagram et al. [12] discovered that the interpolated and moving average value methods provided the adequate estimates than the more commonly used median and mean replacement methods. From the context of analytical calibration, Miller J.N. discussed fundamental statistical approaches for analytical chemistry [13] which gives detailed information in the area of curve fitting and linear regression, which helps to understand the basics of equation modelled in this work (Eq. (2) and (5)).
It is challenging to model simulations by adopting conventional mathematical approaches as several factors influence them. Issues of simulation, Modelling and the importance of ANN as a solution are expanding because of their simplicity in Modeling of process performance, simulation and prediction [14]. Mohammadi et al. [15] used ANNs to model and predict the decolourization of dyes like methyl orange (MO) and methylene blue (MB) by Sn/Zn-TiO2 nanoparticles synthesized by the sol-gel method. The forecasted results of this model were found to be in good agreement with the experimental results. Response Surface Methodology was implemented using Central Composite Design for malachite green removal [16], but very few studies were done with Box-Behnken Design (BBD). The BBD offers optimal results with few experimental runs when compared with other response surface methodology techniques [17].
In the present study use of sol-gel synthesized TiO2 as photocatalyst for the malachite green degradation is explored. To overcome the drawbacks of conventional one parameter at a time approach, which is time consuming, multi parameter variation at a time has been explored by using BBD to achieve the maximum % dye degradation with few experiments. This approach also helps to understand the influence of interactive effects of the two parameters on the Photodegradation of malachite green dye by TiO2 nanoparticles. Additionally, the BBD and the ANNs techniques were validated with statistically optimized experimental results and comparison between the two techniques’ ability to predict the results are discussed.
2. Experimental2.1. Sol Gel Synthesis of TiO2 NanoparticlesSol-gel method is employed to synthesize TiO2 Nanoparticles (NPs). Briefly, 5 mL each of absolute ethanol and glacial acetic acid were mixed well. The mixture was added dropwise to 10 mL of titanium (iv) isopropoxide with continuous magnetic stirring for 30 min and left undisturbed for 5 h. After this aging period a pale-yellow transparent gel was formed. The obtained gel was dried over-night at 70°C in hot air oven to form white powder. Then, the resulting powder upon grinding, an ultra-fine white powder was formed. Finally, the resulting powder was calcined at 450°C for 2 h to increase the crystallinity of the as-formed sample.
2.2. CharacterizationThe crystallite size and phase composition of synthesized TiO2 nanoparticles were determined by X-ray diffraction (Panalytical X’pert-Pro powder diffractometer). The morphological feature and microstructure of nano TiO2 were inspected by scanning electron microscopy (JEOL (JSM-840A)) and transmission electron microscopy (Hitachi H-8100 (LaB6 filament, accelerating voltage up to 200 kV)). UV Visible spectral data and band-gap energy was determined by using a UV-Visible spectrometer (Specord 250 plus, Germany). Elemental composition was detected by an energy dispersive spectrometer (EDS; Keney Sigma TM Quasar, USA)).
2.3. Photocatalytic ExperimentsPhotodegradation of malachite green dye of chemical formula [C6H5C (C6H4N(CH3)2)2]Cl-4, IUPAC name [ (4 – dimethylamino phenyl) phenyl methyl]-N.N-dimethyl aniline was carried out by using the synthesized TiO2 NPs under UV light. A stock solution (10 ppm) was prepared by dissolving malachite green in double distilled water to investigate the degradation efficiency. For each experiment, 10 mL MG solution of 10 ppm concentration was used by varying the parameters like initial dye concentration (20 ppm, 30 ppm and 40 ppm), catalyst dosage, (1 mg, 2 mg, 3 mg), time (20 min, 40 min and 60 min). The solution was stirred well at 300 ± 10 rpm under UV light of distance 10 cm and intensity 250 watts. All the reactions were carried out at constant temperature (2 5± 1°C). After the reaction time, the supernatant liquid was taken out, and the changes in the dye concentration were evaluated by using UV-Vis spectrophotometer (Shimadzu UV-2600) by monitoring absorbance at 617 nm (λmax). The efficiency of photocatalytic degradation of MG dye was calculated by the equation:
In the above equation, Co is initial dye concentration and C is the final dye concentration after photo degradation.
