AbstractA novel N-doped mesoporous carbon (AC-N) was prepared by impregnation method on the basis of cypress-derived activated carbon for methylene blue (MB) removal. The introduction of nitrogen atoms with appropriate proportion could improve MB adsorption capacity of the prepared mesoporous carbon (AC-N). AC-N obtained at optimized modification (1 mol/L urea) had an excellent MB adsorption of 910.95 mg/g and the removal efficiency was ca. 91% at 25°C. Elemental analysis showed that the nitrogen content of AC-N was high up to 2.6%. A large specific surface area (SBET) 1,215 cm3/g and an obvious mesoporous structure with a high mesopore to total pore volume ratio (Vmes/Vtot) 91% was observed on AC-N. X-ray photoelectron spectroscopy (XPS) results indicated a successful doping of nitrogen element into the mesoporous carbon and pyridinic-N, pyrrolic-N and amino-N were existed in AC-N. Scanning electron microscope (SEM) and Fourier transform infrared spectrometry (FTIR) were also used to characterize this prepared adsorbent. MB adsorption kinetics parameters and isotherm results on the produced carbon were well fitted by the pseudo-second-order kinetic model and Langmuir model. According to the thermodynamic analysis, the adsorption of MB on AC-N was found to be an endothermic, spontaneous process in nature.
1. IntroductionMany serious environmental problems caused by industrial effluents have harmful effects on various forms of life, and are attracting more and more attention [1]. Dyes are one kind of typical contaminants existing in industrial wastewater and they are extensively used in various industries such as textile, food, rubber, paper and so on [2]. In the environmental field, a wide range of processing techniques have been applied to remove contaminants from various dyeing effluents, including biological treatment, chemical oxidation [3, 4], coagulation, flocculation and membrane treatment [5]. However, some of the drawbacks of these methods limit their use in industrial production, such as high-cost, slow rate and secondary pollution [6].
Adsorption is a conventional method for separating and removing contaminants from wastewater and it has the advantages of low cost, easy operation, flexible and simplicity of design, and large throughput. Its high efficiency mainly depends on the electrostatic interaction between the adsorbent surface and the adsorbate, van der Waals force, chemical bonds and hydrogen bonds [7].
Activated carbon has been the most commonly used adsorbent in dye wastewater treatment due to its high specific surface area, developed pore structure and abundant surface chemical functional groups [8]. C. He et al. synthesized mesoporous carbon materials and found a maximum adsorption capacity 758 mg/g for MB [9]. The adsorption capacity 609 mg/g for phenol could be achieved on the nitrogen doped mesoporous carbon synthesized by Y. Liu et al [10]. The adsorption characteristics of activated carbon materials depend not only on the precursor material (its surface functional groups and pore structure) but also on the heteroatoms built into the structure. At present, the most important and broadly introduced heteroatom is nitrogen atom, and amides, imides, lactams, pyrrolyl groups and pyridyl groups all belong to nitrogen-containing functional groups introduced [11]. Nitrogen-containing functional groups generally provide basic properties to the adsorbent and enhance the interaction between the adsorbent and the adsorbate molecules, such as dipole-dipoles, covalent bonds, hydrogen bonds [11]. As a simple nitrogen doping method, functional modification on the surface of the prepared activated carbon is introduced by some physical or chemical methods to obtain the desired nitrogen-containing functional groups. Kim et al. adopted a high-temperature ammonia injection method to obtain nitrogen-doped porous carbon with N content up to 2.83%, and the total pore volume and specific surface area of the new carbon increased from 0.281 cm3/g, 724 m2/g to 0.647 cm3/g, 1,458 m2/g, respectively [12]. Chen et al [13]. used urea as a nitrogen source to prepare nitrogen-doped porous carbon by impregnation method, and its N content was up to 8.01%, with the BET surface area raising from 21 to 1,012 m2/g, CO2 adsorption capacity raising from 2.0 to 4.8 mmol/g. Zhuravsky et al. [14] used melamine or urea solution immersion and post-heat treatment to obtain a nitrogen-doped carbon material with a nitrogen content of 6.2 wt.%. EL-Mahdy et al. [15] synthesized nitrogen-doped mesoporous carbons directly from triazine-functionalized resol for CO2 uptake and highly efficient removal of dyes. The ammonia-modified method is highly toxic, and the operating conditions are relatively strict, and the impregnation method is a safe, reliable and non-toxic nitrogen-doping method.
Methylene blue (MB), a typical cationic dye, was selected in our work due to its widespread industrial application, especially in textile dyeing [16]. MB is a stable and non-biodegradable contaminant with an intricate aromatic structure. Its high toxicity and potential carcinogenesis seriously influence the balance of aquatic ecosystems and has latent harm on humans [17]. Various kinds of adsorbents have been tested on the removal of this typical cationic dye from aqueous media [1, 2, 18–20]. Because MB dimension is 1.43 nm × 0.61 nm × 0.4 nm [21], the mesoporous structure (2~50 nm) of the adsorbent is suitable and significant for MB removal. Therefore, the N-doped activated carbon was an excellent adsorbent for MB due to its improved mesoporous structure and suitable functional group properties.
