1. Introduction
During the transformation of released oil, various kinds of physical, biological, and chemical changes happen due to releasing oil into the environment. Collectively the processes as mentioned regarding weathering that explains the action while changing the composition, exposure, execution, and toxicity level of extracted oil [1]. The United States Environmental Protection Agency (USEPA) has found that the soil contaminated with petroleum is harmful to the animals and people’s health that affect the lungs, kidney, liver, and nervous system. It also causes cancer and various disorders concerning the reproductive system and the immune system [2]. To determine the total content of oil, there are various methods like total organic carbon (TOC), total carbon (TC), total petroleum hydrocarbon (TPH), etc. Based on the environmental sciences division UK [3], TPH is a frequently utilized gross parameter to quantify the impurity of the environment created by petroleum hydrocarbons compounds (PHC) products like fuels, oils, lubricants, waxes, and others. There are four primary structural forms in which TPH can be categorized, and these are aliphatic, aromatic, resins, and asphaltenes compounds. Based on the molecular structure, aromatic hydrocarbons are classified into two groups. These groups are high molecular weight (HMW) compounds and low molecular weight (LMW) compounds [4]. For determining the TPH level, the base of traditional wet chemistry methods is the “extraction of contaminant from the sample of soil.” Various methods like gas chromatography-mass spectrometry (GC-MS) gravimetric calculation calibrated by an EPA calibration standard are used to determine the level of TPH in the extracted solution. Moreover, this study was also designed because of the limited information about the concentration of the constituent of petroleum hydrocarbons in the Kuwait oil sand. A major factor that triggers the contamination in Kuwait is weathered burnt crude oil, which has been exposed to the environment for over the past 30 years, causing most of its volatile substances to evaporate into the atmosphere with heavy compounds left as a residue. The Iraqi military extensively laid landmines in Kuwait oilfields as well as other areas around Kuwait in 1991. Unexploded ordnance (UXO) fired during the conflict were also left which need to be cleared. Because of the difficulty of access, no clearance certificates were issued for the wet oil lake features within the oil-contaminated areas. Considering the inability to clear wet oil lakes and the depth of excavation in oil-contaminated dry areas, all areas will be subject to further UXO survey and clearance. In addition, due to shifting sands, all areas will need to be surface swept. Because of this, only a few locations were cleared and approved for sampling, such as south of the Burgan oil field.
The present work aims to evaluate the total petroleum hydrocarbon concentration (TPH) because it can determine a wide range of hundreds of different compounds that are present in crude oil. Furthermore, the reason behind the TPH selection among various other methods is that it is the most relevant method in this case because the core contaminants that are supposed to be analyzed are based on petroleum, which could estimate the oil-pollution level and provides better indication of the pollution-degree extracting from oil.
2. Materials and Methods
2.1. Soil Sampling, Preparation, and Analysis
Weathered contaminated sand samples from the Burgan oil field in the Kuwait desert (29° 06′ −11″ N, 47° 57′56″E) were collected, between January 2021and March 2021. On the first day of each month, a 3-kg sample of oil contaminated sand was collected. Samples of 100 g were sealed in sterile sampling bags and were then stored in the fridge at 3°C until subsequent analysis. Uncontaminated soil was collected from the desert in the Burgan oil field, the soil was sieved through 2 mm openings for 2 min to remove coarse particles. Soil texture for uncontaminated soil was 85% sand and 15% silt, as determined by the hydrometer method ASTM D7928 [5]. Uncontaminated soil was classified as loamy sand and had the following properties (dry weight basis): pH of 7.4 at a ratio 1:1 soil to water [6]. The Uncontaminated soil contained 0.7% organic matter according to Walkley–Black method [7]. Water saturation for this soil was 23.6 cm/h on a dry weight basis, based on ASTM F1815-11 [8]. Weathered oil contaminated sand with properties shown in Table 1 was used for the test.
2.2. Extraction Process
At a ratio of 1:1 w/w, the soil with full of contamination of 1g and the mixing of it with Hydromatrix concentrate was employed (Varian, UK). Then to make the appearance of the mixture dry and powdery, it was ground vigorously. The addition of sample in a 22 mL stainless steel extraction vial was done (Dionex, UK) and partitioned with disposable cellulose filters (Dionex, 52 UK) and acid-washed sand (VWR, UK). Using Dionex method 324 accelerated solvent extractor (ASE) 200 (Dionex, UK) has been used for the process of extraction. The ASE cell is left in an ASE carousel to facilitate the process of extraction. Hexane/acetone, 1:1 (Fisher Scientific, UK) brought into the cell together with the level up to 50 mL while extraction takes place. With the assistance of nitrogen, the flushing out of extract from the cell into a collection bottle. To perform the analysis on the extract, GC-MS was used. Then, the specimen solution with the amount of 1.5 mL was pipetted into the GC autosampler vial (Technopath, UK) and capped by crimp caps.
