AbstractThe present study investigates possible roles of manure-derived biochar (MBC) in anaerobic digestion (AD) of dairy manure. Addition of MBC led to an increase in cumulative methane yield and a decrease in lag phase under all tested conditions (concentration of MBC: 1 and 10 g/L, temperature: 20, 35 and 55°C). For example, the cumulative methane yield in the mesophilic AD with 10 g/L MBC were 24.51% higher than that of the AD without MBC. Additionally, lag phage of mesophilic AD with 10 g/L MBC decreased from 2.08 d to 1.52 d. Microbial community analysis indicated that the addition of MBC to mesophilic and thermophilic AD of dairy manure increased the relative abundance of Ruminofilibacter which related to the hydrolysis. In addition, the addition of MBC to AD potentially stimulated the growth of syntrophic bacteria (e.g., genera Clostridium, Syntrophomonas and Syntrophus) and hydrogenotrophic methanogens (e.g., genera Methanobacterium, Methanolinea and Methanomassiliicoccus). Furthermore, microbial community analysis also suggested that mediate interspecies electron transfer and direct interspecies electron transfer would be accelerated by addition of MBC which showed high electrical conductivity (3230 μS/cm).
1. IntroductionProper management of solid wastes such as sewage sludge, food waste and animal manure from municipal and agriculture sectors is important, since solid wastes pose a significant threat to human health and the environment [1–3]. Furthermore, sustainability is increasingly important, and effective recovery of energy from various solid wastes is stressed as an integral component of waste management. Because solid wastes are not competing with food crops, renewable energy from these sources is considered as one of the most promising alternative energy sources in the near future [4]. Various approaches including physicochemical (e.g. extraction and transesterification), thermochemical (e.g. pyrolysis and gasification) and biochemical (e.g. anaerobic digestion (AD)) processes are being used to obtain energy from solid wastes [4, 5].
AD is a widely applied and mature technology that exploits microbial synergistic metabolisms between a diverse assemblage of bacteria and methanogenic archaea for treatment of the organic fraction of solid wastes [6]. With biogas production, which usually contains about 55 – 65% methane [7], AD can significantly reduce potential emission of greenhouse gases and odors [8]. Nevertheless, some limitations such as low metabolic rate and process stability lead to increasing challenges in its practical application. For instance, the AD of dairy manure has suffered from the low digestion yields, low biogas production rates, and fluctuating performance [9]. Extensive research has been conducted to enhance the efficiency and stability of AD. For instance, pretreatment [10], co-digestion [11, 12] and multi-stage AD (i.e., temperature-phased or separated acidogenic-methanogenic step) [13] have been studied to accelerate the rate-limiting step of AD.
Recently, addition of carbonaceous materials (CMs) to AD has received growing attention because it can enhance methane production without any modification of current AD facilities. Yang et al. [14] and Zhang et al. [15] reported that addition of activated carbon (AC) to AD can promote methane production. It was also demonstrated that graphene oxide (GO) could boost methane yield and production rate in AD via enhanced direct interspecies electron transfer (DIET) [16]. Meanwhile, Zhang et al. [17] reported that the addition of GO to AD reduced methane production due to acute toxicity on the microbial community. As a cost-effective CM, biochar (BC) has high potential as an additive and methane production enhancer in AD. Various BCs derived from pine wood [18], fruit wood [19, 20], sawdust [21, 22], cow manure [23] and chicken manure [24] have been utilized to enhance methane production in AD. However, response of addition of BC to AD is not universal (i.e. negative effects of addition of BC in AD also reported [19, 20]).
Monlau et al. [25] suggested that integrated AD and pyrolysis processes could achieve about 42% higher electricity production than AD as a stand-alone process for treating agricultural residues. In addition, the overall energy consumption and carbon footprint can be reduced by using the same feedstock for pyrolysis and AD. Very recently, Jang et al. [26] reported that the addition of manure-derived BC (MBC) to AD of dairy manure significantly enhanced methane production and yield. However, for a more detailed understanding of MBCs effects on AD of dairy manure in our previous work [26], the responses of the microbial communities need to be clearly investigated. Recently-developed high-throughput sequencing technologies can provide the improved community data in complex biological samples, especially in AD [11, 27].
The main objective of this study was to investigate the microbial communities in the AD of dairy manure with and without MBC via high-throughput sequencing (i.e. MiSeq). The changes in key bacteria and archaea were correlated with AD methane and metabolite production and facilitate an understanding of underlying mechanisms. In addition, unlike other studies, broad spectrum of temperatures including psychrophilic (20°C), mesophilic (35°C) and thermophilic conditions (55°C) were applied. In particular, the result from psychrophilic conditions might be helpful to design the AD coupled with BC using daily manure since many closed lagoon are operated under this conditions.