3. Modelling Tools for Design of Experiments & ANNMany researchers are using many techniques and tools for Design of Experiments such as Central Composite Design, BBD, etc., In the present work, the combination of RSM with BBD has been used. For further optimalization of RSM results Artificial Intelligence (AI) is introduced in the present study to predict the results in and around the experimental boundary.
3.1. Modeling Using BBDBBD with three factors (3 independent variables) each at three levels ( −1, 0, 1), one dependent variable, one block with three replicates at the centre points gives 15 sets of experiments. They are used for the modelling of operational parameters for degradation of MG dye at different initial dye concentration (10, 20 and 30 ppm), catalyst dosage (1, 2 and 3 mg) and time (20, 40 and 60 min). The context of design should be sufficient to fit all requirements such as one squared term, a linear term, a product of two variables and an intercept to fit a quadratic model.
To study the effect of different variables towards their responses and the subsequent optimization studies, BBD has been used. To determine the relationship between the factors and the response variables, the collected data were analyzed by the statistical manner utilizing regression. A regression design is usually employed to model the response as a mathematical function, as shown in Eq. (2):
Response function (2nd order polynomial) was estimated by performing regression analysis, representing the linear, quadratic as well as cross-product response of variables x1, x2, x3 on response. Where β0, β1, β2, β3 are the coefficients estimated from regression and Y is the predicted dependent variable. The analysis was done using Statsoft Inc., Tulsa, USA (Version 6).
3.2. Modelling Using ANNANN is inspired by the working principle of neurons present in the human brain. ANN simulates the brain learning process by using probabilistic mathematical models. ANN operates directly on input-output data [18], and Matlab 2020b (Mathworks, USA) consists of various functions to implement artificial neural network architecture.
Neural network architecture consists of an input layer, one or more hidden layers and an output layer. The neurons in the hidden layer are linked to the input and output layers. The input data fed to neural network architecture is divided into a training set and a testing set. The most common training algorithm used to process ANN is the back-propagation algorithm. In this training algorithm, the error between the output neurons and the actual output (experimental data) of the training set were calculated and propagated backwards until it minimizes the error. After modeling the training data using ANN, the testing data set is used to verify the accuracy of the predictions and Multiple Coefficient of Regression (R2) was calculated. The neural network model is considered as an objective function for optimization [18]. The DIRECT optimization algorithm adopted by Ravi et al. [19] was used for optimizing Malachite green degradation. TOMLAB Optimization Inc, Sweden developed the MATLAB code for implementing DIRECT Algorithm (Global Optimization Technique).
4. Results and Discussion4.1. X-ray Diffraction
Fig. 1. Shows the X-ray diffraction pattern of nano TiO2 prepared by sol-gel method. All the diffraction peaks observed are highly crystalline and can be readily indexed to anatase phase TiO2. The diffraction peaks identified for the anatase phase is in accordance with the standard JCPDS card no. 21-1272. No other diffraction peaks other than those corresponding to TiO2 are observed indicating the phase purity of the sample prepared. The broader diffraction peaks indicate the smaller crystallite size of the catalyst. Further, the crystallite size of the sample was calculated by peak broadening calculations using Scherrer’s formula
Where λ is the X-Ray wavelength, β is the full width at half maxima, θ is Bragg’s angle and K is the shape factor (constant). Its value is 0.9 (for spherical shaped particles). The average crystallite size of TiO2 is calculated and is found to be ~11 nm. The peaks intensity confirms highly crystalline nature of TiO2.