2.3. Measuring the Levels of Weathered Crude Oil by GC-MS
Due to the effectiveness of GC-MS in various major classes of organic samples, it has been employed widely in ongoing research regarding the environment. On a Varian 21-MS IT and a Varian 43GC with an auto-sampler CP-8400 (Fig. 1), analyses of weathered crude oil in terms of TPH were done to observe and assess the soil sample for the traces of petroleum hydrocarbons. For the GC-MS, the assistance of a DB-5 MS capillary column (15 m × 0.32 mm i.d. × 0.1 μm film) was employed. The working of the GC-MS is done by mixing the solution of the specimen with air and helium into the column. Here are these conditions for GC-MS; oven temperature program, 50°C, injector temperature, 300°C; for 2 min – 300°C for 20 min at 5°C/min. 1 μl sample of the sample brought together into the GC inlet by injection in this state it vaporised and swept onto a chromatography column by helium, which is used as the carrier with a constant flow rate of 1.0 mL/min at 300°C and then carried on to the MS console. The electron impact model was used for the ionisation of the sample. The MS was scanned from m/z 35–550, with a 3 min solvent delay and a 150-threshold count.
2.4. Gravimetric Method for Analysis of Weathered Crude Oil
There are two phases of the gravimetric method. Phase one focuses on the extraction of the weathered crude oil from sand, whereas the second phase focuses on determining the total petroleum hydrocarbons recovered percentage from contaminated sand. Here is a procedure through which the oil was extracted from the sand. One gram of full of contaminated soil sample was specified by using a screw-capped bottle with the ratio of (1:1 v/v) of acetone and hexane solvent and the amount of 5mL of this ratio. Then in an ultrasonication bath, it was washed for 5 min. The dry and clean weighed round-bottomed glass flask, the crude oil and the acetone: hexane (1:1 v/v) solution were pipetted. By using fresh solvent for each cycle, the ultrasonication cycle was then revised four times. The dry weight was noted after the air dry of isolated sand for 2 h. The collection apparatus, distillation head with condenser sidearm, water bath, and conical flask all were arranged and fixed. For the rapid evaporation of the acetone: hexane mix, the temperature of the water bath has been kept at 52°C. At last, to calculate the isolated weathered crude oil concentration, the weight of crude oil was reweighted when the round glass flask was dried, and the acetone: hexane mix was recovered.
Where, the weight of the crude oil sediment plus the round flask is Wb, while the weight of the round flask is Wa. While the percentage removal of weathered crude oil is calculated by
