1. Introduction
Organic wastes have become increasingly used for energy production, because of the diminishing reserves of fossil fuels. Waste activated sludge (WAS) is mainly microbial cell biomass. The usage of WAS as an energy source is very important, because it is estimated that about 3 million tons of WAS are generated annually in Korean wastewater treatment plants (WWTPs) accounting for over 50%–60% of total operating costs [1]; and ocean dumping has been prohibited by the London Dumping Convention [2, 3]. The amount of WAS is also expected to continuously increase, since many WWTPs have been constructed over the last few decades.
Among the WAS treatment methods, anaerobic digestion methods are commonly used at most WWTPs to convert organic wastes into energy. Anaerobic digestion of particulate materials and macromolecules follows a four-step sequence: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. In anaerobic digestion systems, WAS is not only stabilized, but also degraded into biogas through a complex biological process regulated by an anaerobic bacteria consortium. These advantages of anaerobic digestion, as well as the corresponding reduction of greenhouse gas emissions, have favored the anaerobic digestion treatment method.
The main fraction of WAS consists of cellular materials. Therefore, the structure of the sludge and the integrity of the agglomerated particles are determined by the amount and the composition of extracellular polymeric substances (EPS) [4]. Theoretically, WAS has great potential for biogas production; but the efficiency of anaerobic digestion has been limited by the hydrolysis of EPS and some non-biodegradable components, which is well known as the rate-limiting step in the anaerobic digestion process [5, 6]. For hydrolysis of WAS, anaerobic bacteria should release extracellular enzymes to break down the rigid cell wall of the microorganisms in WAS [7], leading to longer hydraulic retention time of anaerobic digestion. Thus, to improve digestion efficiency, the most appropriate method is to disrupt the microbial cells in the sludge.
Several methods have been reported to enhance sludge solubilization and improve sludge destruction including acid/alkali [8], heating [9], ozonation [10], and ultrasound [11] methods. These pretreatment methods aim to destroy the cell wall, which makes the intracellular and extracellular cell substances more available in the anaerobic digestion process [6]. However, chemical methods require high operation and maintenance costs [12], the conventional low-temperature thermal treatment requires long reaction time [2], and the ultrasound approach is energy intensive [12]. Consequently, an economical and effective pretreatment method is required.
Microwave (MW) heating is increasingly emerging as an alternative, because it widens the scope of conventional thermal heating with accelerated reaction rates, environmental friendliness, and low overall cost [13, 14]. MW heating is also rapid and volumetric with the whole material being simultaneously heated. In contrast, conventional heating is slow and is introduced into the sample from the surface. The three main effects of MW irradiation (MWI) on reactions are: thermal effects, specific thermal effects, and non-thermal effects. Thermal effects are based on the direct coupling of electromagnetic energy with the molecules of solvents and reagents, whereby the magnitude of the heating depends on the dielectric properties of the molecules. Specific thermal effects arise due to the unique nature of MW. These effects are defined as accelerations of chemical transformations in a MW field; for instance, hot spots and liquid overheating, which could not be achieved by conventional heating, but which are still essentially thermal effects. Non-thermal effects are electrostatic polar effects leading to dipole-dipole type interactions between the dipolar molecules and charges in the electric field [15, 16]. Thus, the non-thermal effect on dielectric materials is a significant increase in rotation of the polarized dipoles in the molecules. This increases the probability of collision between molecules, which generates heat. Thus, the non-thermal effect enhances the reaction rate and reduces the activation energy [17].
In the past decade, several studies on MWI (mainly 2,455 MHz) applied hydrolysis of WAS have been performed. In the MWI reaction, the destruction of microorganisms is generally thought to have been caused by the thermal effects of MW exposure. With the application of MWI, the desired final temperature can be reached faster, because WAS, which is a multiphase medium with high water content, can efficiently absorb MW energy [18]. In some cases, the energy demand of the process can be reduced and the hazardous emission potential is smaller [19]. Several studies have reported an additional non-thermal effect, which is expected to weaken or break the hydrogen bonds of the polymer forming the cell wall of WAS, through the alternation of the electric field of polar substances like water, by 2.455 billion times per second [20–22].