2. Material and Methods2.1. Preparation and Characterization of MBCDairy manure was obtained from Southwestern Dairy Research Center at Tarleton State University (Stephenville, TX; Tarleton dairy farm). It was oven-dried at 60°C before pyrolysis. The MBC was prepared by pyrolysis of dry dairy manure at 350°C for 3 h with heating rate of at 10°C/min under oxygen-limited conditions. After pyrolysis, the MBC was ground and sieved to pass through a 30×40 (420–600 μm) mesh sieve. More detailed information about the manure and preparation of MBC was described previously [26]. Electrical conductivity is determined in a 1:2 MBC: water extract using deionized water. Samples are stirred and allowed to equilibrate for a minimum of 30 minutes after adding the water. The actual determination is made using a conductivity probe. The minerals (e.g., Ca, Fe, K, Mg and Zn) are determined by inductive coupled plasma analysis of a sulfuric acid digest. The elemental analysis was conducted by Robertson Microlit Laboratories (Ledgewood, New Jersey). Chemical characterization showed the MBC included C (11.7%), H (0.56%), O (16.76%), N (1.3%), S (0.05%) and minerals (Ca, 9.1%; Mg, 3.6%; Fe, 0.82%). The MBC possessed low surface area (SBET) of 6.3 m2/g and high electrical conductivity (3230 μS/cm).
2.2. Experimental Set UpAs previously described [26], the batch experiments were carried out using serum bottles with 150 mL of working volume. To enhance the microbial activity in inoculum obtained from the waste lagoon at the dairy farm, it was incubated under mesophilic anaerobic conditions (35°C) for approximately 30 days with the dry dairy manure. Based on the volatile solids (VS), the inoculum to substrate ratio (ISR) was set to 1. Afterwards, two different concentrations of MBC (1 g/L and 10 g/L) and three different temperatures (psychrophilic, 20°C; mesophilic, 35°C; thermophilic, 55°C) were applied to evaluate the effects of addition of MBC on AD of dairy manure. The incubation was terminated when the daily methane production ceased or was < 1% of the total accumulated volume of methane produced. The bottles were incubated for 35 days (35 d) after initial purging with nitrogen. The experimental sets were designated as P0, P1, P10, M0, M1, M10, T0, T1 and T10, where P, M and T represents the operation temperature and the number indicates the concentration of MBC (g/L). All experiments were conducted in duplicate.
2.3. Preparation of Sample for DNA ExtractionThe attached microorganisms on the MBC were obtained as described by Leitch et al. [28]. Briefly, non-attached and loosely attached microorganisms were removed as follows: (1) 5 mL of sample was vortex-mixed for 30 s and pelleted by centrifugation at 700 X g for 5 min, (2) the pellet was re-suspended in PBS buffer containing 8 g/L of NaCl, 0.2 g/L of KCl, 1.14 g/L of Na2HPO4 and 0.24 g/L of KH2PO4 and vortex-mixed for 30 s and pelleted by centrifugation at 700 X g for 5 min, (3) repeating step 2 four times with PBS, (4) repeating step 2 twice with PBS containing of 0.1% of Tween 80, (5) repeating step 2 twice with PBS to remove residual Tween 80. The final pellet was assigned as attached sample and labelled as “A”. For instance, P1A represents the attached sample obtained from P1. The total DNA in the total and attached samples were extracted and purified using a NucleoSpin® Soil kit (MACHEREY-NAGEL, Germany). The purified DNA was stored at −25°C for further analysis.
2.4. High-throughput Sequencing AnalysisPCR amplified microbial communities were sequenced on an Illumina MiSeq instrument using a v3 600 cycle paired-end sequencing kit. Primers 519F (5′-CAGCMGCCGCGGTAA-3′) and 785R (5′-TCCTCCGCTTATTGATATGC-3′) were used to amplify the V4 region of the 16S small subunit ribosomal locus according to the Illumina 16S Metagenomic Sequencing Library Preparation protocol. After sequencing, fastq files were processed using QIIME version 1.9 and USEARCH version 8.0.1 [29, 30]. Sequences were quality filtered with USEARCH to remove single-ton sequences and any sequence with greater than 1 expected error. Chimeric sequences were removed with the uchime ref command in USEARCH using the gold.fasta database as a reference [31, 32]. Reference-based Operational Taxonomic Unit (OTU) picking was conducted in QIIME using the UCLUST algorithm and the Greengenes database version 13.8 [33] with OTU similarity set at 97%. Taxonomy was assigned using the RDP method [34] in QIIME using the Greengenes 13.8 database. Venn diagram for shared OTUs analysis was created using InteractiVenn [35]. The nucleotide sequences obtained were deposited in the Sequence Read Archive accessible through the National Center for Biotechnology Information (NCBI) (PRJNA604470).
