1. Introduction
Arsenic (As) contamination in water is one of the world’s major environmental problems, posing significant risks to human life and ecosystem [1–3]. Arsenic is toxic, carcinogenic and a potent poison to different organisms. Arsenic toxicity is a serious threat to human life in many parts of the world due to contamination of potable water and food crops [4–6]. Arsenic concentration in water is increasing day by day due to various natural as well as intensified environmentally unsound activities, such as volcanic eruption, dissolution of minerals from sedimentary rocks, dilution of geothermal waters, smelting of ores, disposal of As containing industrial wastes, and others agricultural applications [7–9]. Arsenic is used in agriculture as herbicide and plaguicide in the form of synthetic compounds, such as sodium methyl arsenate disodium methyl arsenate, and dimethyl arsenic acid. Discharge of As containing effluent and irrigation of As contaminated ground or surface water are the main culprits for As contamination in land and water ecosystems [9–11] which gets accumulated in food crops, water and finds its way into the human system by way of direct ingestion or through consumer products [10, 12]. A wide range of technologies are available for remediation of metals-contaminated soil and groundwater. General approaches for remediation of metal contamination include: isolation, immobilization, toxicity reduction, physical separation & extraction, adsorption, coagulation and precipitation of metal ions from the contaminated matrix [13, 14]. Although, the above mentioned methods are successful in remediation at certain extents, they are not sustainable with respect to energy input and cost. Thus, development of eco-friendly and cost effective technology for mitigation of As pollution is a R&D priority. Phytoremediation is one of the emerging technologies that offer significant benefit in comparison with the conventional technologies, which employs plants for remediation purpose. It is aesthetically pleasing, ecologically sound and environmentally sustainable and safer for humans [15–17].
Phytoextraction phenomenon is based on the ability of plants to tolerate metal stress, metal accumulation in their above ground biomass, high growth rate and biomass accretion with prolific root system [12]. In the last few years, importance of metal accumulating hyperaccumulator plants have been realized, which are potential candidates to enhance the existing phytoremediation process [18, 19]. In view of this, prospective plant species for metal tolerance and accumulation and other preferred characteristics are being actively tested to determine their metal phytoremediation potential. Different plant species primarily belonging to genus Brassicaceae and grasses have been identified which show tolerance for various metals [18, 20–22]. Some aquatic plants have also been reported having capability to accumulate heavy and toxic metals by different mechanisms, which are potential for purification of metal contaminated water [23–25]. Pteris vittata (brake fern) is the first As hyperaccumulator plant having remarkable ability to accumulate As in its shoots, followed by which 12 other As hyperaccumulator plants have been identified [12, 26]. However, a number of fern species and some plant species from the genus Brassicaceae have also been reported to be As non-tolerant [27, 28]. Due to this paradox, identification of candidate better plants species, for phytoremediation application is a R&D priority.
This investigation was carried out to identify fast-growing, high biomass non-crop plant species for metal phytoextraction from polluted water. Three different plant species, i.e, Pteris vittata, Mimosa pudica, and Eichhornia crassipus were selected for treatment with different As concentration and assess their response with reference to morphological changes of the plant and metal accumulation in their biomass. Hydroponic screening method reported by Kumar et al. [29] was used to identify the potential plant species out of the three varieties.
2. Materials and Methods
2.1. Experimental Setup
Three plant species namely P. vittata, M. pudica and E. crassipus of similar age were selected for the present study. All the three plant species were having 4–6 whirls of leaves, 6–8′ height in case of Pteris and Mimosa and 2–6 leaves and 2–2.5′ height in the case of Eichhornia. These plants were already maintained in the glass house at the Council of Scientific & Industrial Research–National Environmental Engineering Research Institute, Nagpur, India. The plants used for the treatment study were acclimatized in the hydroponic condition in 20% Hoagland nutrient solution with pH 5.4–6 [30] for 2 weeks under controlled temperature and humidity. Subsequently, these plants were transferred into culture vessels containing 500 mL nutrient solution supplemented with 0, 25, 50, 100, 150, and 200 mg/L of arsenate in the form of sodium arsenate (Na2HAsO4·7H2O), control plants were grown in the nutrient solution without As amendment. During the treated duration, volume of the treatment medium was maintained with Milli-Q water. The experimental setups were conducted in a closed manner to prevent loss of As from the liquid medium. The experiments were conducted in glass house under 25°C–30°C temperature and 70%–90% humidity, in natural light intensity and 14:10 (light:dark) photoperiod. After 14 days, plants were harvested, thoroughly washed with tap water followed by distilled water, and separated into fronds and roots. The plant samples were oven dried at 60°C for 72 hr, and dry weight of each sample was recorded to determine the biomass accumulation. Known dry weight of the root and shoot biomass were taken for As estimation.