Where, the initial concentration of the weathered oil in the soil (g) before washing is Wd, while the concentration of weathered crude oil in the soil (g) after washing is Wf.
3. Results and Discussion
3.1. Analysis of weathered Oil Using Gas Chromatography-Mass Spectrometry (GC-MS)
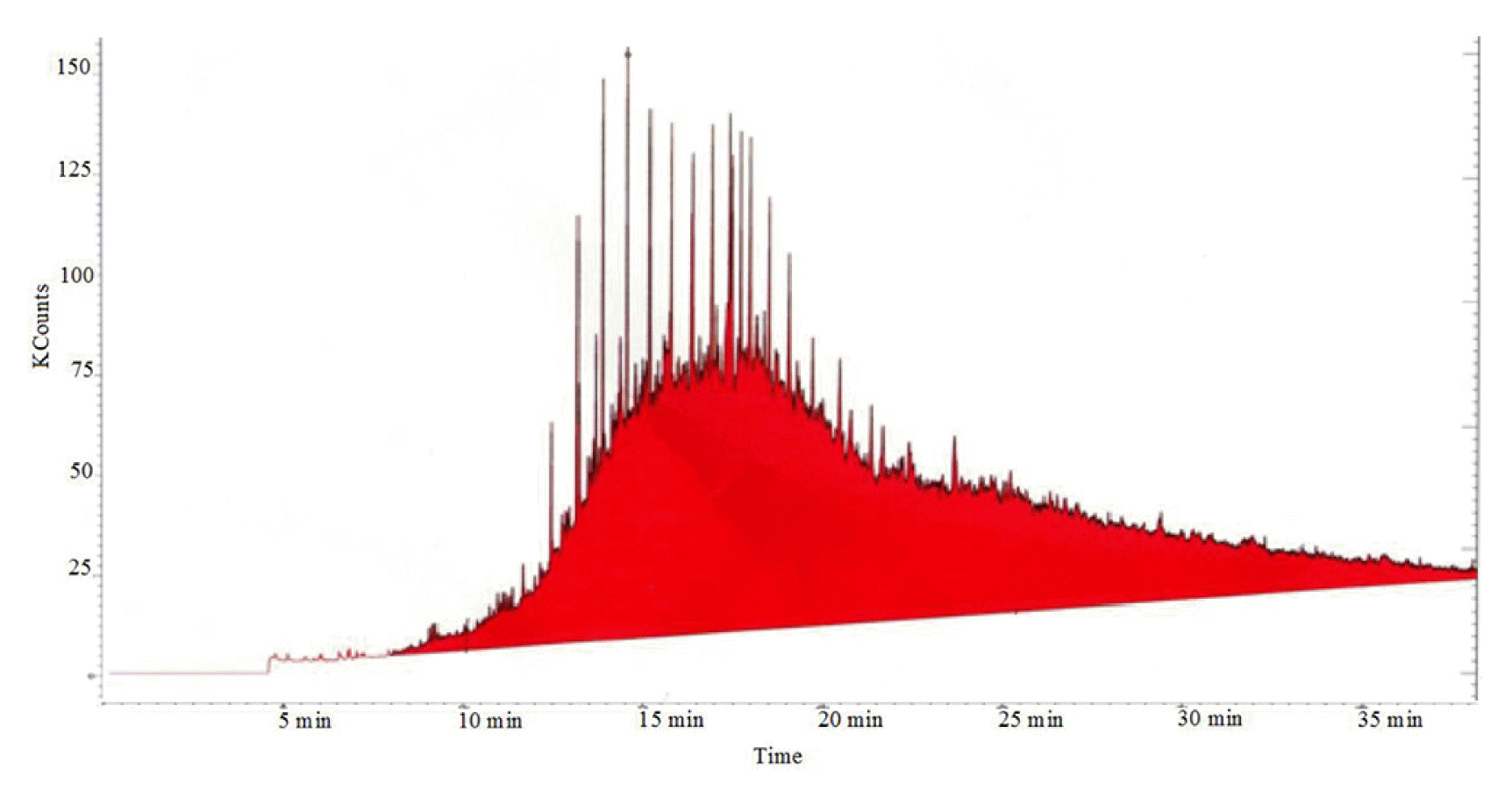
In analytical chemistry, all of the extraction procedures mainly aim to separate the analyte from its speedily, quantitatively, and utilising as little solvent as possible [13]. To extract quantitatively several semi-volatiles comprising, phenols, polychlorinated biphenyls (PCBs), Polycyclic Aromatic Hydrocarbons (PAHs) as well as total petroleum hydrocarbons, and accelerated solvent extraction (ASE) procedure was used [14]. The segregation, identification, and quantification of a complex mixture of chemicals, an instrumental method known as GC-MS containing a gas chromatograph (GC) coupled to a mass spectrometer (MS) was used. To examine hundreds of compounds compared to LMW, this method proves one of the most suitable methods. For the analysis using the GC-MS technique, the compound should be thermally stable and satisfactorily volatile. To analyse weathered oil in the soil, this study was done using GS-MS. The initial results indicated that the GC-MS analyses showed that the TPH concentration variation from 183,432 to 212,993 mg/kg was observed. These results were based on the preliminary study done for the weathered oil contaminated sand. By this analysis, in the sample, no traces of PAH compounds were observed. It was illustrated by the GC profile that after passing 15 min, the level of TPH begins to rise, which shows the fact that the long-chains of hydrocarbons were presented in the sample, Fig. 1.
The sample’s chemical modification is necessary to omit all those compounds that can put an influence the quality of obtained record before conducting the GC analysis. To take a correct and precise calculation for aromatic and aliphatic compounds and protect the GC, the high content of asphaltenes was removed. As most of the constituents could not be resolved, the major portion of the diesel oil was also not specified. These constituents appear as ‘hump’ in the chromatogram, which is named unresolved complex mixture’ (UCM), containing branched, cyclic alkanes, and polar transformation products [15]. The resolved hydrocarbons are referred to as ‘total resolved hydrocarbons’ (TRH). To become TPH, the UCM and TRH were combined. In the GC-MS, the TRH is observed as peaks that are non-degraded hydrocarbons, as Fig. 2 illustrates.