3. Results and discussion3.1. Effect of MBC Addition on Reactor Performance
Table 1 summarizes the major impact of biochar on AD based on the estimated parameters from the modified Gompertz model. As previously reported by Jang et al. [26], the positive effects of MBC on AD were observed under all tested conditions. Compared to P0, M0 and T0, cumulative methane yields were increased to 7.98 and 28.49% in P1 and P10, 5.40 and 24.51% in M1 and M10, and 10.07 and 23.37% in T1 and T10, respectively. In addition, the lag phase was shortened by the addition of MBC under all tested conditions. The shortened lag phases (%) were - 3.05 and - 14.34 in P1 and P10, - 10.10 and - 26.92 in M1 and M10 and - 9.14 and - 24.37 in T1 and T10, respectively. Similarly, Sun et al. [23] reported shortened lag phase and increased methane yield by addition of cow manure-derived BC (2, 6, 10 and 14 g/L) to AD of beer lees. Also, addition of chicken manure-derived BC (5% of TS; 11.91 g/L) to AD of chicken manure increased methane yield, although it increased the lag phase. Li et al. [22] applied 10g/L of sawdust-derived BC in co-digestion of food waste and waste activated sludge. They reported shortened lag phase and increased maximum methane production rate under thermophilic conditions with different ISR (0.33 – 4). Similarly, Wang et al. [21] reported positive effects of sawdust-derived BC (2, 6, 10 and 15 g/L) on mesophilic AD, while decreased methane yield was observed with 15g/L of BC. Cai et al. [20] achieved a shortened lag phase and increased methane yield by addition of fruit wood-derived BC (2, 5 and 10 g/L) to AD of food waste, however negative effects were observed when the concentration of BC and ISR were 2 g/L and 2, respectively. Additionally, negative effects from addition of fruit wood-derived BC (10 g/L) on methane yield in AD of synthetic wastewater (glucose as carbon source (2, 4, 6 and 8 g/L)) were observed [19]. Taken together, the results presented suggest that addition of BC is a potential option to enhance reactor performance of AD. However, to minimize the negative effect, several factors (e.g., ISR, type of BC, concentration of BC, feedstock for AD, temperature for AD and pyrolysis) should be considered before addition of BC in AD. In this study, one ISR and pyrolysis temperature was applied as a conceptual experiment. Thus, further studies are needed to understand the relationship between several factors and reactor performance.
3.2. High-throughput Sequencing DataUsing high-throughput sequencing analysis, a total of 3,974 and 78 OTUs (based on 3% dissimilarity) were obtained for bacterial and archaeal community, respectively. The values in parentheses in this section are for the archaeal community. More specifically, a total of 1840 (16) OTUs were assigned to the initial samples (Fig. S1). A total of 1371 (19), 1390 (19), 1866 (17), 1397 (21) and 1275 (17) OTUs were assigned to P0, P1, P1A, P10 and P10A, respectively. For the samples from AD under mesophilic conditions, a total of 1342 (19), 1475 (16), 1668 (19), 1847 (21) and 1588 (22) OTUs were assigned to M0, M1, M1A, M10 and M10A, respectively. In addition, a total of 1539 (20), 1510 (25), 1461 (21), 1740 (23) and 1121 (21) OTUs were assigned to T0, T1, T1A, T10 and T10A, respectively. The Venn diagram analysis indicated that the shared OTUs for psychrophilic, mesophilic and thermophilic conditions were 591 (9), 671 (10) and 527 (8), respectively (Fig. S1). As expected, lower microbial diversity (i.e. Chao1, species richness (number of observed species), Shannon and Simpson index) in thermophilic conditions was observed compared to those of psychrophilic and mesophilic conditions (Table S1), which is consistent with the literature [36]. The response of microbial diversity to addition of MBC was not universal. For example, it increased in P1, P10, M1 and T10, while it decreased in M10 and T1.
3.3. Relative Abundance of Bacterial Phyla and Archaeal OrderThe relative abundance of bacterial phyla in all samples is shown in Fig. 1 and S2. Overall, 42 bacterial phyla were detected in all samples. In P0 and M0, the phylum Bacteroidetes showed the highest relative abundance. This phylum decreased in P1 (− 19.93%), P10 (− 23.45%), M1 (− 3.79%), while it slightly increased in M10 (+ 0.31%). Unlike P0 and M0, the relative abundance of Bacteroidetes in T0 was lower than 2%, whereas Firmicutes showed the highest relative abundance (72.04%). This was consistent with the data from Lee et al. [36], who reported the predominance of Firmicutes in thermophilic AD. This phylum increased in T1 (+ 7.88%), but decreased in T10 (− 0.32%).
The archaeal community structure (Fig. 1) showed that the most dominant order was Methanobacteriales (ranging 61.25 – 65.17%) followed by Methanosarcinales (ranging 21.43 – 30.00%) and Methanomicrobiales (ranging 6.25 – 10.11%) in all conditions without the addition of MBC (P0, M0 and T0). In P1 and P10, the relative abundance of Methanobacteriales (− 4.44 and − 4.13%) and Methanosarcinales (− 13.33 and − 5.04%) decreased, but the relative abundance of Methanomicrobiales (+ 113.33 and + 51.94%) increased, respectively. Similar to P1 and P10, increase in the relative abundance of Methanomicrobiales (+ 55.56 and + 18.01%) was observed in M1 and M10, respectively. Interestingly, an obvious increase in the relative abundance of Methanosarcinales (+ 34.17 and + 22.03%) was observed in M1 and M10, respectively. Furthermore, a decrease in the relative abundance of Methanomicrobiales (− 33.18%) was observed in T1, whereas the order increased in T10 (+ 12.35%).