Thus, the objective of this paper is to prepare a new N-doped mesoporous carbon through impregnation method for MB adsorption. The enhancing of its adsorption capacity on MB removal through preparation optimization was further discussed. The physical and chemical properties of the produced N-doped mesoporous carbon were characterized by elemental analysis, N2 physisorption, scanning electron microscope (SEM), Fourier transform infrared spectrometry (FTIR) and X-ray photoelectron spectroscopy (XPS). In order to further explore the MB adsorption mechanism, kinetics, isotherms and thermodynamic models in the adsorption process were also studied.
2. Materials and Methods2.1. Preparation of Mesoporous CarbonCypress-derived activated carbon was prepared from discarded cypress sawdust (43.10% C, 5.31% H, 0.11% N, 0.13% S, and 51.35% O) as described in our previous work [16], and designated as AC-0. The raw material (cypress sawdust) was crushed and sieved (18 mesh), and then dried at 105°C for 24 h. After that, the sample was activated by H3PO4 (the mass ratio of raw material and H3PO4 was 1.0:1.9) for 24 h at room temperature. The carbonization process was carried out by loading the sample into a tubular oven and heating to 550°C under N2 flow (150 mL/min) for 90 min. The AC-0 was sieved (200 mesh) and dried at 105°C for subsequent experiments.
2.2. Preparation of N-doped Mesoporous CarbonN-Doped mesoporous carbon was prepared using impregnation method. The nitrogen source was provided by urea, nitric acid and melamine, respectively. When AC-0 was treated with urea, 5 g samples were impregnated with 250 mL urea solution of different concentrations (0.25 ~2.00 mol/L) under 35°C for 24 h, dried at 105°C for about 6 h, and then the obtained sample was activated under N2 flow (200 mL/min) at 450°C for 50 min. When nitric acid was used as the source of nitrogen, 10 g AC-0 was stirred with 100 mL nitric acid of various concentrations (1 ~7 mol/L) at 80 °C for 2 h, and then the obtained product was washed several times with deionized water continuously until the solution pH was 6~7. When AC-0 was treated with melamine, 3g AC-0 was mixed with 10 mL melamine solution, and the concentrations of melamine were among 0.08 ~ 0.64 mol/L. The precursor then was stirred at room temperature for 5 h. The obtained product was activated under N2 flow (200 mL/min) at 850°C for 30 min, and then washed with boiling deionized water and dried to constant weight. All the N-doped carbons were storied in desiccator for subsequent utilization.
In addition, commercial wooden activated carbon (CMC) was purchased from Huansheng Carbon Industry Co., Ltd, Henan province in order to compare its adsorption performance with N-doped carbons. Detailed parameters of CMC are shown in Table S1. All the samples were passed through 200 mesh sieves (75 μm) before utilization.
2.3. Characterization of N-doped Mesoporous CarbonThe elemental analysis of the material was carried out using an elemental analyzer (Euro EA 3000). The specific surface area and pore size distribution analysis of prepared samples were measured by N2 adsorption-desorption method at 77K using a Micromeritics instrument (ASAP 2460, Quanta chrome, USA). The morphology and textural structure of the prepared samples was studied by the scanning electron microscope experiment using JSM-7500F (JEOL, Japan). The surface functional groups of prepared samples were identified by Flourier Transform Infrared Spectrometry (FTIR) using the Nicolet 6700 Infrared Spectrometer. X-ray photoelectron spectroscopy (XPS) experiment was performed on an AXIS Ultra DLD (KRATOS) spectrometer using Al Kα radiation (1,486.6 eV) operated at an accelerating power of 150 W.
2.4. Batch Adsorption Experiments0.5 g/L prepared samples were added into a 100 mL Erlenmeyer flask containing 50 mL MB solution (500 mg/L) and shaken for 60 min in a thermostatic oscillator (150 rpm) at 25°C. Adsorption kinetics was carried out at 25°C and the initial MB concentration was 500 mg/L. The adsorption isotherms were obtained by batch experiments at various temperatures (15~55°C). After pre-determined time reached, the adsorbent was filtered out and the residual MB concentration of the supernatant was analyzed to obtain the adsorbent removal efficiency. The absorbance of MB solution was monitored by an ultraviolet spectrophotometer (UV-1100, Mapada) at wavelength of 665 nm and the concentrations were calculated using a standard curve plotted beforehand. Furthermore, effects of initial MB concentration (100~1,600 mg/L) and adsorption time (0~180 min) on the MB adsorption capacity were also investigated.
The equilibrium adsorption amount qe (mg/g), was determined as following:
where C0 (mg/L), Ce (mg/L), V (L) and M (g) were the initial and equilibrium concentration of MB, MB solution volume and the mass of adsorbent, respectively.
Each experiment was duplicated twice under identical conditions and the mean values were recorded. It was also confirmed that the Erlenmeyer flask did not affect the adsorption of MB at all.