During the measurement, this method faced some difficulties. ‘Blocked after each run’ was the main challenge caused by the GC column. So, this difficulty gave rise to the need for an alternative way for analysing TPH from samples of soils, as provided in the next passage.
In comparison to the non-weathered samples, the chemical and physical properties of the weathered contaminated soil changed over time, resulting in contaminated soil with a larger oil molecular weight and greater density and viscosity [15, 16]. It was then assumed that the organics and non-organics (such as sulfur, carbon residues, aromatics, asphaltenes, and metals) in the oil have concentrated more than those in the original oil. By the early 1970s, the term unresolved complex mixture (UCM) was used to describe a broad class of petroleum-based environmental contaminants [16]. This “hump-fit” approach to distinguishing different sources of UCM has been used for over four decades. The method provides little information about the chemical composition of UCM. Additional information is required to accurately determine the source of UCM contamination, determine the extent of UCM weathering or biodegradation, and evaluate the residual toxicity. The most volatile compounds are removed via evaporation, polar and water-soluble compounds are removed via dissolution, furthermore, biodegradation attacks the linear alkanes, branched alkanes, and then the cycloalkanes and aromatics [17]. This hump is formed by recalcitrant petroleum compounds that do not degrade. A GC-MS study was carried out on weathered and non-weathered soil samples to measure the concentration of these compounds in the sand. Fig. 1 indicates the majority of carbon numbers more than C16 was increased. With the unresolved complex mixture in Fig. 2, the weathered samples reasonably represent real-life contaminated soil. This finding is similar to Urum et al. [18], this may be due to the nature of contamination. Lighter n-alkanes are found in the lowest concentrations. As their molecular weight increases, their concentration increases Fig. 1. The alkane n-C10 is found as a minor constituent with a concentration between 420.5 mg/kg and 662.8 mg/kg. The concentration of n-C16 and > n-C30 was about 15,700 mg/kg and 28,800 mg/kg, respectively. Such a characteristic is typical of hydrocarbons of natural origin, such as oil spills [19]. Diesel n-alkane peaks are seen in the chromatogram since the peaks were preferentially extracted from sand using combinations of solvents. The shape and range of this hump are almost identical to those observed by Reddy at the same site 19 years earlier (see Fig. 2) [20]. The peaks visible on top of the UCM may be caused by other abundant contaminants such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and substituted benzotriazoles [21, 22]. Numerous PAHs were also identified in this sample, and they form a band across the GC-MS chromatogram from phenanthrene (peak 4) to benzo[ghi]perylene (peak 12).
3.2. Analysis of Weathered Crude Oil Using Gravimetric Method
For the distinction of TPH in impure soil with petroleum hydrocarbon products, there is no standard method yet available [23]. For this purpose, various researches [24, 25] have been conducted and utilized. ASE, Soxhlet extraction, or ultrasonication was involved in most of the techniques that were used for the separation of oil from the sand in the laboratory. With the help of a suitable solvent, ultrasonication has been chosen for the extraction of the oil from the contaminated sand. It is conveniently available with high extraction efficiency and is economical. Using the gravimetric technique, the amount of weathered crude oil can be calculated.
3.2.1. Different sample weight
Beginning from the most polluted sample, the selection of 5 g and 1 g of the oil contaminated samples was based on the level of contamination present in them. Three cycles were required to clean out 1 g contaminated soil, while six cycles were needed to clean 5 g contaminated soil. After completing the washing cycle and extraction, the weight of crude oil and the weight of the oil-contaminated soil sample were calculated through equation 1. Moreover, the data is given in Table 2.
With 15 mL of the solvent, the one gram of sample took 30 min to clean, while with 150 mL of solvent, the 5 g sample took 60 min to clean. By gradually increasing the weight of the sample soil, the compatibility of the oil contaminants and extraction of solvent was not likely to be altered. So that is why in this study, 1 g was recommended.