3.4. Relative Abundance of Bacterial and Archaeal GeneraTo further explore possible functions of the classified members detected in this study, the taxonomic distribution of bacterial and archaeal communities was determined at genus level (Figs. 2 and S4). The genus Ruminofilibacter was frequently detected in AD of manure [37] and increase of relative abundance of this genus at 50°C was observed but decreased at 55°C compared to 25°C [38]. Similarly, this genus showed highest the relative abundance in P0 (14.50%) and M0 (10.67%), whereas it was lower than 1% in T0 (0.08%). The relative abundance of Ruminofilibacter decreased in P1 (− 29.75%) and P10 (− 32.39%), whereas it increased in M1 (+ 6.12%), M10 (+ 3.89%), T1 (+ 83.91%) and T10 (+ 78.29%). Considering the putative role of this genus (i.e. hydrolysis) [38], higher hydrolysis activity which resulted in higher methane production might be induced by addition of MBC under mesophilic and thermophilic conditions.
Enhanced methane production was highly related to the relationship between syntrophic bacteria and hydrogenotrophic archaea [16]. As shown in Fig.2, genus Clostridium, a well-known syntrophic bacteria (i.e. metabolize organic acids to acetate with the production of H2), was a common member in all samples. The increase in relative abundance of this genus was observed in samples with MBC compared to those without MBC. In particular, genus Clostridium was most abundant under thermophilic conditions and their relative abundance increased in T1 (+ 24.27%) and T10 (+ 25.57%). In addition, other syntrophic bacteria from genera Syntrophomonas and Syntrophus were detected in all samples. These genera are frequently detected in AD and are able to convert propionic and butyric acid to acetic acid and H2 [39, 40]. Interestingly, these genera increased in T10 while decreased in M1. The increase in genus Syntrophomonas was observed in P1 (+ 32.48%) and P10 (+ 68.34%). Similarly, Dang et al. [40] reported that the carbon-based conductive materials potentially stimulated the growth of syntrophic bacteria in AD. Furthermore, the enrichment of syntrophic bacteria with addition of MBC correlated with the VFAs concentration in AD (i.e. lower concentration of propionic acid and TVFAs) (Fig. S3).
The archaeal community structure at genus level is shown in Fig. 2. In all samples, Methanobacterium was the most dominant archaeal genus. This genus is often abundant in AD and participates in hydrogenotrophic methanogenesis, converting CO2 and H2 into CH4 [16]. This genus was increased in all conditions with addition of MBC except M1 and T10. In contrast to the data from Wang et al. [21], who added sawdust BC to AD, an increase of genus Methanolinea (known hydrogenotrophic methanogen) was observed in the MBC-added conditions, except T1. For instance, higher abundance of genus Methanolinea was observed in M1 (+ 110.00%) and M10 (+ 66.28%) than in M0 (7.95%). In addition, increase in hydrogenotrophic methanogens from genus Methanomassiliicoccus was observed in MBC added conditions, except P1. Interestingly, a remarkable increase in genus Methanomassiliicoccus was observed in T1 (+ 168.46%) and T10 (+ 515.38%). This result was consistent with the data from Dang et al. [40] who reported the expansion of genus Methanomassiliicoccus with addition of carbon-based conductive materials. In contrast, a gradual decrease in this genus was found (Sun et al. [23]) under mesophilic conditions with increased loading of cow manure-derived BC. Collectively, the results suggest that enhanced syntrophic relationships induced by addition of MBC may play an important role in modifying AD performance [14, 40].
As shown in Fig. 2, the relative abundance of genus Methanosaeta (i.e. well known as aceticlastic methanogens) in P0, M0 and T0 was 21.43%, 22.73% and 6.25%, respectively. With the addition of MBC, this genus was decreased in P1 (− 12.08%), P10 (− 4.48%) and T1 (− 3.36%), suggesting that the pathway of CH4 production by aceticlastic methanogens was diminished. However, increase in genus Methanosaeta was observed in M1 (+ 34.17%), M10 (+ 22.03%) and T10 (+ 34.27%). This result was consistent with data from Peng et al. [41] and Wang et al. [21] who reported the enrichment of genus Methanosaeta with adding magnetite and granular AC and sawdust BC under mesophilic conditions, respectively. However, Lin et al. [16] reported a decrease in this genus when adding graphene under mesophilic conditions. Unlike genus Methanosaeta, genus Methanosarcina, which has flexible switching metabolisms (i.e., both aceticlastic and hydrogenotrophic methanogenesis), was observed only under thermophilic conditions. In T0, the relative abundance of genus Methanosarcina (23.75%) was higher than that of genus Methanosaeta (6.25%). Considering the higher concentration of TVFAs in T0 than M0 and P0, this might be due to the relatively higher tolerance of genus Methanosarcina to accumulation of organic acids than genus Methanosaeta [42]. With addition of MBC, decrease in this genus was observed in T1 (− 35.01%) and T10 (− 55.83%).