4.2. Microstructural and Optical CharacterizationsMorphology of synthesized TiO2 nanoparticles was studied by using a scanning electron microscope (SEM), and it is shown in Fig. 2(a). The surface structure of the catalyst was found to be agglomerated mass of particles with varying size of agglomeration. Careful observation of SEM micrographs reveals the porous agglomerated clusters with fine voids on the structure. In order to evaluate the size of the particles, transmission electron micrographs are recorded and are shown in Fig. 2(b)–(c). From TEM images, it can be inferred that the particles are nearly spherical and a few particles are irregular in shape. However, irrespective of the shape of the agglomeration, the particles can be seen with particles connected to each other. Agglomerations of particles are of typical nano-sized particles in order to decrease the high surface energy. The particle size from the TEM micrographs was calculated to be in the range of 30–40 nm. Furthermore, the HR-TEM image (Fig. 2(c)) shows clear lattice fringes with well observable atomic planes. The d-spacing from the HR-TEM was calculated to be 0.356 nm and corresponds to (101) plane of anatase TiO2. The TEM and HR-TEM results are in accordance with the PXRD and SEM results.
UV–Visible absorption spectra of Sol-gel derived TiO2 nanoparticles are depicted in Fig. 3(a). The absorbance spectrum of the sample reveals an absorption band in the UV region ranging from 270–390 nm. Further Wood and Tauc relation (Eq. (4)) is used to determine the optical energy gap of the prepared samples.
Where a, h, n, Eg and k represent the absorption coefficient, Planck’s constant, frequency, optical energy band gap and constant (k = 1/2, 2 and 3/2 for direct, indirect and direct -indirect forbidden transitions), respectively. The optical band gap of sol-gel derived TiO2 nano particles is estimated by plotting (ahn)2 Vs hn (Fig. 3(b)). It can be observed from Tauc plot that the optical energy band gap value is found to be 3.1 eV and matches with the reported values for anatase TiO2.
5. Photocatalytic StudiesAs a preliminary study to know the approximate range of parameters (which is required for DOE) the experiments have been conducted by one parameter at a time approach. Influence of each parameter on dye degradation is discussed in section 5.1 to 5.3 and the interactive effects of the parameters (by RSM) on dye degradation are discussed in section 6.1 to 6.3.
5.1. Effect of Catalysts DosageTo detect the effect of catalyst dosage, the repeated experiments were conducted under UV light. The amount of the TiO2 catalyst ranging from 1 to 6 mg at constant dye volume (10 mL solution of 10 ppm) at natural pH (5.16) with mixing speed of 300 ± 10 rpm has been taken. Initial and final concentrations of dye have been determined by using UV/visible spectrometer. From Fig. 4(a) it can be seen that as the amount of catalyst increased the percentage of dye degradation also increased. The increase is found up to 3 mg beyond which there is little degradation. This can be attributed to the increased active sites of the catalyst with a high dosage. However, further increased dosage of the catalyst did not show effective degradation. The reasons are, the deposition of dye on the catalyst and the dye decomposition rate by the catalyst are influenced by several active sites and the absorption of photons by the catalysts [20, 21]. High catalyst loading enhances the rate of generation of electron-hole pairs and thereby increases the degradation rate. However, the addition of high dosage decreases the penetration of light by photocatalysts suspension. High catalyst dosage can also not affect degradation rate because of a smaller number of dye molecules to react.
5.2. Effect of Time
Fig. 4(b) illustrating the effect of contact time on the degradation of MG dye by TiO2. The experiments were conducted under UV light at different contact time intervals (10 min to 100 min at the step size of 10) by keeping catalyst amount constant, dye concentration and pH constant. As time increased the percentage of dye degradation also increased to the maximum extent, after that it remains almost constant. This is because, as time increases the contact time between the molecules of dye and catalysts also increases which enhances the degradation rate. The availability of active sites of the catalyst will be initially more for the dye molecules to react; after some time, the catalyst surface becomes saturated due to the deposition of more and more dye molecules. So, the increased time shows no effect on dye degradation [22].
