AbstractTo treat leather industry wastewater (LIW) containing high nitrogen concentration, eight aerobic denitrifiers were isolated from sludge existing in an LIW-treatment aeration tank. Among them, one strain named as KH8 had showed the great ability in denitrification under an aerobic condition, and it was identified as Pseudomonas aeruginosa R12. The aerobic denitrification ability of the strain KH8 was almost comparable to its anaerobic denitrification ability. In lab-scale aerobic denitrifications performed in 1-L five-neck flasks for 48 hr, denitrification efficiency was found to be much improved as the strain KH8 held a great majority in the seeded cells. From the nitrogen balance at the cell-combination ratio of 10:1 (the strain KH8 to the other seven isolates) within the seeded cells, the percentage of nitrogen loss during the aerobic denitrification process was estimated to be 58.4, which was presumed to be converted to N2 gas. When these seeded cells with lactose were applied to plant-scale aeration tank for 56 day to treat high-strength nitrogen in LIW, the removal efficiencies of CODCr and TN were achieved to be 97.0% and 89.8%, respectively. Under this treatment, the final water quality of the effluent leaving the treatment plant was good enough to meet the water-quality standards. Consequently, the isolated aerobic denitrifiers could be suitable for the additional requirement of nitrogen removal in a limited aeration-tank capacity. To the best of our knowledge, this is the first report of aerobic denitrifiers applied to plant-scale LIW treatment.
1. IntroductionNitrogenous substances remaining in the industrial and domestic wastewater have attracted attention because of the role of nitrogen in eutrophication of receiving waters. Biological nitrification-denitrification is known to be one of the most economical processes for nitrogen removal [1]. Aerobic nitrification and anaerobic denitrification are generally consisted of this process because the nitrogenous substances existing in wastewater are mostly in the form of ammonium ion. It has commonly been accepted that these two biochemical processes are separately conducted for the sake of their different requirements for the reaction parameters, such as dissolve oxygen, substrate sources and retention time [2]. Nowadays, however, a host of reports dealing with a potential way to combine them into an integrated one has been informed for savings in oxygen for nitrification and carbon requirements for denitrification: Ammonium oxidation [3]; aerobic denitrification [4, 5]; nitrification-denitrification [6]; and nitrite nitrification [7]. The utilization of these bacteria is apparently more cost-effective and manageable than the conventional process.
In the nitrogen removal process, various microorganisms participate, and the nitrification process has been considered to be carried out mainly by ammonia- and nitrite-oxidizing bacteria that are obligately aerobic and chemoautotrophic. Nevertheless, a number of heterotrophic microorganisms have been reported to nitrify many types of nitrogen compounds [8]. Thus, understanding the characteristics of bacteria used in the biological nitrification-denitrification process is very important in order to remain high treatment efficiency at all times, and the possible microbial nitrogen conversions should be provided. Currently, aerobic denitrification becomes the center of public interest due to its potential application [9]. Therefore, the isolation and characterization of aerobically denitrifying bacteria have been actively dealt, and the representatively reported bacteria were: Acinetobacter calcoaceticus [10], Pseudomonas stutzei [11, 12], Bacillus subtilis [13], Agrobacterium [14], Marinbacter [15], Pseudomonas mendocina [16], Paracoccus versutus [9], Acinetobacter sp., [17] and Klebsiella pneumoniae [18]. It has been known that these bacteria can not only reduce nitrate under the aerobic condition but also convert ammonium to nitrogen gas via hydroxylamine, nitrite nitrate and nitrous oxide in order. With the advancement of research in the characteristics of these bacteria, the heterotrophic nitrification and aerobic denitrification processes have been clarified to some extent [16]. As a result, it is more feasible that the biological nitrogen removal could be conducted in one aerobic reactor [9].
For feasibility of commercialization, the aerobic denitrifiers have to be indispensably applied to the wastewater treatment plant and examined for their potential. To our best knowledge, no direct application of aerobic denitrifiers to wastewater treatment plant has been performed in spite of many studies on them. Accordingly, the performance of aerobic denitrifier applied to leather industry wastewater (LIW) treatment was tested as a first aim. The LIW is characterized to contain complex pollutants with low biodegradability, and its effluent has to meet the permissible discharge standard for total nitrogen [19]. During the biological nitrogen removal process, the imbalance between carbon and nitrogen concentration in the influent is generally occurred. Hence, high nitrogen concentration contained in the LIW was taken into consideration in this study. In addition, biological properties depend on the size of reactor, although the metabolic patterns remain unchanged [20]. For this reason, candidate aerobic denitrifiers were isolated from the sludge suspended in the aeration tank of LIW treatment plant, the characteristics of their aerobic denitrification in a lab-scale were examined from the nitrogen balance, and their potential ability for N removal in the plant-scale LIW treatment was performed in this study.