3.5. Microorganisms on the MBCIn recent studies, formation of biofilms on carbon-based materials such as BC and AC in AD was frequently observed [14, 21, 43, 44]. The relative abundance of several bacterial and archaeal genera on MBC are summarized in Table 2. Interestingly, several syntrophic bacterial genera including Clostridium, Syntrophomonas, Syntrophobacter and Syntrophus were detected on MBC under all temperature conditions, while genus Symbiobacterium was detected only under thermophilic conditions. Among them, genus Clostridium was most dominant under the mesophilic and thermophilic conditions, while the relative abundance of genus Syntrophus was slightly higher than that of genus Clostridium under the psychrophilic conditions. In addition, several hydrogenotrophic methanogens were detected on MBC under all temperature conditions. In particular, genera Methanobacterium and Methanomassiliicoccus were dominant on MBC under all temperature conditions. The formation of biofilms includes the syntrophic bacteria and hydrogenotrophic methanogens on MBC suggesting that the mediated interspecies electron transfer (MIET, i.e. using hydrogen and formic acid as sole electron shuttles for methane production) might be accelerated by decreasing distance between the microorganisms [43].
According to previous research [45, 46], carbon-based conductive materials can act as both electron acceptor and donor in AD. In addition, compared to electrical conductivity of granular AC (3000 μS/cm [45]) and pine wood-derived BC (2.11 – 4.41 μS/cm [47]), MBC showed relatively high electrical conductivity of 3230 μS/cm mainly due to its high nutrient and metal compositions [48]. Also, Pan et al. [24] reported higher electrical conductivity of chicken manure-derived BC (1420 – 1730 μS/cm) than wheat straw-derived BC (210 – 460 μS/cm) and fruit wood-derived BC (320 – 590 μS/cm). It has been reported that genus Geobacter can transfer electrons to genus Methanosaeta and Methanosarcina through extracellular pili or conductive materials [49, 50]. Similar to genus Geobacter, genus Pseudomonas is recognized as electrogenic bacteria which can covert VFAs to electric current, while it has poorly conducting pili [51]. Therefore, MBC could be an alternative for conductivity pili. Once electron transfers occur to genera Methanosaeta and Methanosarcina, they can reduce CO2 to CH4, although they are considered aceticlastic methanogens [21]. As shown in Table 2, genera Geobacter and Pseudomonas were detected on MBC although their relative abundance was lower than 0.4%. In addition, archaeal genera Methanosaeta and Methanosarcina were detected on MBC with high relative abundance. Taken together, DIET on MBC could be one of the possible mechanisms for the enhancement of methane production in AD with addition of MBC. To date, the possibility of DIET by other potential electron-donating bacteria (e.g., Clostridium and Syntrophomonas), which are frequently found in the anodes of bio-electrochemical processes, is still unclear [43, 52]. Thus, further study is needed to verify that those bacteria are responsible for DIET.
4. ConclusionsThe addition of MBC in AD was found to improve methane production and to enhance reactor stability. Estimated parameters from the kinetic model suggested that the addition of MBC shortened the lag phase of AD. Microbial community analysis indicated that growth of syntrophic bacteria and hydrogenotrophic methanogens was potentially enhanced by MBC. In addition, the microbial community on the MBC suggested the possibility of DIET in AD with MBC.
AcknowledgmentThis work was supported by the Texas A&M University Chancellor’s Research Initiative Fund.
NotesAuthor Contributions H.M.J (former postdoctoral researcher at Texas A&M University, currently assistant professor at Jeonbuk National University) conducted all the experiments. H.M.J and E.K (associate professor at Texas A&M University) analyzed the experimental data. J. B (assistant professor at Texas A&M University) prepared the DNA samples for sequencing while helping the analysis of bioinformatic information. H.M.J and E.K prepared, reviewed and edited the manuscript. References1. Jang HM, Cho HU, Park SK, Ha JH, Park JM. Influence of thermophilic aerobic digestion as a sludge pre-treatment and solids retention time of mesophilic anaerobic digestion on the methane production, sludge digestion and microbial communities in a sequential digestion process. Water Res. 2014;48:1–14.
2. Shin H, Han S, Song Y, Lee C. Performance of UASB reactor treating leachate from acidogenic fermenter in the two-phase anaerobic digestion of food waste. Water Res. 2001;35:3441–3447.
3. Kafle GK, Chen L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016;48:492–502.
4. Appels L, Lauwers J, Degrève J, et al. Anaerobic digestion in global bio-energy production: potential and research challenges. Renew Sust Energ Rev. 2011;15:4295–4301.
5. Monlau F, Francavilla M, Sambusiti C, et al. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl Energ. 2016;169:652–662.
6. Jang HM, Ha JH, Park JM, Kim MS, Sommer SG. Comprehensive microbial analysis of combined mesophilic anaerobic–thermophilic aerobic process treating high-strength food wastewater. Water Res. 2015;73:291–303.