3. Results and Discussion3.1. Optimization of N-doped Carbon PreparationAC-0 was prepared according to our previous study, nitrogen (melamine, nitric acid and urea as nitrogenous source) was provided by the impregnation method onto the carbons. Fig. 1 shows the effect of nitrogenous sources on adsorptive capacity of N-doped mesoporous carbon for MB removal.
As seen from Fig. 1, MB removal efficiency for three N-doped carbon samples were obviously different after modification. Nitric acid modification played no effect on MB adsorption, while melamine modification greatly inhibited MB adsorptive performance on the produced carbon. Only urea could improve MB removal efficiency in a certain addition amount. As shown in Fig. 1 (a), N-doped mesoporous carbon with high urea impregnation concentration (1 or 2 mol/L) had better MB adsorption than AC-0. However, with low urea concentration impregnation (0.25 and 0.5 mol/L), MB removal efficiency changed slightly. A relative lower nitrogen addition was insufficient to provide enough active sites or prompt the electronegativity of the material which was crucial to MB adsorption. Therefore, 1 mol/L urea was optimized as the nitrogen-doping operation and the product was recorded as AC-N.
3.2. Characterization of N-doped Mesoporous CarbonThe elemental analysis of sample carbons modified with different urea concentration was listed in Table 1. The main elements in AC-0 were C (87.8%), H (2.1%) and O (10.1%), and completely free of N. After nitrogen doping, the content of N in treated samples was increased to 1.0–2.6%, which indicated that nitrogen was successfully introduced into those mesoporous carbons. It was worth noting that the nitrogen content was 2.6% when urea concentration was 1 mol/L, while with a higher urea concentration of 2 mol/L, the nitrogen content was only 1.9%. The successful N-doping by impregnation method is mainly due to urea adsorption and capillary pressure caused by liquid surface tension, which facilitate the active agent entering the mesoporous carbon. The doping of N element can effectively provide more active sites for the adsorbent and greatly improve the electronegativity of the sample [22]. MB is one kind of cationic basic dyes [2] and the stronger the electro-negativity of the adsorbent, the more favorable to attract MB from solutions.
SEM pictures showed that there was no significant difference in samples morphology. As illuminated in Fig. S1, there are similar relatively dense layered structures and many small particles are observed on AC-0 and AC-N’s surface, which may be caused by the carbonization process.
N2 adsorption-desorption isotherms of AC-0 and AC-N were given in Fig. S2. It can be clearly observed that the adsorbed amount increased evidently as the pressure raised. Furthermore, the hysteresis loop was observed for both samples [23]. It was attributed to the capillary condensation and occurred in mesopores, which confirmed that the AC-0 and AC-N were typical mesoporous carbon materials. The pore size distribution of the prepared samples was shown in Fig. S3.
Textural properties of two samples calculated from isotherms were summarized in Table 2. Mesoporous activated carbons had a large specific surface area before and after nitrogen doping. The specific surface area (SBET) were 1,221.31 and 1,214.98 m2/g for AC-0 and AC-N, respectively. These two samples exhibited an obviously mesoporous structure with a high mesopore to total pore volume ratio (Vmes/Vtot), while the volume ratio of AC-N (91%) was a little higher than AC-0’s (88%). In general, there was no significant difference in specific surface area, total pore volume, mesopore /micropore volume and average pore size between those two samples. It was in accordance with the SEM observation.
The extremely strong adsorption capacity of mesopore for MB removal is mainly reflected in the interconnected two-dimensional ordered mesoporous channels, which can effectively reduce the steric hindrance during mass transfer and is beneficial for MB adsorption. Moreover, N-doped mesoporous carbon has higher adsorption potential than AC-0 due to the doping of nitrogen-containing functional groups in its pores [24].
FT-IR spectra of AC-0 and urea-modified AC-N were presented in Fig. 2. As shown in Fig. 2, the band at 3,400 cm−1 was attributed to the stretching of O-H in H2O [25], the band at 1,573 cm−1 was associated to the stretch of C=O [26], the band at 1,158 cm−1 was assigned to C-OH single bond stretching vibration [25], and the band at 895 cm−1 was attributed to the C-H bending vibration on the aromatic ring [27]. As seen from Fig. 2, the main peaks on AC-0 still existed on AC-N after nitrogen doping, indicating that the main structure of the mesoporous carbon did not suffer obvious damage during the urea impregnation process. However, after nitrogen doping, the peaks at 1,573 cm−1 and 1,158 cm−1 on the AC-0 gave a red shift to 1,560 cm−1 and 1,140 cm−1 on AC-N, respectively. It indicated that the function group structure of the mesoporous carbon had undergone certain changes after nitrogen introduction.
N1s XPS spectra was shown in Fig. 3. There were mainly three types of N in AC-N. The peak at 398.6 eV can be assigned to pyridinic-N in which nitrogen atom is bonded to C atom in the sp2 hybridization [28], the peak at 400.2 eV can be associated to pyrrolic-N [29, 30], and the peak at 399.5 eV is attributed to amino-N [31]. The change of functional groups on mesoporous carbon is closely related to the adsorption performance for MB treatment. Amine, imine and pyridine functional groups found in N-doped mesoporous carbon are helpful to improve the adsorption performance by enhancing the affinity of the material with the adsorbate [32].