2. Materials and Methods2.1. Isolation of Potential Aerobic DenitrifiersThe sludge used to isolate the denitrifying bacteria was taken from an aeration tank in the LIW treatment plant located in Busan, Korea. The sludge sample was agitated to obtain homogeneous suspension in sterile 0.2% NaCl. One mL of the suspended liquid was pipetted into a 10-mL tube that contained ‘PYK medium’: 5 g peptone, 3 g yeast extract and 2 g KNO3 in 1 L tap water (pH 7.0). Another 1 mL of the suspended liquid was also pipetted into a 10-mL tube that contained ‘Succinate medium’: 4 g succinate, 1 g (NH4)2SO4, 0.3 g KH2PO4, 0.1 g K2HPO4, 0.2 g Mg SO4·7H2O and 2 g KNO3 in 1 L tap water (pH 7.0). After 3 days incubation at 30°C and 180 rpm, the liquid culture was spread with a platinum loop onto the same media solidified with 1.5% nutrient agar. The separated colonies formed on the agar plates were picked up serially, and a purified isolate was obtained by repeated streaking onto fresh agar plates. Each isolate was maintained on the agar plate at 4°C, and transferred to a fresh agar plate every 2 weeks until use.
The ability of aerobic denitrification for each isolate was tested by the use of the syringe technique under an aerobic condition [21]. First, 1 mL of each isolate was inoculated into a 50-mL glass syringe containing a 20-mL sterile PYK medium. At the beginning, 10 mL of pure O2 was supplied using a Hamilton gastight syringe, after which the syringe reactor was incubated at 30°C and 180 rpm for 72 hr. Oxygen was supplied into the syringe reactor twice more before depleted. To measure the moles of gas produced by denitrification, 20 μL of gas sample was taken periodically using a 50 μL gas-tight syringe and analyzed by gas chromatography (GC). At the same time, a syringe reactor under an anaerobic condition was also tested in parallel. The number of moles of gas produced in the syringe reactor was calculated by the ideal gas law.
2.2. Identification of One Potent IsolateThe isolate that produced the largest amount of N2 gas in the syringe test was primarily identified on the basis of colony morphology, Gram reaction, and microscopic examination.
The specific identification of the isolate was performed using 16S-rDNA sequence analysis. Chromosomal DNA of the isolate was extracted from cells grown in the given medium with AccuPrep® Genomic DNA extraction kit (Bioneer, Korea) as the manufacturer’s instructions. PCR amplification of the DNA using the 27F (5″-AGAGTTTGATCCTGGCTCAG-3″) and 1492R (5″-GGTTACCTTGTTACGACTT-3″) primers was performed with a DICE model TP600 PCR thermal cycler (Takara, Japan) as described by Kim et al. [21], and the 16S rDNA genes were determined by the Macrogen Company (Korea). These sequences were compared with GenBank (National Center for Biotechnology Information, USA) entries using the Advanced BLAST similarity search option [22], which is accessible from the homepage of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). BioEdit Sequence Alignment Editor version 5.0.9 was used to verify the alignment and remove all positions with gaps before calculating distances with DNAdist programme in PHYLIP (version 3.5c). Phylogenetic tree was gained on the ground of the given sequences and their close relatives using the neighbor joining method with 1,000 bootstrap replicates.
2.3. Aerobic Denitrification Experiment in a 1-L Five-Neck FlaskThe characteristics of aerobic denitrification by the isolates were investigated in 1-L five-neck flasks. The flask was comprised of five necks, and at each neck, a thermometric sensor, a pH probe, tubing for sampling, oxygen-inlet tubing connected to a membrane filter (0.2-μm pore size), and oxygen-outlet tubing were installed. To avoid contamination from foreign microorganisms, two consecutively connected 1-L flasks containing 10 N NaOH were placed for the discharging gas. This flask set-up was placed in a hot stirring bath system (Eyela, Japan) and maintained at 30 ± 0.2°C. Stirring the medium inside the flask was accomplished with the Variomag Telesystem (H+P Labortechnik AG, Germany) at 500 rpm. Oxygen (1.5 kgf/cm2) was supplied continuously into the flask from an oxygen tank (80% purity), and 10-fold diluted Antifoam 204 was used when the foam occurred severely.