2.2. Effect of As on Plant Growth and As Removal Efficiency
To determine the effect of As on plant growth, the fresh weight and height of plant at initial and final day of treatment were measured. Plant height was measured from the crown to the apex of the highest shoot. Physiological and morphological changes of the plants with respect to As exposure were also recorded by visual observations. Relative growth rates (RGR) of the plants were calculated as per the formula given in the Eq. (1).
where, W0 and W1 are the initial and final weights and t1 and t0 are the beginning and end of treatment durations, respectively. Two indices, i.e., bioaccumulation factor (BF) and translocation factor (TF) were calculated to estimate the phytoextraction ability of selected plants. The BF for As accumulation in plant biomass was determined as the ratio of As accumulated in the biomass to that of the treated nutrient solution. The TF was calculated as the ratio of As accumulated in shoot to that of root to evaluate the plant’s ability to translocate metal from roots to the aerial part to determine its phytoextraction potentiality.
2.3. Arsenic Determination in Plant Samples
Known weight of dried plant biomass (0.1–0.5 g) was grinded in mortar and pestle, mixed with 10 mL concentrated nitric acid and digested in Microwave Digestion (Ethos 900; Milestone Inc., Shelton, CT, USA) under 300 W for 15 min. The digested samples were cooled and volume adjusted to 50 mL with Milli-Q water, filtered through Whatman Grade 42 filter paper and stored in capped polypropylene bottles until As estimation. Arsenic content of the digested samples were analyzed using Inductively Coupled Plasma Optical Emission Spectrophotometer (ICP-OES; PerkinElmer, Waltham, MA, USA) against blank and 2, 5, and 10 ppm As standards (Merck Cat. No. OC486486).
2.4. Statistical Analysis
All experiments were carried out in three replicates. Data presented here are expressed as the mean ± SD of three independent experiments. Statistical analysis was carried out using MINITAB 16 software and treatment effects were determined by analysis of variance using Tukey test given in the software.
3. Results and Discussion
3.1. Comparative Growth Performance of Selected Plants
The hydroponic screening experiment was carried out to test the effect of As on growth and metal accumulation in three non-crop plants belonging to different habitats, i.e., Mimosa (terrestrial) and Pteris and Eichhornia (lithophytic and macrophyte), respectively. In addition to the ability for efficient extraction of As from liquid medium, growth performance which is indicative of biomass accumulation is one of the essential features for identification of prospective plants for successful As phytoremediation application. Comparative growth performance of control and treated experimental plants were evaluated during the study period of two weeks and presented in this paper. Out of the three plants, As treated Eichhornia and Mimosa plants showed toxicity symptoms in all concentration of As. Leaves started turning yellow and wilted after one week, whereas the control plants remain green and normal. At higher As concentration, onset of toxicity was early. In case of these two plants, leaves dried within two weeks, whereas in case of Pteris the plant remains green even after one month. This finding shows that Pteris is more tolerant to As stress in comparison to other two plants. Moreover, Pteris was able to survive even in 200 mg/L As concentration, although toxicity symptoms were visible lately.
One of the factors that plays important role in phytoextraction efficiency is plant biomass accretion under metal stress condition. This parameter was also considered in this study to evaluate stress response of the investigated plants to determine their As phytoextraction potentiality in addition to tolerance of the stress. Growth performances of these three plants with respect to height of the plant were compared at initial and final day of As treatment (Fig. 1). Out of these three plants, in Pteris root and shoot elongation was clearly evident and it flourished in all studied As concentration. Nevertheless, in lower concentrations of As up to 50 mg/L, Pteris biomass increased as compared to control plant. On the contrary, Mimosa and Eichhornia plants didn’t show significant growth with respect to plant height in most concentration of As. Although Mimosa plant survived in 25 and 50 mg/L As, but at higher As concentrations toxicity was prominent. The RGR of the studied plants treated with all concentrations of As was determined with reference to controls (Table 1). The results clearly indicate that in all As treatment concentrations Pteris showed highest RGR in comparison to the other two plant species.