3.3. Adsorption Mechanism3.3.1. Adsorption kineticsIn order to determine the equilibrium time for maximum adsorptive capacities of our materials and to study the kinetics of the adsorption process, MB adsorption on AC-0, AC-N and commercial activated carbon (CMC) was studied as a function of contact time, and the results was shown in Fig. 4. It can be seen that MB removal efficiency by AC-0, AC-N and CMC all increase quickly with the adsorption time increment in the first 60 minutes. For instance, 60.5% removal efficiency was reached within five minutes after the adsorption starting, and the equilibrium was achieved in ca. 60 minutes for the AC-N. In the first 30 minutes, MB removal efficiency of AC-0 and AC-N were almost same, while it was significantly higher of AC-N than that of AC-0 after 30 minutes. At 60 minutes, MB removal efficiency on AC-N was stable and high up to 91%, and was larger than AC-0’s (87%) and CMC’s (75%). During the initial reaction stage, many vacant surface sites on the activated carbon were available and the adsorption rate was very fast. Most MB was adsorbed within 60 minutes, but with time lapse, an equilibrium was reached and most of the available sites were occupied by MB [33]. A similar observation and same equilibrium of 60 minutes were reported before [1].
Under identical adsorption conditions, MB removal efficiency on AC-0 increased ca. 11% compared with CMC’s, and the one on AC-N increased ca.5% compared with AC-0’s. MB removal efficiency on AC-N was higher than both on AC-0 and CMC. Both AC-0 and AC-N had good adsorption capacity for MB due to their high specific surface area and developed porosity, while compared to AC-0, AC-N displayed enhanced removal efficiency with modification of nitrogen-containing functional groups on it.
Adsorption kinetics can explain the solution uptake rate and show the adsorption efficiency of the adsorbents [34], which is essential for the practical design of an adsorption system [16]. In the present study, the kinetic data were modeled using pseudo- first-order kinetic, pseudo-second-order kinetic, and intra-particle diffusion model, which were expressed by the Eq. (2), (3) and (4), respectively.
where qe and qt represented the MB adsorption capacity of adsorbent at equilibrium and time t (min), respectively, and k1 (min−1) was the equilibrium rate constant of pseudo-first-order kinetic, and the slope and intercept of the plot of ln (qe − qt) versus t were used to determine k1.
where k2 (g/(mg.min)) was the pseudo-second order rate constant, which was calculated by plotting t/qt versus t.
where kid was the intra-particle diffusion rate constant and C was a constant related to the boundary layer thickness.
For MB adsorption on AC-0, AC-N and CMC, the plots of kinetics model fitting were shown in the Fig. S4~S5, the kinetic parameters and the coefficient (R2) were calculated and listed in Table S2. As shown in Table S2, R2 values of pseudo-second-order kinetic for three carbons were all high above 0.999, and the calculated value qe was much close to the experimental value. MB adsorption process for three samples was fitted well by the pseudo-second-order kinetic model, which suggested that MB adsorption might depend on the availability of the active sites. The pseudo-second-order kinetic behavior suggested that the adsorption mechanism was a rate controlling step, and mainly involved valency forces through electrons sharing between the hydrophilic edge sites of activated carbon and MB cations [35]. In addition, the adsorption might be controlled by the chemisorption due to the interaction between the MB and the activated carbon [36, 37]. The results were also in agreement with Poormand’s study for MB adsorption [1].
As described by the intra-particle diffusion model, if the fitting plot (Eq. (4)) gives a straight line, the adsorption process is affected by intra-particle diffusion; if this line passes through the origin, intra-particle diffusion is the rate determining step [38]. As shown in Table S2, the fitting line was straight but did not cross the origin and the value C for intra-particle diffusion model was not zero, which indicated that the intra-particle diffusion also participated in the adsorption process, but not a major part.
3.3.2. Adsorption thermodynamicsEffect of temperature on MB adsorption was studied by varying the temperature over a range of 15~55°C. Thermodynamic adsorption parameters, such as free Gibbs Energy change (ΔG0), enthalpy change (ΔH0), entropy change (ΔS0), was calculated using Gibbs-Helmholtz equation and Van’t Hoff equation [38, 39].
where KD is the adsorption equilibrium constant and can be calculated by plotting ln(qe/Ce) versus qe and extrapolating to zero qe. Where T is the adsorption temperature in kelvin (K), R is the universal gas constant (8.314 J /(mol·K)). The values of ΔH0 and ΔS0 were determined from the intercept and slope of plot ln KD versus 1/T.
Fig. S6 and Table S3 displayed the plots of adsorption thermodynamics model fitting and these thermodynamic parameters for MB adsorption on AC-0, AC-N and CMC. As shown in Table S3, a negative ΔG0 indicated that MB adsorption was spontaneous. The values of ΔG0 decreased with temperature, thus the adsorption became more favorable at higher temperatures. The positive value of enthalpy change ΔH0 revealed that the adsorption of MB on carbons was endothermic. The positive value of ΔS0 represented an increasing randomness at the solid/solution interface during the adsorption process [36].