For the preparation of inoculums, each isolate was separately cultivated in a sterile PYK medium until a late-log growth phase. Then, equal amounts (5 mL) harvested from each of eight isolates were combined together and inoculated to the flask containing the PYK medium under an aseptic condition. To examine the effect of one potent isolate on the aerobic denitrification, a flask inoculated with the combined cells at the ratio of 10:1 (one potent isolate to other seven isolates) was cultivated in parallel. During the experiments, the culture broth was sampled periodically from the flask by a peristaltic pump using Tygon tubing, and the changes in the reaction parameters were measured. From these measurements, the percentage of nitrogen converted to N2 gas by the isolated aerobic denitrifiers was calculated as the following formula:
where the concentration of final TN was the total concentrations of NH4+–N, NO2−–N, NO3− –N, organic N and N in biomass at the final stage of aerobic denitrification. The biomass composition was assumed to be C5H7O2N.
2.4. Application of Aerobic Denitrifiers to LIW Treatment PlantTo investigate the potential of the isolated aerobic denitrifiers in plant-scale treatment, they were applied to a LIW treatment plant (Busan, Korea) in which effluent quality was difficult to meet the N regulatory standard. The process flow diagram of the LIW treatment plant is shown in Fig. 1. The LIW treatment plant was comprised of collection tank (180 m3), equalization tank (10,472 m3), four relay tanks (758 m3), three clarifiers (6,666 m3), fermentation and synthesis tank (1,769 m3), eight-stage aeration tank (18,572 m3), two sludge thickeners (2,773 m3), two bio-contact tanks for treatment of nitrogen by microalgae (1,144 m3), silica tank for coagulation of colloids (450 m3), carbon tank for adsorption of organic compounds (336 m3), mineral tank for deterioration of organic compounds (453 m3) and industrial water tank (593 m3). The maximal capacity of this plant for LIW treatment was 9,000 m3 per day. The existing treatment for this LIW had been conducted by the use of microbial consortium (Korean patent: 10-1231977) consisting of Bacillus, Paenibacillus, Leuconostoc, Kurthia, Sphingobacteirum, etc. The microbial consortium was seeded daily into equalization tank (0.2%, v/v), fermentation and synthesis tank (0.04%, v/v), aeration tank (0.02%, v/v) and two sludge thickeners (each 0.04%, v/v), respectively. This treatment was set as a control experiment in this study.
Against the control, the experiments using additional input of isolated strains were performed for better N removal of this LIW to meet the N regulatory standard. For the preparation of seed culture, each isolated strain was actively cultivated at 30°C and 180 rpm in a 1-L flask containing the PYK medium, and then cells were collected after centrifugation. The collected cells were combined at a 10:1 ratio of one potent isolate to other seven isolates, based on the result of lab-scale experiment. After 1 day acclimation in the PYK medium at 30°C and 180 rpm under an aerobic condition, the actively grown cells were transferred to a 150 L reactor under an aseptic condition. With addition of 50-mg lactose/L, the seed culture for the plant-scale experiment was further cultivated in a 3-ton tank for 3 days under an aerobic condition. Finally, the proliferated cells prepared in this way were seeded into the aeration tank in two different ways: 1) the proliferated cells of 12 m3 were seeded at the first day and then the cells of 3.6 m3 were seeded daily from the second day to the end (designated as ‘treatment 1’), and 2) The cells were seeded as the above strategy and moreover 0.6-m3 lactose as a carbon source was additionally supplied every 3 days (designated as ‘treatment 2’). The aeration tank was divided into eight stages, and the hydraulic residence time was approximately 42.2 hr (with maximal flow rate of 440 m3/hr). Samples were periodically taken from the aeration tank, and the changes of the reaction parameters were measured according to standard methods for the water pollution. Experiments were carried out for eight weeks.