On the other hand, other studies have focused on the kinetics of MW-assisted hydrolysis of WAS. Stuerga and Gaillard [23] explained the acceleration in the reaction rate in condensed states under MWI compared to conventional heating by enhancement of the collision rates in the condensed phases, which causes transfer between rotational energy levels and reaction acceleration. Sucrose hydrolysis by MW and conventional heating was revealed as a first-order chemical reaction [15]. In the kinetic study of thermohydrolysis for excess activated sludge according to temperature conditions (423–523 K), the thermal dissolution of raw activated sludge particles was shown to follow a first-order reaction [24].
The hydrolysis rates of WAS in a MW-assisted system can be affected by various factors. These differ widely for the same output power and irradiation time, because the amount of MW absorption of samples differs according to the type of cavity, waveguide, temperature, and the presence of an isolator [25]. In addition, as the hydrolysis rates are affected by the characteristics of WAS, the hydrolysis rates have diverse effects on MW-assisted WAS hydrolysis [20, 26].
Thus, this study evaluated the effect of MW on hydrolysis of WAS at different initial solid concentrations. After MW pretreatment of WAS, the increase in the soluble chemical oxygen demand (SCOD) concentration may be due to aggravating deag-glomeration and the transfer from non-soluble organic material into soluble organic material [27]. Accordingly, the solubilization of WAS with MWI at different initial solid concentrations was quantified by evaluating the volatile suspended solids (VSS) decrease and the SCOD increase. Eskicioglu et al. [28] reported that soluble total nitrogen (T-N) increased from the hydrolysis of the protein component of bacterial cells. Thus, nutrients concentrations were also analyzed to verify the disintegration of the bacterial cell and its protoplasm.
2. Materials and Methods
2.1. Waste Activated Sludge
Samples of raw WAS were collected from the secondary settling tank (WAS 1) and centrifugal thickener (WAS 2) of Suyoung Municipal Sewage Treatment Plant (MSTP) in Busan, Korea, which treats sewage at a flow rate of 450,000 m3/day with combined processes of activated sludge and membrane bioreactor. The collected WAS samples were promptly transported to the laboratory and sieved with mesh No. 24 (800 μm). High solid concentration sludge (WAS 3) was prepared with centrifugation of the thickened sludge. Every experiment was performed within 5 hr after collection of WAS samples. Table 1 presents the characteristics of the WAS samples collected from Suyoung MSTP.
2.2. Experimental Conditions of WAS Hydrolysis by MW
To evaluate the effects of initial solid concentration on the hydrolysis of WAS, three different sludge samples were prepared as shown in Table 1. Each WAS sample was irradiated using an MW oven system (Synthos 3000; 2,455 MHz, output power range 0–1.2 kW, maximum temperature 350°C maximum pressure 6.0 bar) equipped with eight cylindrical pressure vessels made of quartz. Each sample vessel had 80 mL effective volume and 5 mm thickness of vessel wall.
Then, 50 mL of each WAS sample was placed in a sample vessel and irradiated for 0, 3, 6, and 10 min at 600 W output power to determine the optimum irradiation time for the hydrolysis of WAS. In previous studies, although the MWI time was widely varied according to the biomass kinds, many researchers limited the irradiation time to within 10 min, because of the rapid biomass heating by MW [13, 29, 30].