3.4. Adsorption IsothermAdsorption isotherms are helpful in explaining adsorbent–adsorbate relationship and adsorbate molecule distribution at equilibrium in the liquid and solid phases [37]. Langmuir (Eq. (8)), Freundlich (Eq. (9)) and Temkin (Eq. (10)) isotherms were used to fit the experimental data and the results were listed in Table S4.
Langmuir model is developed to represent monolayer sorption on the actives sites and expressed by the following equation [40]:
where Ce (mg/L), kL (L/mg) and qm (mg/g) were the equilibrium concentration of MB, Langmuir constant and the monolayer capacity of the adsorbents, respectively.
Freundlich model deals with physicochemical sorption on heterogeneous surfaces. It was given by the flowing form [41]:
where kF ((mg/g)/(L/mg)1/n) and n were Freundlich constant and the heterogeneity factor, respectively.
Temkin model suggests a linear decrease of adsorption energy with adsorption capacity decreasing, which is similar to Freundlich model. It was depicted by the following equation [42]:
Where b (J/mol) and AT (L/mg) was the Temkin constants related to heat of sorption and equilibrium binding, T is the adsorption temperature in kelvin (K), and R is the universal gas constant (8.314 J /(mol·K)).
Fig. S7~S9 and Table S4 showed the plots of adsorption isotherms model fitting and these parameters for MB adsorption on AC-0, AC-N and CMC. As seen from Table S4, the highest R2 value was obtained in Langmuir model for three materials. Accordingly, MB adsorption on three activated carbon materials was monolayer adsorption. As shown in Table S4, CMC is a little more favorable for MB adsorption than AC-0 (ca. 6.9% higher MB maximum adsorption capacity compared to AC-0). However, the adsorption capacity of AC-N is significantly higher than CMC (ca. 14.7% higher than CMC), which improved the effect of N modification as we discussed in the text.
3.5. Adsorption ExperimentsThe N-doped mesoporous carbon prepared by waste biomass material, using discarded cypress sawdust obtained from furniture manufacturing, was a cheaper promising adsorbent. The comparison of MB adsorption capacity for different adsorbents was summarized in Table 3. It was quite interesting that for activated carbons prepared from hydrothermal methods, their MB adsorption capacities were lower than 550 mg/g, even though they had outstanding high SBET (> 1,200 m2/g) [35]. As listed in Table 3, the hydrothermal pre-treated carbon possessed an extremely higher SBET (1,744 m2/g), but its MB adsorption capacity and mesoporosity were only 526 mg/g and 49.9% [43], respectively, which were obviously smaller than the ones of AC-N prepared in this work. Due to lack of active sites associated with methylene blue in the pore structure and very low mesoporosity, those carbons displayed poor MB adsorption in the test. While the carbon prepared from coconut shell had a relatively high mesoporosity, the adsorption of MB onto it was only 200 mg/g [44]. A modified water treatment sludge was prepared and its MB adsorption capacity could be high up to 909 mg/g [1]. Thus, the excellent adsorption capacity of AC-N was attributed to abundant mesopores and doping with nitrogen atoms, which increased adsorption active sites and enhanced MB adsorption. AC-N had great potential to utilize in dyes wastewater treatment.
4. ConclusionsA N-doped mesoporous carbon was successfully synthesized for MB removal from aqueous solution in this work. In the process of N-doped mesoporous carbon preparation, the nitrogen source and concentration for modification were studied and optimized. With impregnation in 1 mol/L urea solution, the produced N-doped mesoporous carbon AC-N had a higher removal efficiency around 91% and MB adsorption capacity of 910.95 mg/g. The specific surface area (SBET) was 1,214.98 m2/g for AC-N, and a high mesopore to total pore volume ratio (Vmes/Vtot) of 91% was displayed in AC-N. MB adsorption capacity on AC-N enhanced by the introduction of nitrogen atoms. FTIR indicated that the structure of AC-N had certain surface group changes by N-doping. XPS results indicated that nitrogen element was successfully doped into the mesoporous carbon and existed in the form of pyridinic-N, pyrrolic-N and amino- N. The kinetics, isotherms, and thermodynamics parameters for MB adsorption on AC-N were investigated to further understand the adsorption process. The experimental data were well fitted by the pseudo-second-order kinetic model and Langmuir isotherm. The adsorption thermodynamic results indicated that the adsorption process was endothermic and spontaneous. In summary, AC-N prepared from industrial wastes cypress sawdust and urea provided a low-cost adsorbent with high adsorption capacity for MB waste-water treatment.
References1. Poormand H, Leili M, Khazaei M. Adsorption of methylene blue from aqueous solutions using water treatment sludge modified with sodium alginate as a low-cost adsorbent. Water Sci Technol. 2017;75:281–295.
2. Mouni L, Belkhiri L, Bollinger J-C, et al. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl Clay Sci. 2018;153:38–45.
3. Moussavi G, Yazdanbakhsh A, Heidarizad M. The removal of formaldehyde from concentrated synthetic wastewater using O-3/MgO/H2O2 process integrated with the biological treatment. J Hazard Mater. 2009;171:907–913.