5.3. Effect of Initial Dye Concentration
Fig. 4(c) shows the influence of different dye concentration on the percentage dye degradation. The experiments were conducted under UV light at different initial dye concentration (10 ppm to 50 ppm at the interval of 10 ppm) by keeping other parameters constant. From Fig. 4(c) it is clear that the increased initial dye concentration decreased the rate of dye degradation. Increased dye concentration enhances the colour intensity and thereby screens the light penetration into the solution, which leads to the less availability of photons for the degradation reaction. Another reason is that some of the photons were absorbed by the many molecules of MG dye itself and thereby reduces the availability of photons to the catalyst surface. This leads to the reduction in the amount of excited TiO2 and holes also. So lesser number of holes enters the dye solution and reacts with the hydroxide ions and gives hydroxyl radicals, the primary and vital reactive oxygen species for the photo-oxidation of dye molecules [23].
6. Mathematical Function using Design of Experiments and Response Surface MethodThe experimental data were analysed using multiple regression (statistical method) in order to find the relationship between the factors and response variables. The obtained response values produced by BBD were fitted in different models such as linear, square, 2-way interaction. The regression Eq. (5) obtained is as follows:
The linear terms of x1, x3 showed positive values and also shown a synergistic effect on the response, x2 is negative value while the squared term x12 shows the negative value and antagonistic effect, while x22, x32 are positive values. The interaction terms x1x3, x2x3 were found to have an adverse effect on the response, whereas the interaction effect of x1x2 was positive.
Analysis of variance (ANOVA) results suggest that the model was of significant importance (Table 1) as per F-test with the low p- values < 0.05, and significant R2 value 0.95322. The experimental and the prediction of response values are also connecting, as shown in Table 2, which confirms statistical validation of experimental data.
6.1. Interactive Effects of Initial Dye Concentration and Catalyst Dosage
Fig. 5(a), (b) and (c) represents contour plots to verify the combined effects of two factors by keeping other factors as constant. Fig 5(a) shows the effect of initial dye concentration and catalyst dosage on % degradation. The degradation percentage increases as an increase in the dosage of the catalyst at optimal initial dye concentration around 20 ppm, as shown in Fig. 5(a). This can be attributed to the increased number of available dye molecules to react with catalyst. However, after optimum value (20 ppm) the increased dye concentration leads to the increase in the intensity of molecules which covers the outer surface of the reaction mixture and thereby scatters the light which in turn decreases the penetration of the sufficient photons into the solution, which leads to the decrease in the dye degradation rate [24].
Similarly, as the amount of catalyst increases the number of active sites over the catalyst surface available for the dye molecules to react increases and hence enhances the degradation rate. After optimum range (3 mg) in the Fig. 5(a), the catalyst molecules aggregate themselves and thereby shields the photons penetration to reach the dye molecules. This leads to less interaction between the molecules of the dye and active sites of catalyst surfaces [25].
6.2. Interactive Effect of Contact Time and Initial Dye Concentration
Fig. 5(b) represents the effect of irradiation time and initial dye concentration on percentage dye degradation. At 20 ppm of initial dye concentration, the percentage of dye degradation is also increasing as time increases. the dye degradation increased by extending the time up to 60 min this is because as the irradiation time increases, the number of photons absorbed by the catalyst surface increases, this, in turn, enhances the reactive oxygen species responsible for photodegradation and hence leads to more significant dye degradation [26]. Besides, the available time for the interaction between the dye molecules and the active oxygen species over the catalyst surface increases leads to more significant degradation. Suppose the initial dye concentration crosses some optimal range 20 ppm in the Fig. 5(b) the degradation rate decreases even as the time increases. In that case, this is because the increased dye concentration enhances the colour intensity and there by shields the light penetration into the solution and the less availability of photons for the degradation to occur.
6.3. Interactive Effects of Contact Time and Catalyst Dosage
Fig. 5(c) represents the influence of irradiation time and catalyst dosage on percentage dye degradation. The highest percentage of dye degradation was observed at the catalyst amount of 3 mg in Fig 5(c). It can be attributed to the increased number of active sites on the TiO2 surface, which increases the highly reactive oxygen species like hydroxide and superoxide radicals and hence more degradation. After a certain amount (saturation amount), the available dye molecules are insufficient to fill all the active sites of the catalyst. Besides, more amount of the catalyst leads to the particles aggregation and thereby leads to the light scattering, which reduces the number of available photons for the photodegradation, hence lesser dye degradation [27]. The irradiation time is another crucial factor which influences the photodegradation, as the irradiation time increases the number of photons available for the reaction increases. Another reason is that, the increased time increases the interaction time required for the dye molecules, and the catalyst molecules increases and hence enhances the reaction. After an optimum time, it does not affect photodegradation reaction because all the active sites of the catalyst surface become saturated due to the deposition of a more significant number of dye molecules. Hence further increase in time does not affect dye degradation [28].