2.5. AnalysesThe dry-cell weight (DCW) was determined by weighing the cell pellet after being dried in an oven at 105°C for 12 hr. The cell pellet was prepared by centrifuging a 5 mL sample of broth culture at 5,000 rpm for 10 min and then by decanting the supernatant after washing twice with distilled water. To measure the nitrogen and carbon dioxide gases produced by aerobic denitrifiers in the syringe experiments, 20 μL samples (injection volume) were taken for GC/TCD (Perkin Elmer Instruments) analysis. The columns used were a ‘molecular sieve 13X’ and ‘carboxen 1000’ for nitrogen and carbon dioxide, respectively. In the analyses of both gases, the following conditions were equally applied: the carrier gas was helium at a flow rate of 30 mL/min while the injector and detector temperatures were 100 and 200°C, respectively. However, the oven temperature for nitrogen gas was 40°C, whereas that for carbon dioxide gas was initially 40°C for 3 min and then increased to 170°C at a rate of 30°C/min.
In the aerobic denitrification experiments, the following parameters were analyzed: 5-day biological oxygen demand (BOD5), chemical oxygen demand-dichromate (CODCr), total phosphorus (TP), suspended solids (SS), and total nitrogen (TN), total Kjeldahl nitrogen (TKN), ammonium, nitrite and nitrate. These analyses were carried out according to standard methods for the water pollution [23]. The concentration of dissolved oxygen (DO) and the value of oxidation-reduction potential (ORP) were monitored with an YSI DO probe (Model 58; USA) and Isteck ORP probe (Model 730P; Korea), respectively.
3. Results and Discussion3.1. Screening of Potential Aerobic DenitrifiersBy repeated streaking on agar plates, eight strains were purified. The eight isolates were given the names KH1 to KH8, and their characteristics of colonies and morphology were tabulated in Table 1. Each isolate exhibited its own characteristics of colony and morphology distinctively. Among these strains, the strain KH8 exhibited the most prominent ability of N2 gas production in the syringe experiment. For the efficient treatment of nitrous concentration contained in LIW, N2 gas as an end metabolite must be produced from the nitrous compounds at a higher conversion rate. Hence, the denitrification ability of the strain KH8 under an aerobic condition was further examined against that under an anaerobic condition. As shown in Table 2, 72.1-μmole N2 gas was produced anaerobically for 72 hr of cultivation with 28.3-μmole CO2 gas. Under the aerobic condition, N2 production was not active at initial stage. After then, however, N2 was steadily produced, and 64.2-μ mole was produced after 72 hr with a much higher amount of 411.9-μmole CO2. This indicates that the strain KH8 needed some time to adapt to an aerobic condition but its denitrification ability under an aerobic condition was almost comparable to the anaerobic denitrification ability. For this reason, the strain KH8 was identified and characterized in later experiments.
3.2. Identification of One Potent IsolateThe species-specific identification of the isolate was performed using 16S rDNA sequence analysis. The 1,418 bp-sized fragment of the 16S rDNA gene of the isolate was amplified and sequenced. Homology searches revealed that the isolate KH8 was closely related to Pseudomonas aeruginosa R12 (DQ073454; 100% similarity) and P. aeruginosa LCD12 (FJ194519; 99% similarity). The phylogenetic tree based on the partial 16S rDNA gene showed the relation of the isolate KH8 and other related strains (Fig. 2). From these distinctive features, the isolate KH8 was designated as P. aeruginosa R12.