3.2. As Accumulation in Plant Biomass
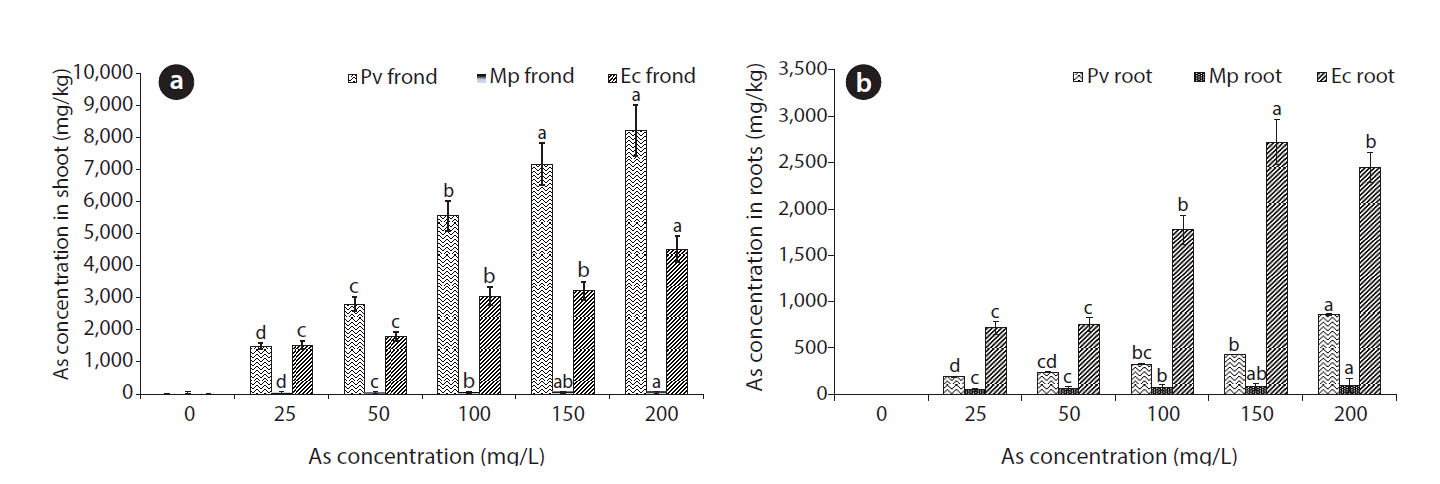
After 2 weeks, the older and green leaves of the plants were taken for As estimation as described in the materials and methods. The results of As accumulation inside the shoot and root of both control and As treated plants of Pteris, Eichhornia, and Mimosa species are presented in Fig. 2. P. vittata plant shows the highest As accumulation in shoot, i.e., 8,223 mg/kg dry biomass. Arsenic accumulation in Eichhornia shoot, i.e., 4,517 mg/kg biomass was nearly half that of Pteris; whereas, Mimosa plant show very low accumulation in its shoots. On the other hand, in the case of Pteris, extent of Arsenic accumulation in shoots increased with increase in concentration. As accumulation in roots also followed the same trend as that of shoot. Previous studies have reported Pb, Cu and Cd phytoextraction ability of Mimosa [31] with the adsorption efficiency 71%, 81% and 33%, respectively. Ashraf et al. [32] have also reported Zn accumulation in M. pudica, i.e., 38.94 mg/kg biomass. Although Mimosa is reported to be accumulator for the other heavy metals our study shows that it is not suitable for extraction of As. Previously, Eichhornia has been utilized for As removal from water matrix which showed 1.8 ± 0.5 mg As/kg removal under the assay conditions at 0.15 mg/L concentration of As [33]. They suggested that high As removal efficiency of Eichhornia was due to high biomass. Our results also show high As accumulation by Eichhornia and this findings is similar to the other studies reported earlier.