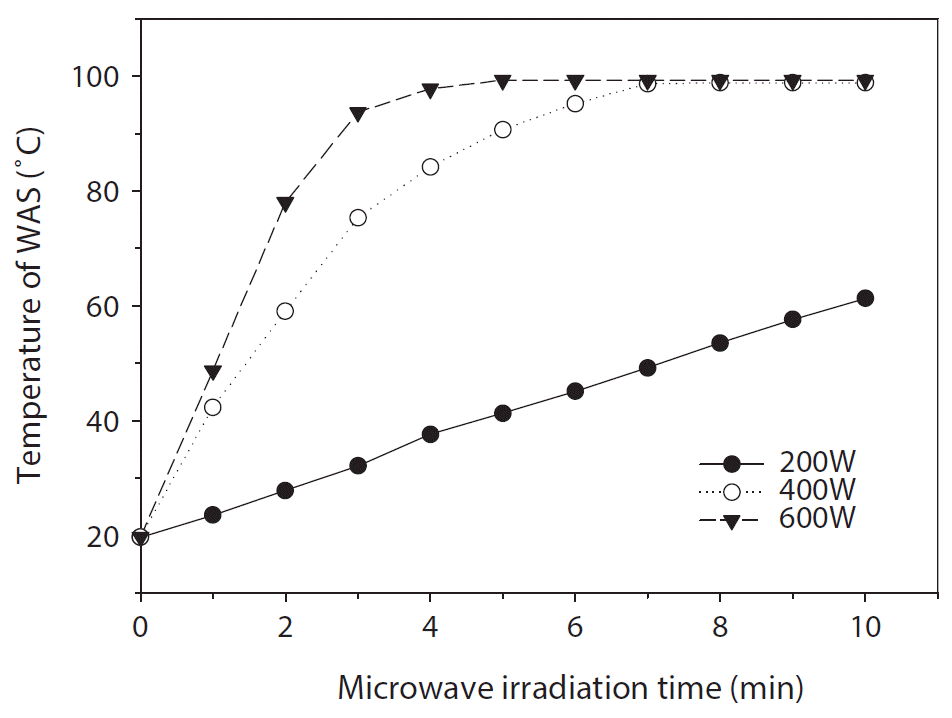
As an output power of 600 W is generally used in the magnetron of commercial kitchen-type MW ovens, this type of MW could be a cost-effective method for the application of an MW hydrolysis system to MSTPs. Furthermore, in our preparatory experiments, the temperature of WAS 2 increased close to the boiling point within 2.3 min at 600 W MW output power. Temperature profiles for different MW output powers are shown in Fig. 1. The WAS temperature is the most important parameter in the hydrolysis of sludge, because it significantly influences the floc disintegration and cell lysis [30, 31]. In the experiment on MW heating for thickened sludge, the sludge temperature approached the boiling point within 2 min irradiation at 750 and 900 W output power, but the sludge temperature was under 90°C at 500 W output power with 2 min irradiation [30].
As the dielectric heating mechanism of MWI could rapidly increase the temperature of the heated materials, the WAS temperature for a pressure vessel could reach a higher temperature than the boiling point in a few minutes of irradiation. Therefore, the target temperature of WAS was fixed at 100°C (373 K) for all experiments to avoid the carbonization reaction of humic materials abundant in sludge [32]. The experimental conditions are summarized in Table 2.
2.3. Analytical Methods
All experiments were performed in triplicate to minimize random errors and the results are presented in mean values. Hydrolysis efficiencies were evaluated, through VSS decrement and SCOD increment of irradiated WAS samples. Total suspended solids (TSS), VSS, and SCOD were analyzed by the procedure of Standard Methods [33]. The changes of T-N and total phosphorus (T-P) concentrations were also monitored by the procedure of Standard Methods. Hydrolysis of sludge induces the increment of T-N and T-P caused by cell wall destruction and hydrolysis of protein and protoplasm [26, 28]. Volatile fatty acids (VFAs) and pH profiles were monitored to explain the MWI-induced pH variation of the sludge. VFAs were determined by HPLC (Ultimate 3000; Dionex, Sunnyvale, CA, USA) equipped with an Aminex HPX-87H column.
3. Results and Discussion
3.1. Characteristics of WAS Hydrolysis and SCOD Increase by MWI
Recently, a new criterion of WAS hydrolysis composed of floc disintegration and cell lysis was proposed in the pretreatment of WAS by ultrasonication [31]. According to this criterion, as WAS is mainly composed of EPS and bacterial cells, floc disintegration, and cell lysis occur simultaneously in WAS hydrolysis. However, it was suggested that EPS disintegration is predominant in the initial period of hydrolysis and that cell lysis occurs subsequently, because the bacterial cells are surrounded and protected by EPS. MW could also disintegrate the WAS structure and release proteins, carbohydrates, and lipids into the bulk liquid of sludge; and this hydrolysis should increase the SCOD, T-N, and T-P concentrations [29, 34].