3.3. Chartacteristics of Isolates in Lab-Scale Aerobic DenitrificationThe changes in reaction parameters were examined during the aerobic denitrification using different compositions of isolated strains as seed culture. The characteristics of aerobic denitrification starting with equal amounts of isolated strains under an aerobic condition are shown in Fig. 3(a). The initial DO was measured to be 5.8 mg/L under the continuous supply of oxygen. As the aerobic denitrification started, DO levels decreased to 0.3 mg/L within 2 hr, then fluctuated, and maintained over 0.9 mg/L after 32 hr. This implies that the supplied oxygen did not match the amount of DO consumed by isolates during the active reaction, probably due to the low solubility of oxygen [24]. As DO levels dropped, ORP decreased from 56.7 to −44.0 mV within 1 hr, decreased further to −148.4 mV after 4 hr, then increased and maintained at positive values after 10.5 hr. This indicates that the decrease in ORP value was related to the decrease in DO. The pH was 7.24 at the beginning, and decreased to 7.09 within 1 hr. After this, the pH increased to 8.01 until 5 hr due to alkalinity production by denitrification [25]. After 5 hr, the pH decreased steadily to a final value of 6.20. During the experiment for 48 hr, the concentration of CODCr reduced from 10,695 to 1,632 mg/L, the concentration of TN reduced from 2,355 to 1,767 mg/L, and the concentration of TKN reduced from 1,333 to 588 mg/L. From these data, the removal efficiencies of CODCr, TN and TKN were estimated to be 84.7%, 25.0% and 55.9%, respectively. The resulting CODCr/TN ratio decreased from 8.6 (at the beginning) to 2.0 (at the end). It is known that the C/N ratio may influence the metabolic pathway of organic matter utilization [26]. Accordingly, the decrease in the C/N ratio in a later stage of aerobic denitrification implies that external carbon may be necessary for high efficiency of N removal. Along with these reaction parameters, profiles of NH4+–N, NO2−–N and NO3−–N concentrations are also shown in Fig. 3(a). The concentration of NH4+–N increased from 73.7 to 354.0 mg/L for first 12 hr, and then decreased slowly to the end. On the other hand, the concentration of NO3−–N decreased from 270.7 to 104.6 mg/L for first 12 hr, and then increased slowly to the end. The concentration of NO2−–N as an intermediate metabolite in aerobic denitrification maintained low for first 12 hr, then increased to 202.7 mg/L after 24 hr, but decreased to almost zero at the end. From the analyses of all parameters, it was found that active aerobic denitrification took place in the first 12 hr when equal amounts of eight isolates were used as inoculums.
At a cell-combination ratio of 10:1 (the isolate KH8 to the other seven isolates), the characteristics of the aerobic denitrification are shown in Fig. 3(b). From the beginning of the experiment, DO levels maintained below 1 mg/L for the first 5.5 hr even under the continuous supply of oxygen. After then, the DO levels increased and maintained over 2.5 mg/L until the end. Due to active denitrification, the ORP values decreased as DO levels dropped. The ORP decreased to −182 mV after 3 hr, then increased steadily, and maintained at positive values after 11 hr. This was followed by a slight increase to 113.3 mV after 48 hr. The initial pH was 7.24, and decreased slightly to 7.20 within 1 hr. After then, the pH increased steadily to 8.34 until 24 hr. This duration of increase in pH was much longer than that exhibited during the experiment using equal amounts of isolated strains (shown in Fig. 3(a)). This indicates that higher alkalinity production resulted from stronger denitrification by the dominant action of the isolate KH8. After 34 hr, the pH somewhat decreased to a final value of 7.75. In particular, severe foams were generated between 1 hr and 6 hr. As a result, the changes in DO, ORP and pH appeared mostly in the first 12 hr. During the 48-hr reaction, the concentration of CODCr reduced from 7,381 to 1,099 mg/L, the concentration of TN reduced from 3,513 to 1,464 mg/L, and the concentration of TKN reduced 1,471 to 728 mg/L, respectively. From these data, the removal efficiencies of CODCr, TN and TKN were estimated to be 85.1%, 58.3% and 50.5%, respectively. Although there was no significant difference in the CODCr removal, the TN removal efficiency doubled in comparison with that (25.0%) obtained from the experiment using equal amounts of isolated strains (shown in Fig. 3(a)). Thus, this result indicates that the increase in the composition of isolate KH8 in the seeded cells resulted in improvement of N removal efficiency. The initial ratio of CODCr/TN was 5.5, but it decreased to 3.3 after 12 hr. After then, the C/N ratio continued to decrease, and was calculated to be 2.2 at the end. This result indicates that the aerobic denitrification efficiency was influenced by C/N ratio [27]. Along with these reaction parameters, changes in NH4+–N, NO2−–N and NO3−–N concentrations were also examined. The concentration of NH4+–N increased from 101.1 to 524.0 mg/L for first 12 hr, and then decreased to 144.9 mg/L at the end. On the other hand, the concentrations of NO3−–N and NO2−–N decreased from 202.3 to 18.0 mg/L and from 24.5 to 0 mg/L for first 12 hr, respectively. After then those concentrations increased slowly to 82.9 and 477.0 mg/L at the end, respectively. Therefore, the final concentration of NO3−–N was almost nine times lower, compared with the result shown in Fig. 3(a), indicating that aerobic denitrification was adequately taken place by the dominant isolate KH8.