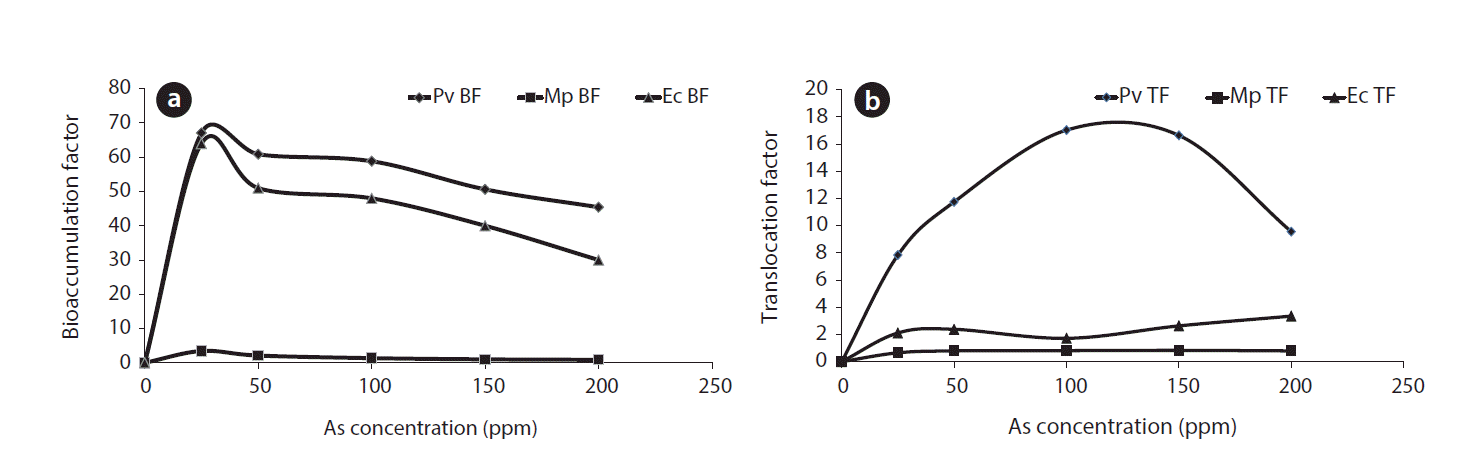
It is reported that As hyperaccumulator plants can accumulate As up to 2% dry weight of their biomass in the above ground parts with As TF usually exceeding 1 [12, 34]. Arsenic tolerance by a plant system depends on its inherent ability for avoidance of metal entry to the plant body as well as biochemical adaptation to tolerate intracellular As stress. The plant with high BF and TF >1 has been categorized as hyperaccumulator [35]. Pteris and Eichhornia plant have high As BF in the biomass, i.e. 67 and 64, respectively, at 25 mg/kg in Fig. 3(a). Further, the BF increased up to 50 mg/L As concentration and high TF indicted more As accumulation in shoot in comparison to roots. Fig. 3(a) also shows that Pteris and Eichhornia has As BF >1 which signifies one of the characteristics of hyperaccumulators; whereas, Mimosa has BF <1 indicating as As non-hyperaccumulator. BF for As in case of Mimosa was significantly different in comparison to other two plants. Arsenic translocation to the above ground biomass was highest in case of P. vittata showing TF up to 17, i.e. highest in comparison to other two plants (Fig. 3(b)). Arsenic treated Eichhornia plant died within 7 days unlike Pteris, which were green up to one month. Our finding shows that Pteris plant is tolerant up to 100 mg/L As. On the other hand, the plant continues to grow with increasing plant biomass under 50 mg/L As stress. Therefore, As extraction and accumulation in the plant biomass was possible simultaneously. Based on these findings it is certain that phytoextraction potentiality of this P. vittata genotype is more in comparison to the other two plants.
4. Conclusions
The results of this investigation show that Pteris and Eichhornia have significant capacity for As accumulation in the biomass, but Pteirs has more tolerance to As toxicity. Arsenic accumulation was found to be more in shoot of Pteris; whereas, in case of Eichhornia, it was more in roots. The Pteris and Eichhornia plants accumulated 8,223 ± 791 and 4,517 ± 402 mg As/kg of dry shoot biomass and 859 ± 79 and 2,452 ± 162 mg in roots per kg of dry shoot biomass of the respective plants. They are more potential to accumulate As in plant biomass in comparison to Mimosa plant. The results indicate that Pteris plant has tolerance limit up to 100 mg/L As and has high As accumulation in the biomass with high BF up to 67. Under As stress, As accumulation in Pteris and Eichhornia plant biomass increased with increase in treatment concentration up to 200 mg/L. However, Eichhornia plant died after treatment with 50 mg As/L medium. High BF shows that both the plants can be used for As phytoextraction, but P. vittata is more suitable in comparison to Eichhornia due to higher TF for accumulation in above ground biomass and biomass accretion under As stress. The results of this study, along with the fact that this plant species are capable of thriving in As amended water support that P. vittata and E. crassipes could be the suitable species for sustainable As removal from contaminated water.