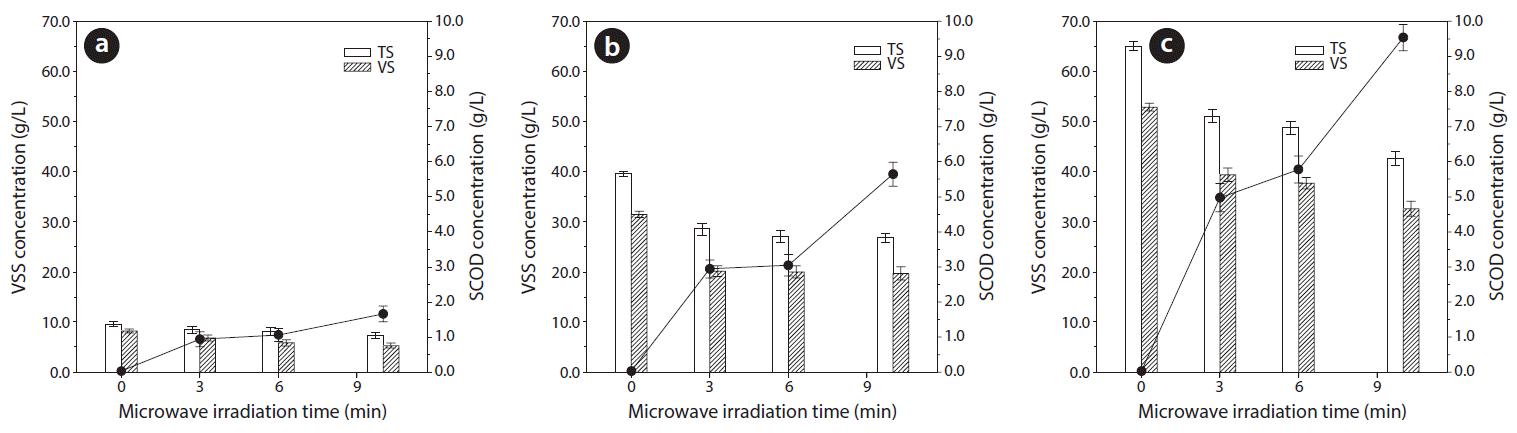
Fig. 2 presents the VSS and SCOD profiles according to MWI time for each WAS condition. The concentrations of VSS decreased during MWI for all WAS conditions presenting the hydrolysis of VSS with increasing SCOD concentrations. However, the slope of VSS decreases and SCOD increases clearly differed according to MWI time. During the MWI time of 3 to 6 min, the VSS decreases and SCOD increases occurred very slowly compared to the initial 3 min MWI (0 to 3 min), or the final 4 min MWI (6 to 10 min) for all WAS conditions. The ratios of SCOD increase to VSS decrease (g SCOD/g VSS) during the period of 3 to 6 min for all WAS conditions (with 0.262–0.438 g SCOD/g VSS) were also lower than those during the initial 3 min MWI (0.257–0.651 g SCOD/g VSS), and the final 4 min MWI (0.476–0.560 g SCOD/g VSS).
These results could be explained by the 2-step disintegration of WAS hydrolysis composed of the initial disintegration of WAS floc and the subsequent lysis of bacterial cells [31]. Therefore, the initial rapid decrease of VSS during 0 to 3 min MWI was attributed to the disintegration of WAS floc. Further, MWI during 3 to 6 min was considered to have weakened the difficult-to-biodegrade cell wall structure, so that the cells were solubilized during 6 to 10 min MWI [29, 31]. Similar results of SCOD increments were observed in a previous study [35] with secondary sludge at 11 min MWI time.