To examine the characteristics of denitrifying isolates under an aerobic condition, nitrogen balances for their aerobic denitrifications were investigated at different compositions of seeded cells (Table 3). At the cell-combination ratio of 1:7, N loss was estimated to be approximately 32.8% during the 12-hr aerobic denitrification, with the cell concentration of 9 mg/L. This result was presumed that 32.8% of the initial N in the culture medium was converted to N2 gas by the denitrifying activities of isolated strains under an aerobic condition. However, this aerobic denitrification slowed down during the next 36 hr. The N loss taking place in the aerobic denitrification was somewhat improved when the composition of isolate KH8 was increased within the seeded cells (at the cell-combination ratio of 10:1). The percentage of N loss was 42.5 during the first 12 hr, and it was 58.4 during an entire period of this experiment. From these results, the potential of isolate KH8 was found to be obvious in aerobic denitrification. The similar results were found in the previous studies using potential aerobic denitrifiers [10, 13, 20].
3.4. Application of Isolates to Plant-Scale LIW TreatmentTo examine the potential ability of the isolates for better N removal, plant-scale aerobic denitrification using the isolates was performed in the aeration tank under three different types of treatments (control, treatment 1 and treatment 2). Fig. 4 shows the concentration profiles in the influent and effluent during the entire course of the experimental study. The LIW flowed into this treatment plant had the following characters: pH of 8.5, DO of 1.0 mg/L, CODCr of 3,000 mg/L, BOD5 of 2,500 mg/L, TN of 450 mg/L, NH4+–N of 400 mg/L, TP of 50 mg/L, SS of 3,000 mg/L, and undetectable NO2−–N and NO3−–N. After the LIW passed through equalization tank, it flowed into fermentation and synthesis tank, and then the eight-stage aeration tank, as shown in Fig. 1. The influent and effluent water quality of the aeration tank under each treatment was tabulated in Table 4. From the control experiment, the influent and effluent concentrations of DO, BOD5, TP and SS were found to be 1.0 and 2.4 mg/L (DO), 1,248 and 71 mg/L (BOD5), 33 and 20 (TP), and 5,174 and 2,976 mg/L (SS), respectively. The increase in SS concentration was due to the return sludge, and this outcoming SS settled down in tertiary clarifier. When the method of treatment 1 (additional input of isolates, as described in the section of 2.4) or treatment 2 (additional input of isolates and C-source, as described in the section of 2.4) was applied, DO levels increased slightly and the removal efficiency of BOD5 also increased slightly. However, the removal efficiencies of TP and SS by these treatments were least different. During the experimental period, the variances in pH, CODCr and TN were obvious among the different types of treatments. The best removal efficiencies of CODCr (97.0%) and TN (89.8%) were achieved by the treatment 2. This TN removal efficiency was much higher than that (51%) obtained from the control experiment. Along with higher TN reduction, a higher pH value (7.34) revealed in the effluent at day 56 under the treatment 2, because more active denitrification occurred under an aerobic condition. In all the treatments, NO2−–N was not detected in the effluent at day 56, implying that it converted to N2 by denitrification. The lowest concentrations of NH4+–N (20mg/L) and NO3−–N (8.9 mg/L) in the effluent at day 56 were achieved by the treatment 2, in proportion to the TN removal efficiency.
To understand the characteristics of the isolated aerobic denitrifiers in the aeration tank during the plant-scale operation, the major parameters were monitored. The profiles of pH, CODCr and TN values under three different types of treatments are shown in Fig. 4, and those of NH4+–N, NO2−–N and NO3−–N are shown in Fig. 5. As shown in Fig. 4(a) (in case of control experiment), the pH was 7.20 at the beginning and it ended at 7.03 without significant variance. This result indicates that poor denitrification resulted in pH decrease at the end [28]. The influent concentration of CODCr fluctuated because it was dependent upon daily LIW content. The influent TN was in a range of 290–300 mg/L, and was removed steadily. As it turned out, the removal efficiencies of CODCr and TN were 92.8% and 51%, respectively. The influent C/N ratios were in a range of 3.7–4.0. Hence, the composition of carbons and nitrogens in the influent was not significantly fluctuated. These removal efficiencies were improved when the isolated aerobic denitrifiers were additionally seeded into the aeration tank (in case of treatment 1, as shown in Fig. 4(b)). Those efficiencies were estimated to be 95.6% and 84.2%, respectively. The intense reductions in CODCr and TN started after 14 day, with the increase of pH. The influent pH at 7.18 decreased to 6.78 after 14 day due to strong nitrification. After then, the pH increased steadily to 7.19 due to the alkalinity production by denitrification. These results indicate that the isolated aerobic denitrifiers needed at least 14 day to be established in the aeration tank. The influence of the isolated aerobic denitrifiers on denitrification in the aeration tank was more obvious when these cells were seeded with lactose as carbon source (in case of the treatment 2, as shown in Fig. 4(c)). With addition of a carbon source, the influent C/N ratios (3.9 to 4.2) increased slightly. In this treatment, the pH decreased during the first 14 day, and then it increased by the strong denitrification. The effluent pH revealed 7.34, which was higher by 0.3 than that (7.03) obtained from the control experiment. The similar results could be found in the previous report of heterotrophic nitrification and aerobic denitrification for treatment of high-strength ammonium [29]. By this improved denitrification, the removal efficiencies of CODCr (97.0%) and TN (89.8%) increased further.