4. Modirshahla N, Hassani A, Behnajady MA, Rahbarfam R. Effect of operational parameters on decolorization of Acid Yellow 23 from wastewater by UV irradiation using ZnO and ZnO/SnO2 photocatalysts. Desalination. 2011;271:187–192.
5. Feng Y, Zhou H, Liu G, et al. Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour Technol. 2012;125:138–144.
6. Xin G, Wang M, Chen L, et al. Synthesis and properties of zeolite/N-doped porous carbon for the efficient removal of chemical oxygen demand and ammonia-nitrogen from aqueous solution. Rsc Advances. 2019;9:6452–6459.
7. Liu H, Liu W, Zhang J, Zhang C, Ren L, Li Y. Removal of cephalexin from aqueous solutions by original and Cu (II)/Fe (III) impregnated activated carbons developed from lotus stalks Kinetics and equilibrium studies. J Hazard Mater. 2011;185:1528–1535.
8. Kim S-I, Yamamoto T, Endo A, Ohmori T, Nakaiwa M. Adsorption of phenol and reactive dyes from aqueous solution on carbon cryogel microspheres with controlled porous structure. Microporous Mesoporous Mater. 2006;96:191–196.
9. He C, Hu X. Anionic Dye Adsorption on Chemically Modified Ordered Mesoporous Carbons. Ind Eng Chem Res. 2011;50:14070–14083.
10. Liu Y, Chen M, Hao YM. Study on the adsorption of Cu (II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem Eng J. 2013;218:46–54.
11. Shen W, Fan W. Nitrogen-containing porous carbons: synthesis and application. J Mater Chem A. 2013;1:999–1013.
12. Kim HS, Kang MS, Yoo WC. Highly Enhanced Gas Sorption Capacities of N-Doped Porous Carbon Spheres by Hot NH3 and CO2 Treatments. J Phys Chem C. 2015;119:28512–28522.
13. Chen J, Yang J, Hu G, et al. Enhanced CO2 Capture Capacity of Nitrogen-Doped Biomass-Derived Porous Carbons. ACS Sustain Chem Eng. 2016;4:1439–1445.
14. Zhuravsky SV, Kartel MT, Puziy OM. Synthesis and catalytic properties of N-containing synthetic carbons on a basis of copolymer of styrene and divinylbenzene. Loureiro JM, Kartel MT, editorsCombined and Hybrid Adsorbents: Fundamentals and Applications. NATO Science for Peace and Security Series C-Environmental Security; 2006. p. 201–206.
15. EL-Mahdy AFM, Liu T-E, Kuo S-W. Direct synthesis of nitrogen-doped mesoporous carbons from triazine-functionalized resol for CO2 uptake and highly efficient removal of dyes. J Hazard Mater. 2020;391:122163.
16. Wang M, Xie R, Chen Y, Pu X, Jiang W, Yao L. A novel mesoporous zeolite-activated carbon composite as an effective adsorbent for removal of ammonia-nitrogen and methylene blue from aqueous solution. Bioresour Technol. 2018;268:726–732.
17. Nair V, Vinu R. Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresour Technol. 2016;216:511–519.
18. Mashkoor F, Abu N. Magsorbents: Potential candidates in waste-water treatment technology - A review on the removal of methylene blue dye. J Magn Magn Mater. 2020;500:19.
19. Somsesta N, Sricharoenchaikul V, Aht-Ong D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater Chem Phys. 2020;240:14.
20. Aly K, Sayed MM, Mohamed MG, et al. A Facile Synthetic Route and Dual Function of Network Luminescent Porous Polyester and Copolyester Containing Porphyrin Moiety for Metal Ions Sensor and Dyes Adsorption. Microporous Mesoporous Mater. 2020;298:110063.
21. Pelekani C, Snoeyink VL. Competitive adsorption between atrazine and methylene blue on activated carbon: the importance of pore size distribution. Carbon. 2000;38:1423–1436.
22. Wilson BE, He S, Buffington K, Rudisill S, Smyrl WH, Stein A. Utilizing ionic liquids for controlled N-doping in hard-templated, mesoporous carbon electrodes for high-performance electrochemical double-layer capacitors. J Power Sources. 2015;298:193–202.
23. Zhou Q, Jiang X, Guo Y, Zhang G, Jiang W. An ultra-high surface area mesoporous carbon prepared by a novel MnO-templated method for highly effective adsorption of methylene blue. Chemosphere. 2018;201:519–529.
24. Tang W-W, Zeng G-M, Gong J-L, et al. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci Total Environ. 2014;468:1014–1027.
25. Njoku VO, Foo KY, Asif M, Hameed BH. Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem Eng J. 2014;250:198–204.
26. Bedin KC, Martins AC, Cazetta AL, et al. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem Eng J. 2016;286:476–484.
27. Li M, Li W, Liu S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr Res. 2011;346:999–1004.
28. Mei S, Gu J, Ma T, et al. N-doped activated carbon from used dyeing wastewater adsorbent as a metal-free catalyst for acetylene hydrochlorination. Chem Eng J. 2019;371:118–129.