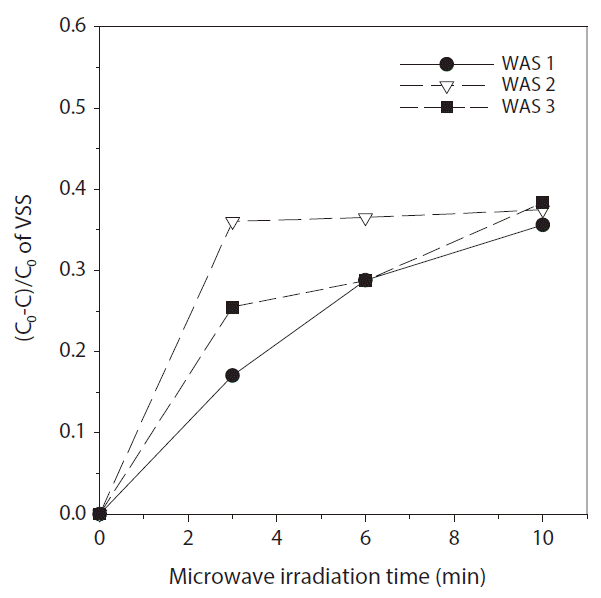
VSS solubilization efficiencies [(C0-C)/C0 × 100] according to the kinds of WAS are shown in Fig. 3. After 10 min irradiation with 600 W output power, total VSS solubilization efficiencies were similar at 35.6%–38.4% for all WAS conditions. In 60 min WAS ultrasonication with 100 W output power corresponding to a specific energy of 10 min MW with 600 W output power, VSS solubilization of 37% was reported [31]. However, the VSS solubilization varied according to the kinds of WAS and irradiation periods. The maximum solubilization of 36.0% during 0 to 3 min for WAS 2 corresponded to WAS in the centrifugal thickener of MSTPs. In a previous study [29], the increase of the SCOD/TOCD ratio by 1,000 W MWI for 6 min was also higher for 3.0% TS (0.143) than that for 1.0% TS (0.117).
3.2. Variation of Nutrients Concentration during MWI
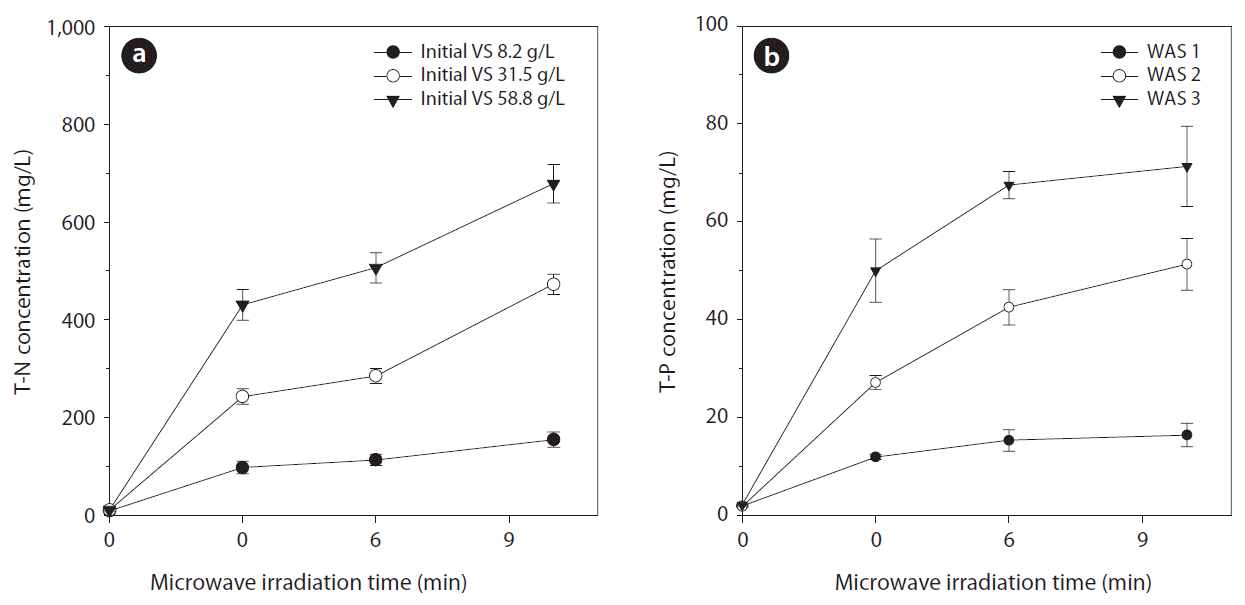
Fig. 4 presents the variation of T-N and T-P concentrations according to MWI time for different WAS. As MWI time was extended, the T-N concentration was increased up to 154.6 mg/L for WAS 1 (initial T-N 8.9 mg/L), 472.5 mg/L for WAS 2 (initial T-N 10.2 mg/L), and 678.3 mg/L for WAS 3 (initial T-N 10.4 mg/L). Soluble T-N was induced from the hydrolysis of the protein component of EPS and bacterial cells [21, 28] and the T-N concentration profiles of this study corresponded well to the SCOD increments with increasing MWI time. The T-N/SCOD ratios ranged from 0.070 to 0.101, which matched well with the empirical formula of bacterial cell mass C5H7O2N and endogenous respiration. The theoretical nitrogen to COD ratio of endogenous respiration is 0.106 from the stoichiometric equation (C5H7O2N + 5O2 → 5CO2 + 2H2O + NH3 + energy). As a total ammonia nitrogen (free ammonia + ammonium ion) level of 1,700–1,800 mg/L inhibits anaerobic digestion, which leads to operation failure [36, 37], the maximum T-N concentration obtained in this study will not exert any inhibition effect on the subsequent anaerobic digestion. The T-P concentration was also increased up to 16.4 mg/L for WAS 1 (initial T-P 1.9 mg/L), 51.3 mg/L for WAS 2 (initial T-P 1.8 mg/L), and 71.3 mg/L for WAS 3 (initial T-P 2.1 mg/L).
3.3. Variation of pH during MWI