As shown in Fig. 5(a), the concentrations of NH4+–N, NO2−–N and NO3−–N were changed in the control experiment. The influent NH4+–N concentration in a range of 240–255 mg/L converted to NO2−–N and NO3−–N by nitrification, and final concentration of effluent NH4+–N was 40 mg/L. Although the influent did not contain NO2−–N, its concentration in the effluent at day 0 was 10 mg/L due to the effect of nitrification. However, NO2−–N was not detected after 40 day. The NO3−–N was detected first in the fermentation and synthesis tank. As a result, the initial concentration of NO3−–N flowing into the aeration tank was 53 mg/L. The initial NO3−–N of 50 mg/L in the effluent increased rapidly to 165 mg/L during the first 14 day, but it did not decrease adequately after 56 day, implying that active denitrification did not occur to offset the nitrification. This reduction of NO3−–N after 14 day was obvious in the result of treatment 1 (Fig. 5(b)). The effluent NO3−–N of 165 mg/L at 14 day decreased steadily to 20.1 mg/L until 56 day, implying that active denitrification occurred in the aeration tank. Along with NO3−–N, the concentration in the effluent NH4+–N decreased from 251 to 35 mg/L in parallel. Similarly, the effluent NO2−–N also decreased, and was not detected after 45 day. This trend was more obvious in the result of the treatment 2 (Fig. 5(c)). The concentration of effluent NO3−–N increased from 49 to 184 mg/L during the first 14 day, and then decreased rapidly to 8.9 mg/L after 56 day. At the same time the effluent NH4+–N decreased from 247 to 20 mg/L for 56 day. The effluent NO2−–N of 9.6 mg/L at the beginning decreased slightly, and was not detected after 47 day. Therefore, more active denitrification by the treatment 2 resulted in the best N removal efficiency among the three different types of treatments. Besides, variances in the values of parameters were not significant after 42 day operation. This result implies that the seeded aerobic denitrifiers for the removal of excess N existing in LIW were stabilized in the aeration tank for six weeks. It is important that operation under a stabilized system is a key to reliable N treatment [30]. Suneethi and Joseph [31] reported that a large-scale anaerobic ammonium oxidation (ANAMMOX) process was stabilized in 130 day after ANAMMOX bacteria were seeded. Compared with this result, the stabilization of the seeded isolates in this study was completed in a shorter time although the LIW contained refractory organic N compounds to some extent [32]. Under the treatment 2, the final water quality in the effluent leaving the treatment plant was as follows: pH 7.3, DO of 3.0 mg/L, CODCr of 30 mg/L, BOD5 of 24 mg/L, TN of 18 mg/L, NH4+–N of 12 mg/L, NO2−–N of 0 mg/L, NO3−–N of 6 mg/L, TP of 5 mg/L, and SS of 15 mg/L, all of which were below the water-quality standard concentrations. As a result, efficient LIW treatment was achieved by additional input of isolated aerobic denitrifiers with a carbon source. Therefore, the isolated aerobic denitrifiers could be an attractive candidate for application to wastewater treatment plants in which the N removal is difficult after nitrification due to poor denitrification or advanced treatment is required for N under the existing aeration-tank capacity.