7. Noorollahi Y, Kheirrouz M, Asl HF, Yousefi H, Hajinezhad A. Biogas production potential from livestock manure in Iran. Renew Sust Energ Rev. 2015;50:748–754.
8. Levis JW, Barlaz MA, Themelis NJ, Ulloa P. Assessment of the state of food waste treatment in the United States and Canada. Waste Manag. 2010;30:1486–1494.
9. Atandi E, Rahman S. Prospect of anaerobic co-digestion of dairy manure: a review. Environl Technol Rev. 2012;1:127–135.
10. Carrère H, Dumas C, Battimelli A, et al. Pretreatment methods to improve sludge anaerobic degradability: a review. J Hazard Mater. 2010;183:1–15.
11. Jang HM, Ha JH, Kim MS, et al. Effect of increased load of high-strength food wastewater in thermophilic and mesophilic anaerobic co-digestion of waste activated sludge on bacterial community structure. Water Res. 2016;99:140–148.
12. Fitamo T, Treu L, Boldrin A, et al. Microbial population dynamics in urban organic waste anaerobic co-digestion with mixed sludge during a change in feedstock composition and different hydraulic retention times. Water Res. 2017;118:261–271.
13. Shin SG, Han G, Lim J, Lee C, Hwang S. A comprehensive microbial insight into two-stage anaerobic digestion of food waste-recycling wastewater. Water Res. 2010;44:4838–4849.
14. Yang Y, Zhang Y, Li Z, et al. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J Clean Prod. 2017;149:1101–1108.
15. Zhang J, Zhang L, Loh KC, Dai Y, Tong YW. Enhanced anaerobic digestion of food waste by adding activated carbon: Fate of bacterial pathogens and antibiotic resistance genes. Biochem Eng J. 2017;128:19–25.
16. Lin R, Cheng J, Zhang J, et al. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour Technol. 2017;239:345–352.
17. Zhang J, Wang Z, Wang Y, et al. Effects of graphene oxide on the performance, microbial community dynamics and antibiotic resistance genes reduction during anaerobic digestion of swine manure. Bioresour Technol. 2017;245:850–859.
18. Shen Y, Linville JL, Ignacio-de Leon PAA, Schoene RP, Urgun-Demirtas M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J Clean Prod. 2016;135:1054–1064.
19. Luo C, Lü F, Shao L, He P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015;68:710–718.
20. Cai J, He P, Wang Y, Shao L, Lü F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag Res. 2016;34:409–416.
21. Wang G, Li Q, Gao X, Wang XC. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour Technol. 2018;250:812–820.
22. Li Q, Xu M, Wang G, et al. Biochar assisted thermophilic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour Technol. 2018;249:1009–1016.
23. Sun C, Liu F, Song Z, et al. Feasibility of dry anaerobic digestion of beer lees for methane production and biochar enhanced performance at mesophilic and thermophilic temperature. Bioresour Technol. 2019;276:65–73.
24. Pan J, Ma J, Liu X, et al. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour Technol. 2019;275:258–265.
25. Monlau F, Sambusiti C, Antoniou N, Barakat A, Zabaniotou A. A new concept for enhancing energy recovery from agricultural residues by coupling anaerobic digestion and pyrolysis process. Appl Energ. 2015;148:32–38.
26. Jang HM, Choi Y-K, Kan E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour Technol. 2018;250:927–931.
27. Jang HM, Lee J, Choi S, et al. Response of antibiotic and heavy metal resistance genes to two different temperature sequences in anaerobic digestion of waste activated sludge. Bioresour Technol. 2018;267:303–310.
28. Leitch ECM, Walker AW, Duncan SH, Holtrop G, Flint HJ. Selective colonization of insoluble substrates by human faecal bacteria. Environ Microbiol. 2007;9:667–679.
29. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335.
30. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461.
31. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200.
32. Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504.
33. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072.
34. Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2008;37:D141–D145.
35. Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC bioinformatics. 2015;16:169.
36. Lee J, Shin SG, Han G, Koo T, Hwang S. Bacteria and archaea communities in full-scale thermophilic and mesophilic anaerobic digesters treating food wastewater: Key process parameters and microbial indicators of process instability. Bioresour Technol. 2017;245:689–697.
37. Wang M, Zhou J, Yuan YX, et al. Methane production characteristics and microbial community dynamics of mono-digestion and co-digestion using corn stalk and pig manure. Inter J Hydrog Energ. 2017;42:4893–4901.
38. Lin Q, De Vrieze J, Li J, Li X. Temperature affects microbial abundance, activity and interactions in anaerobic digestion. Bioresour Technol. 2016;209:228–236.
39. Zhao Z, Zhang Y, Holmes DE, et al. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour Technol. 2016;209:148–156.
40. Dang Y, Sun D, Woodard TL, et al. Stimulation of the anaerobic digestion of the dry organic fraction of municipal solid waste (OFMSW) with carbon-based conductive materials. Bioresour Technol. 2017;238:30–38.