3.2.2. Type of solvent
To do the process of flushing out of crude oil from contaminated sand in a short time with high extraction efficiency and a less harmful product, this technique involved some ‘determining suitable solvents. A wide range of solvents like dichloromethane (DCM), chloroform, and hexane can be used. For the current experiment, DCM, DCM: hexane (1:1 mix), and acetone: hexane (1:1 mix) was the selected solvents. In the isolation of organic compounds from soil, these solvents were found to be the most influential solvent than other solvents [26]. By using the primary method elaborated in Section 2.4, the selected solvents were characterized using the same procedures as employed above and conducted in three trials. Different solvents trials were made, and in three washes, the ultrasonication process cleans out 1 g of contaminated soil. The sand weight for all 3 samples and processed oil was calculated after completing the solvent recovery process, as presented in Table 3.
Based on Table 3, for the solvent ratio of acetone: hexane (1:1 v/v), the highest concentration of the crude oil on average was 341,000 mg/kg. As chloroform affects the central nervous system and the people who have experienced exposure to a certain amount of chloroform turned unconscious, so it isn’t suggested in this study. The ratio of acetone: hexane has high efficiency of extraction because of its dependence on the mixture solvent polarity for removing oil contaminants. Additionally, to extract petroleum products from the soil, many authors have used them [27–30]. The mixture of acetone: hexane (1:1 v/v) was revealed by this experiment to be the suitable solvent system of choice for extracting oil from impure sand.
3.2.3. Different ultrasonication cycles
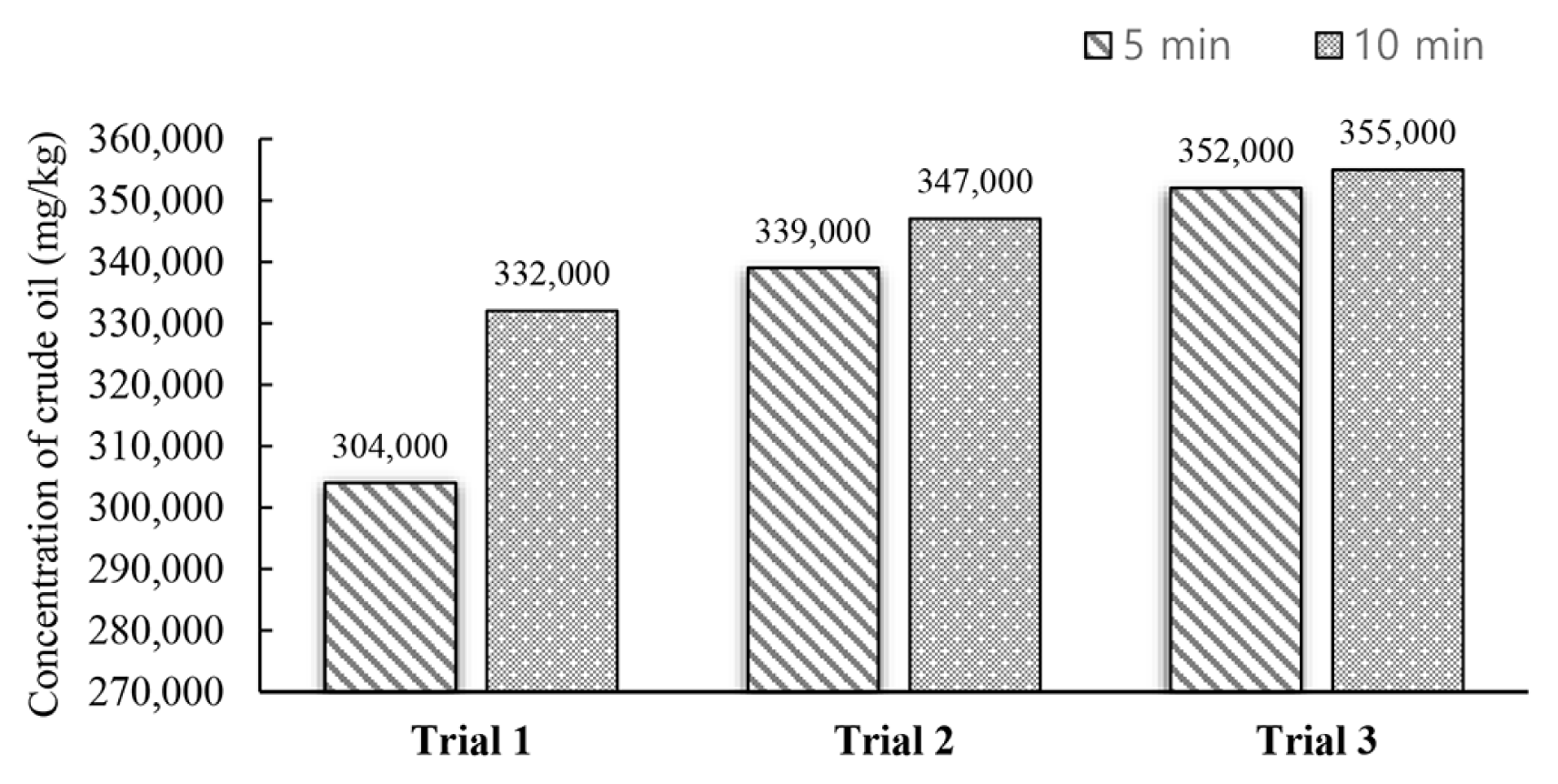
To explore the fact that whether a decrement of the cycle optimised, the process of extraction was the main aim behind the use of various ultrasonication cycles. Three cycles and each cycle based on 10 min were required for the 5 mL of acetone: hexane (1:1 v/v) in the previous experiments (Section 3.2.2). A comparison was then made between the results of this experiment with an alternative experiment that needed four cycles. Each cycle was based on the short time of 5 min for cleaning the 1 g of soil sample using 5 mL of the ratio of acetone: hexane (1:1 v/v) solvent. So, a total volume of 20 mL of solvent was the basic need to complete the process of extraction as the test shows that the 4 cycles were necessary if the washing of sample was done with the rate of 5min/cycle. So 30 mL of solvent was needed for the entire process as the washing of samples required three cycles, and each cycle is based on 10 min, and the results are shown in Fig. 3.
Regardless of the washing cycle time, no significant distinctions in weathered crude oil’s concentration were observed, based on Fig. 3. The mean values for 5 and 10 min were calculated as 344,000 and 331,000, respectively (Post-hoc Scheffe test, P < 0.05) showed by the results. Three cycles, 15 mL of solvent, and 10 min/cycle for washing 2 samples per hour were required by the previous experiment (Section 3.2.2). More solvent with less time was used by the alternative experiment. So, the washing of three samples can be done in 60 min. Hence, the result recommended the choice of the 5-min cycle over a 10-min cycle.