29. Mohamed MG, El-Mahdy AFM, Ahmed MMM, Kuo SW. Direct Synthesis of Microporous Bicarbazole-Based Covalent Triazine Frameworks for High-Performance Energy Storage and Carbon Dioxide Uptake. Chem Plus Chem. 2019;84:1767–1774.
30. Wu JY, Mohamed MG, Kuo SW. Directly synthesized nitrogen-doped microporous carbons from polybenzoxazine resins for carbon dioxide capture. Polym Chem. 2017;8:5481–5489.
31. Li OL, Chiba S, Wada Y, Panomsuwan G, Ishizaki T. Synthesis of graphitic-N and amino-N in nitrogen-doped carbon via a solution plasma process and exploration of their synergic effect for advanced oxygen reduction reaction. J Mater Chem A. 2017;5:2073–2082.
32. Wei J, Zhou D, Sun Z, Deng Y, Xia Y, Zhao D. A Controllable Synthesis of Rich Nitrogen-Doped Ordered Mesoporous Carbon for CO2 Capture and Supercapacitors. Adv Funct Mater. 2013;23:2322–2328.
33. Zolfaghari G, Esmaili-Sari A, Anbia M, Younesi H, Amirmahmoodi S, Ghafari-Nazari A. Taguchi optimization approach for Pb(II) and Hg(II) removal from aqueous solutions using modified mesoporous carbon. J Hazard Mater. 2011;192:1046–1055.
34. Zhu H-Y, Jiang R, Xiao L, Li W. A novel magnetically separable gamma-Fe2O3/crosslinked chitosan adsorbent: Preparation, characterization and adsorption application for removal of hazardous azo dye. J Hazard Mater. 2011;179:251–257.
35. Foo KY, Hameed BH. Mesoporous activated carbon from wood sawdust by K2CO3 activation using microwave heating. Bioresour Technol. 2012;111:425–432.
36. Ezzeddine Z, Batonneau-Gener I, Pouilloux Y, Hamad H. Removal of methylene blue by mesoporous CMK-3: Kinetics, isotherms and thermodynamics. J Mol Liq. 2016;223:763–770.
37. Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochemistry. 1999;34:451–465.
38. Khanday WA, Marrakchi E, Asif M, Hameed BH. Mesoporous zeolite-activated carbon composite from oil palm ash as an effective adsorbent for methylene blue. J Taiwan Inst Chem Eng. 2017;70:32–41.
39. Bulut Y, Aydin H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination. 2006;194:259–267.
40. Langmuir I. The constitution and fundamental properties of solids and liquids Part I Solids. J Am Chem Soc. 1916;38:2221–2295.
41. Freundlich H. Concerning adsorption in solutions. Zeitschrift Fur Physikalische Chemie--Stochiometrie Und Verwandtschaftslehre. 1906;57:385–470.
42. Johnson RD, Arnold FH. THE TEMKIN ISOTHERM DESCRIBES HETEROGENEOUS PROTEIN ADSORPTION. Biochimica Et Biophysica Acta-Protein Structure and Molecular. Enzymology. 1995;1247:293–297.
Fig. 1Effect of nitrogen impregnation on MB removal for different carbons: a-urea, b-nitric acid, c-melamine (initial MB concentration: 500 mg/L adsorbent dose: 0.5 g/L, 25°C, 60 min). 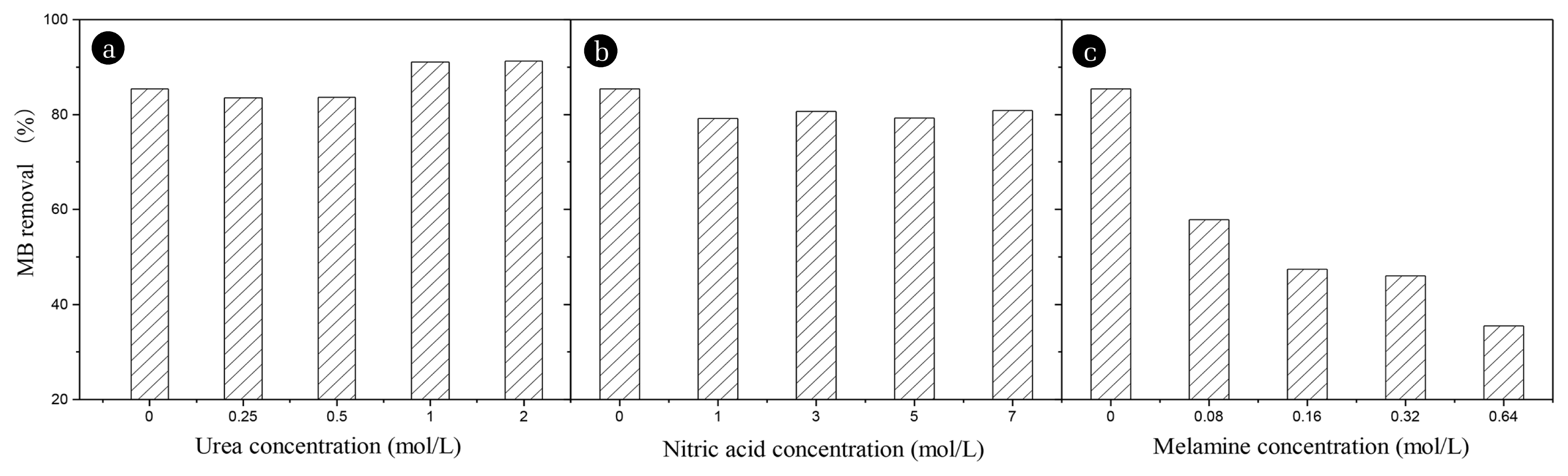
Fig. 4Effect of reaction time on MB removal for different carbons (initial MB concentration: 500 mg/L, adsorbent dose: 0.5 g/L, 25°C). 