This analysis showed that BBD could optimize the process of degradation; however, further to improve the data obtained from the second-order polynomial Eq. (5) obtained from BBD, RSM and the ANN have been used.
7. ANN ApproachFurther modeling and optimization of data which is generated by second order polynomial Eq. (5) was done by using ANN and DIRECT Algorithm [19] to determine the better multiple coefficients. the details such as Input layer, two Hidden layers (6 neurons in each layers), and output layer of ANN shown in Fig. 6. Ravi et al. [19] have suggested Eq. (6), which was used for computation of weights and bias using neural network. The weights and bias are used for optimization using DIRECT Algorithm suggested by Jones et al. [29], at maximum R2 value.
The above equation was used into DIRECT algorithm for optimization [30]. Here weights are represented as w1 and w2, biases as b1 and b2. The predicted value from the neural network as ‘y’, row vector xv1 represent the transpose of the vector with a dimension of (3x1).
The experimental and predicted values are listed in Table 2. When compared the values generated from the ANN model with the BBD, the predicted number of values from ANN model are closer to the experimental values than BBD. The comparison of the predicted values of BBD and ANN with the regression line can be seen in Fig. 7. It is clear that the ANN is a more efficient model and precisely estimated the experimental values. Similar studies in literature also proved the better performance of ANN when compared with BBD approach using RSM [30]. According to the BBD, the maximum achievable percentage degradation is 88.61 (R2 value 0.95322) and with further optimization using ANN and DIRECT algorithm [29] the optimal value yield from ANN found to be 88.8622 (R2 value 0.97073) for maximum dye degradation. As a part of testing the model, we conducted experiments at the final optimized conditions; we could able to achieve 89% dye degradation. The difference of optimum value predicted from optimum conditions of ANN (using Eq. (6)) and the computed optimum value from the RSM for optimum parameters of ANN is 0.1378 which is less than the error values from the repeatability of the experiment, hence the validity of the model.
8. ConclusionsIn summary, the work demonstrates the potential use of TiO2 as photocatalyst for the degradation of Malachite green dye. The powder X-ray diffraction studies showed that the sol-gel derived nano TiO2 resulted in Anatase phase without impurities. Scanning electron micrographs and Transmission electron microscopic images revealed an agglomerated cluster of particles and nano size of the TiO2. RSM was effectively carried out for the optimization of photocatalytic experimental parameters based on BBD model and ANN model. Statistical based analysis using Box-Behnken methodology and the ANN model proposed successfully predicted the malachite green dye degradation using TiO2 as photocatalyst. The optimum parameters for the maximum degradation efficiency (90%) were found to be TiO2 nanoparticle dosage 3 mg; reaction time 60 min; and under the exposer to UV light irradiation for 20 ppm of initial dye concentration. The predicted values form the BBD and ANN models excellently matched with these values. Hence, these results show that the optimization using Box-Behnken approach and ANN approach are excellent tools for evaluating the optimal conditions for pollutant degradation and in this case, ANN model was found to be superior to BBD model.
NotesAuthor Contributions C.K.C. (Ph.D. student) conducted all experimental work and prepared original manuscript draft, T.N.P. (Professor) supervised the research, initial plan and revised the manuscript, R.R.S.K. (Assistant professor) contributed in conceptualization and revision. R.H.K. (Assistant professor) contributed in analysis, writing and revision of manuscript. Reference1. Kumar A, Chaturvedi AK, Yadav K, et al. Fungal phytoremediation of heavy metal-contaminated resources: current scenario and future prospects. Yadav A, Singh S, Mishra S, Gupta A, editorsRecent Advancement in White Biotechnology Through Fungi Fungal Biology. Springer; Cham: 2019. p. 437–461.