In Fig. 5, with increasing MWI time, pH was decreased for all WAS and this pH decrease corresponded well to the VSS decrease and formation of acetic acid. For the relatively lower VSS decrease during 3 to 6 min MWI time, the pH decreased and acetic acid formation also declined in this period. During 10 min MWI, the pH decreases were 6.72 to 6.30 for WAS 1, 6.55 to 6.10 for WAS 2, and 6.59 to 6.15 for WAS 3. The acetic acid concentration was increased up to 10.84 mM for WAS 1, 22.47 mM for WAS 2, and 19.53 mM for WAS 3. In consideration of the Ka value of acetic acid (acid equilibrium constant, 1.75 × 10−7), the acetic acid concentration of each experiment induced pH decrease through the ionization of hydrogen ions. The ratio of the hydrogen ion concentration calculated from the acetic acid concentration of each sample, to the theoretical hydrogen ion concentration needed to obtain the observed pH decrease, had the range of 0.707–0.762. Therefore, the pH decrease in the hydrolysis of WAS by MWI was attributed to the acetic acid formation. However, as the pH values after 10 min of MWI were over 6.10 for all WAS, pretreated WAS by MWI could be applied to anaerobic digestion with no inhibition effect.
4. Conclusions
The study results reveal the strong effects of MW time on the hydrolysis of WAS. For all initial solid concentrations (WAS 1, 2, and 3), the VSS solubilization efficiencies were similar at 35.6%–38.4%. However, the profiles of VSS hydrolysis were clearly divided into three steps during the 10 min MWI time. While the initial 3 min and the final 4 min of MWI showed rapid hydrolysis of VSS, the MWI time of 3 to 6 min resulted in very low hydrolysis. These results supported a 2-step mechanism of WAS hydrolysis consisting of WAS floc disintegration and bacterial cells lysis. This 2-step hydrolysis is expected to be verified by quantification of the EPS (WAS floc) and RNA (cells) in further study. The efficiency of WAS hydrolysis was maximized at 36.0% from 3 min MWI time for WAS 2. On the basis of the empirical formula of bacterial cells, the profiles of T-N and SCOD concentrations corresponded well during the MWI. After 10 min MWI, the T-N concentration and pH value for all WAS were in suitable ranges for anaerobic digestion. Therefore, WAS pretreatment by MWI is expected to be successfully applied to anaerobic digestion.