Fig. 5Ce vs qe at MB equilibrium adsorption onto the produced carbons (initial MB concentration: 100~1,600 mg/L, adsorbent dose: 0.5 g/L, 25°C, 60 min) 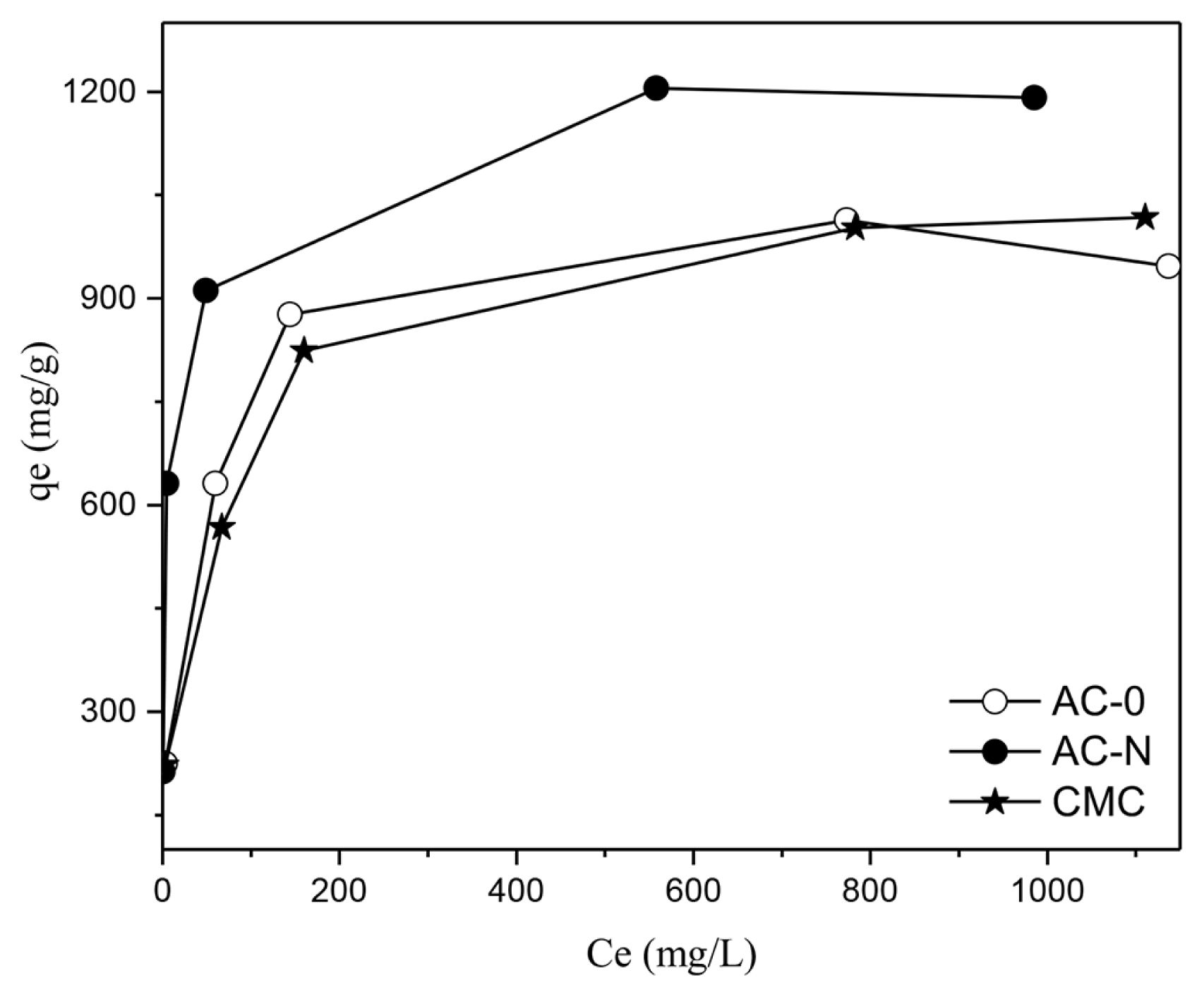
|
|
|||||||||||||||||||||||||||||||||||||||