2. Borker P, Salker AV. Photocatalytic degradation of textile azo dye over Ce1−xSnx O2 series. Mater Sci Eng B. 2006;133:55–60.
3. Srivastava S, Sinha R, Roy D. Toxicological effects of malachite green. Aquat Toxicol. 2004;66(3)319–329.
4. Hoffmann MR, Martin ST, Choi W, Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995;95(1)69–96.
5. Muhd JN, Bagheri S, Bee AHS. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci World J. 2014;2014:1–25.
6. Jang YJ, Simer C, Ohm T. Comparison of zinc oxide nanoparticles and its nano-crystaline particles on the photocatalytic degradation of methylene blue. Mater Res Bull. 2006;41(1)67–77.
7. Marci G, Augugliaro V, Lopez-Munoz MJ, et al. Preparation characterization and photocatalytic activity of polycrystalline ZnO/TiO2 systems surface, bulk characterization, and 4-nitrophenol photodegradation in liquid–solid regime. J Phys Chem B. 2001;105(5)1033–1040.
8. Mathur S, Barth S. Molecule-Based Chemical Vapor Growth of Aligned SnO2 Nanowires and Branched SnO2/V2O5 Heterostructures. Small. 2007;3(12)2070–2075.
9. Zheng L, Zheng Y, Chen C, et al. Network structured SnO2/ZnO heterojunction nanocatalyst with high photocatalytic activity. Inorg Chem. 2009;48(5)1819–1825.
10. Pirdashti M, Curteanu S, Kamangar MH, Hassim MH, Khatami MA. Artificial neural networks: applications in chemical engineering. Rev Chem Eng. 2013;29(4)205–239.
11. Sumpter BG, Getino C, Noid DW. Theory and applications of neural computing in chemical science. Annu Rev Phys Chem. 1994;45(1)439–481.
12. Pigram GM, MacDonald TR. Use of neural network models to predict industrial bioreactor effluent quality. Environ Sci Technol. 2001;35(1)157–162.
13. Miller JN. Basic statistical methods for analytical chemistry. Part 2. Calibration and regression methods. A review. Analyst. 1991;116(1)3–14.
14. Elmolla ES, Chaudhuri M. The use of artificial neural network (ANN) for modelling, simulation and prediction of advanced oxidation process performance in recalcitrant wastewater treatment. Hui Chi Leung Patrick, editorIntechOpen; 2011. p. 105–124.
15. Mohammadi R, Eskandarloo H, Mohammadi M. Application of artificial neural network (ANN) for modeling of dyes decolorization by Sn/Zn-TiO2 nanoparticles. Desalination Water Treat. 2015;55(7)1922–1933.
16. Osouleddini N, Heydari M, Motevalli MD, Khosravi T. Application of artificial neural networks and response surface methodology for analysis of malachite green removal from aqueous solution using phosphoric acid-modified pumice powder: kinetic and isotherm studies. Desalin Water Treat. 2020;178:296–311.
17. Maran JP, Manikandan S, Mekala V. Modeling and optimization of betalain extraction from Opuntia ficus-indica using Box–Behnken design with desirability function. Ind Crops Prod. 2013;49:304–311.
18. Nagata Y, Chu KH. Optimization of a fermentation medium using neural networks and genetic algorithms. Biotechnol Lett. 2003;24(21)1837–1842.
19. Dasari VRRK, Donthireddy SRR, Nikku MY, Garapati HR. Optimization of medium constituents for Cephalosporin C production using response surface methodology and artificial neural networks. J Biochem Technol. 2009;1(3)69–74.
20. Akyol A, Yatmaz HC, Bayramoglu M. Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl Catal B: Environmental. 2004;54(1)19–24.
21. Byrappa K, Subramani AK, Ananda S, Rai KL, Dinesh R, Yoshimura M. Photocatalytic degradation of rhodamine B dye using hydrothermally synthesized ZnO. Bull Mater Sci. 2006;29(5)433–438.