3.2.4. Validation of the gravimetric method
In 5 mL fresh crude oil, one gram of clean sand was mixed. In four wash cycles (Section 2.4) the combination of crude and sand oil was washed with the solvents of amount of 20 mL in 20 minutes. The weight of sand and recovered oil was calculated after completing the solvent recovery process, as shown in Table 3. The previous experiment (Section 2.4) recovery of the crude oil was about 97.20%, as shown by the results of Table 4.
Solvent extraction has also been investigated for treating soils contaminated with hydrocarbons in the diesel range using mixed solvents ethyl acetate–acetone–water and mixed solvents acetone–hexane [31, 32]. Based upon the outcomes of their study, acetone and hexane were considered as promising solvent extraction for removing heavy oil fractions as well as petroleum hydrocarbons from contaminated soils. The objective of the present work is to propose substitution solvent for the extraction of hydrocarbons compounds from soil. Furthermore, this study proposed that four principal characteristics influence the choice of method to recover the crude oil: (1) Reduction of the cost of the extracting solvent by decreasing the quantity of solvent (2) the solvent should dissolve the crude oil in the shortest time (3) high extraction efficiency and (4) select the solvent that has the least side effect. In this research, the volatility of the selected solvents was already high, so the temperature of the extraction was not examined. The most important physicochemical parameters in the choice of an appropriate solvent for hydrocarbons compounds extraction were reviewed and selected based on their hazard characteristics. This study found that the extraction efficiency of acetone: hexane (1:1 v/v mix) was higher than those obtained with dichloromethane DCM or mixture of DCM:hexane (1:1 v/v mix). The reason for this might be the nature of the weathered oil contamination.
4. Conclusions
During the measurements, some difficulties were faced by this study. ‘Blocked after each run’ was the main problem faced due to the GC column. Hence there was a need for another way for the TPH examination in impure soil. While the extraction of weathered oil from contaminated soils, the mixtures of (acetone: hexane v/v) in a mixed solvent were effective. This experiment indicates that by increasing the soil sample’s weight or the contact time, the compatibility of the solvent extraction and contaminants of oil were unlikely to be altered. For the extraction of three samples in 60 min the developed gravimetrical method used more than one solvent in a short span. Limited locations in the Kuwait oil field were cleared and approved, therefore, the developed extraction process needs to be repeated for other locations once it’s become clear to validate the accuracy of the extraction process.