41. Peng H, Zhang Y, Tan D, et al. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour Technol. 2018;249:666–672.
42. De Vrieze J, Hennebel T, Boon N, Verstraete W. Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour Technol. 2012;112:1–9.
43. Capson-Tojo G, Moscoviz R, Ruiz D, et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour Technol. 2018;260:157–168.
44. Qin Y, Wang H, Li X, Cheng JJ, Wu W. Improving methane yield from organic fraction of municipal solid waste (OFMSW) with magnetic rice-straw biochar. Bioresour Technol. 2017;245:1058–1066.
45. Liu F, Rotaru AE, Shrestha PM, et al. Promoting direct interspecies electron transfer with activated carbon. Energ Environ Sci. 2012;5:8982–8989.
46. Lovley DR. Syntrophy goes electric: direct interspecies electron transfer. Ann Rev Microbio. 2017;71:643–664.
47. Chen S, Rotaru A-E, Shrestha PM, et al. Promoting interspecies electron transfer with biochar. Sci Rep. 2014;4:5019.
48. Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol. 2012;107:419–428.
49. Rotaru A-E, Shrestha PM, Liu F, et al. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl Environ Microbiol. 2014;80:4599–4605.
50. Rotaru A-E, Shrestha PM, Liu F, et al. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energ Environ Sci. 2014;7:408–415.
Fig. 1The relative abundance (%) of bacterial phyla (a) and archaeal order (b). Sequences showing percentages of reads < 1% of all samples were grouped into “Others”. More detailed values of inhibition and promotion ratios (%) of the relative abundance of bacterial phyla and archaeal order compared to non-BC added conditions (P0, M0 and T0) are summarized in Fig. S3. 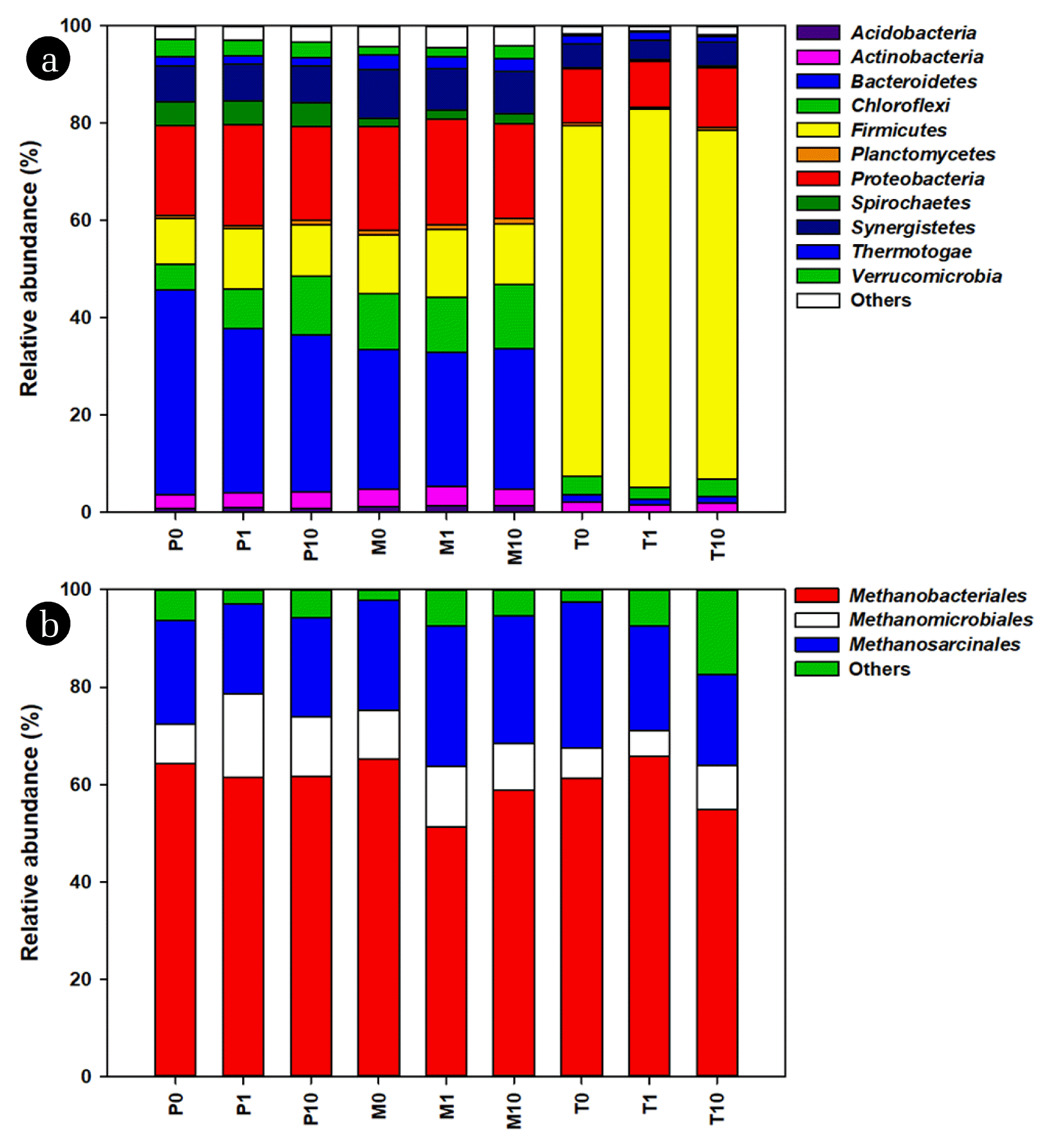
Fig. 2(a) Heat map showing the relative abundance of the top 20 bacterial genera (b) and the top 10 archaeal genera. More detailed values of inhibition and promotion ratios (%) of the relative abundance of bacterial phyla and archaeal order compared to non-BC added conditions (P0, M0 and T0) are summarized in Fig. S4. 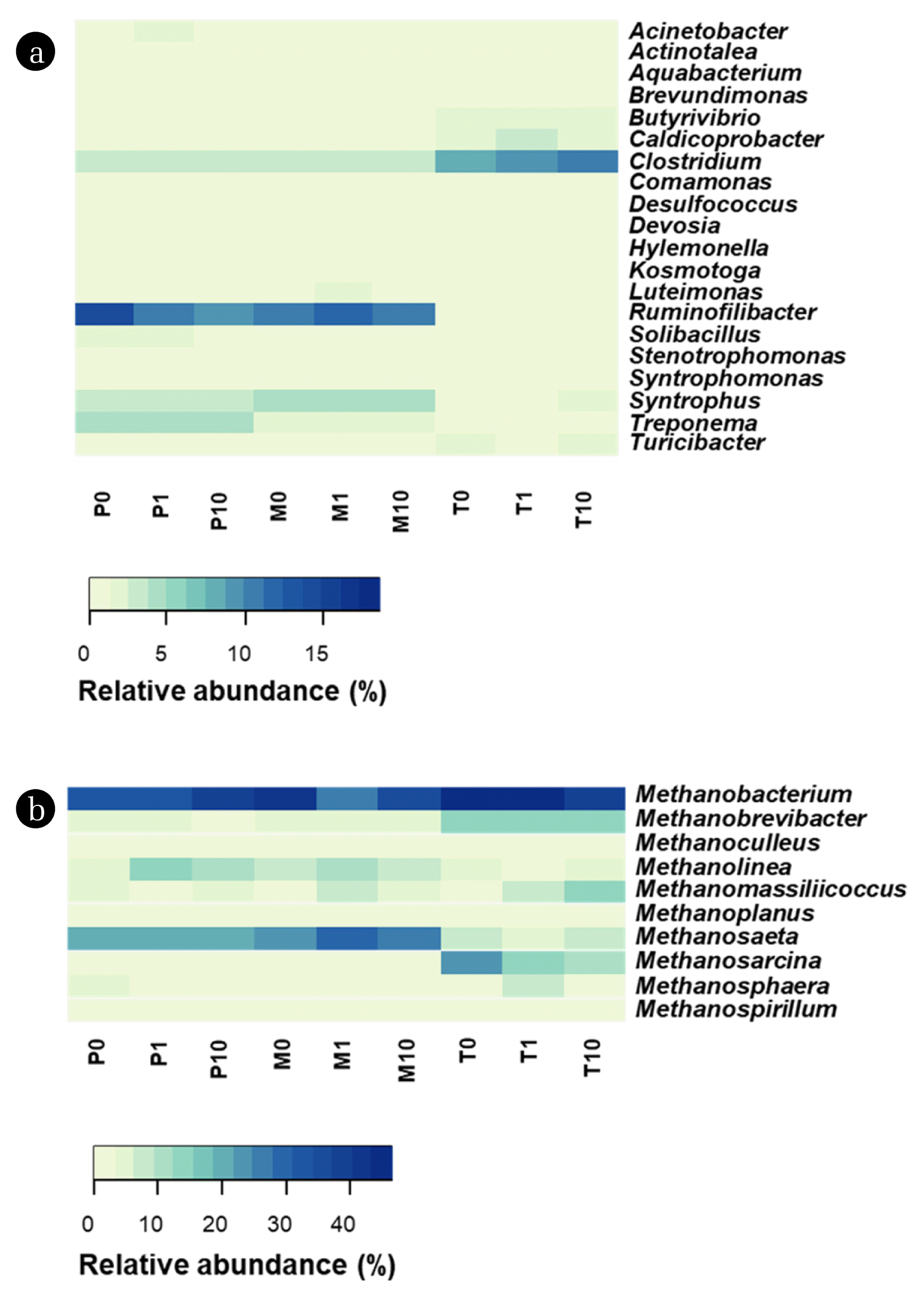
Table 1Summary of Major Impacts of Various Biochars on AD.
Table 2The Relative Abundance of Several Microorganisms on MBC
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||