22. Chaibakhsh N, Ahmadi N, Zanjanchi MA. Optimization of photocatalytic degradation of neutral red dye using TiO2 nanocatalyst via Box-Behnken design. Desalin Water Treat. 2016;57(20)9296–9306.
23. Malik PK, Saha SK. Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep Purif Technol. 2003;31(3)241–250.
24. YasiniArdakani S, Abghari R, Mirjalili M. TiO2@ CoFe2O4 Nanofiber for the Photocatalytic Degradation of Direct Red 80. Phys Chem Res. 2019;7(2)309–325.
25. Mohamed FF, Allah PM, Mehdi AP, Baseem M. Photo removal of Malachite Green (MG) using advanced oxidation process. Res J Chem Environ. 2011;15(3)65–70.
26. Aisien FA, Blessing A. Photocatalytic degradation of 2, 2, 4 trimethyl pentane (isooctane) in aqueous solution. Iran J Neonatol. 2014;4(4)1–7.
27. Giwa A, Nkeonye PO, Bello KA, Kolawole KA. Photocatalytic decolorization and degradation of Cl Basic Blue 41 using TiO2 nano particles. J Environ Prot Ecol. 2012;3(9)1063–1069.
28. Talebi S, Chaibakhsh N, Moradi-Shoeili Z. Application of nanoscale ZnS/TiO2 composite for optimized photocatalytic decolorization of a textile dye. J Appl Res Technol. 2017;15(4)378–385.
Fig. 4Effect of (a) catalyst dose (b) contact time (c) initial dye concentration on degradation of Malachite degradation in presence of TiO2 catalyst. 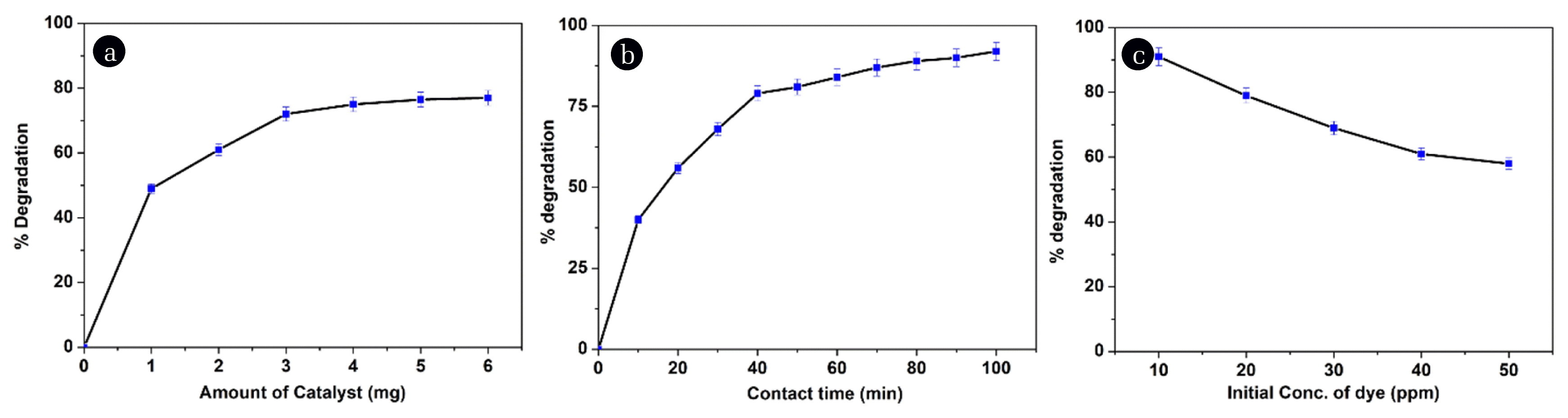
Fig. 5Contour plots of degradation against (a) dye v/s dosage (b) dye v/s time (c) dosage v/s time. 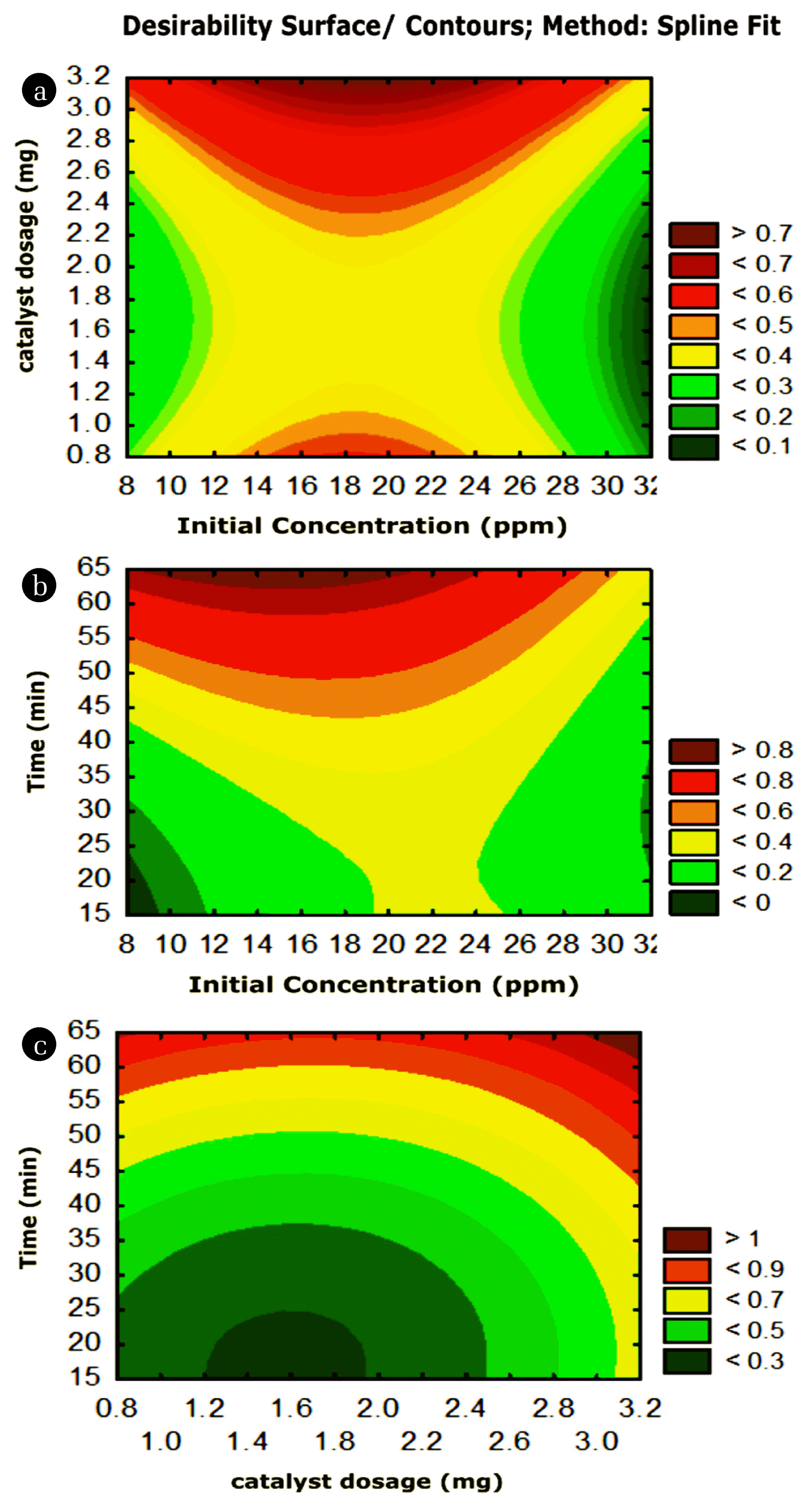
Table 1Analysis of Variance (ANOVA) Table 2Box Behnken Experimental Designs, Effect of Initial Concentration, TiO2 Dosage and Time |
|
||||||||||||||||||||||||||||||||||||||||