4. ConclusionsIn this study, eight aerobic denitrifiers were newly isolated to treat LIW containing high-strength N efficiently. When one potent isolate (the strain KH8) held a great majority (at the cell-combination ratio of 10:1) within the seeded cells, TN removal was much improved and 58.4% of initial N was presumed to be converted to N2 gas, based on the N balance calculated from the lab-scale aerobic denitrification. When these seeded cells with lactose were applied to plant-scale aeration tank for the treatment of high-strength N contained in LIW, the system could be stabilized after 42 day and the N removal efficiency was approximately 38% higher than that obtained from the existing process. Consequently, the isolated aerobic denitrifiers could be an attractive candidate for application to the efficient removal of high-strength N from wastewater.
AcknowledgmentsThis work was supported by Pukyong National University Research Foundation Grant (“Autonomous Ingenuity Research” in Year 2013).
References1. Gupta AB, Gupta SK. Simultaneous carbon and nitrogen removal from high strength domestic wastewater in an aerobic RBC biofilm. Water Res. 2001;35:1714–1722.
2. Third KA, Gibbs B, Newland M, Cord-Ruwicch R. Long-term aeration management for improved N-removal via SND in a sequencing batch reactor. Water Res. 2005;39:3523–3530.
3. Schmidt I, Sliekers O, Schmid M, et al. New concept of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiol Rev. 2003;27:481–492.
4. Robertson LA, Kuenen JG. Thiosphaera pamtotropha gen. nov. sp. nov., a facultatively anaerobic, facultatively autotrophic sulphur bacterium. J Gen Microbiol. 1983;129:2847–2855.
5. Su JJ, Liu BY, Lin J, Yang CP. Isolation of an aerobic denitrifying bacterial strain NS-2 from the activated sludge of piggery waste-water treatment systems in Taiwan possessing denitrification under 92% oxygen atmosphere. J Appl Microbiol. 2001;91:853–860.
6. van de Graaf AA, Mulder A, de Bruijn P, Jetten MS, Robertson LA, Kuenen JG. Anaerobic oxidation of ammonium is a biologically mediated process. Appl Environ Microbiol. 1995;61:1246–1251.
7. Eum Y, Choi E. Optimization of nitrogen removal from piggery waste by nitrite nitrification. Wat Sci Technol. 2002;45:89–96.
8. Focht DD, Verstraete W. Biochemical ecology of nitrification and denitrification. Adv Microb Ecol. 1977;1:135–214.
9. Shi Z, Zhang Y, Zhou J, Chen M, Wang X. Biological removal of nitrate and ammonium under aerobic atmosphere by Paracoccus versutus LYM. Bioresour Technol. 2013;148:144–148.
10. Zhao B, He YL, Hughes J, Zhang XF. Heterotrophic nitrogen removal by a newly isolated. Acinetobacter calcoaceticus HNR. Bioresour Technol. 2010;101:5194–5200.
11. Wan C, Yang X, Lee DJ, Du M, Wan F, Chen C. Aerobic denitrification by novel isolated strain using NO2−–N as nitrogen source. Bioresour Technol. 2011;102:7244–7248.
12. Zheng M, He D, Ma T, et al. Reducing NO and N2O emission during aerobic denitrification by newly isolated Pseudomonas stutzeri PCN-1. Bioresour Technol. 2014;162:80–88.
13. Yang XP, Wang SM, Zhang DW, Zhou LX. Isolation and nitrogen removal characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Bacillus subtilis A1. Bioresour. Technol. 2011;102:854–862.
14. Chen Q, Ni J. Ammonium removal by Agrobacterium sp. LAD9 capable of heterotrophic nitrification-aerobic denitrification. J Biosci Bioeng. 2012;113:619–623.
15. Zheng HY, Liu Y, Gao XY, Ai GM, Miao LL, Liu ZP. Characterization of a marine origin aerobic nitrifying-denitrifying bacterium. J Biosci Bioeng. 2012;114:33–37.
16. Zhu L, Ding W, Feng LJ, Dai X, Xu XY. Characteristics of an aerobic denitrifier that utilizes ammonium and nitrate simultaneously under the oligotrophic niche. Environ Sci Pollut R. 2012;19:3185–3191.
17. Yao S, Ni J, Ma T, Li C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour Technol. 2013;139:80–86.
18. Padhi SK, Tripathy S, Sen R, Mahapatra AS, Mohanty S, Maiti N. Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int Biodeter Biodegr. 2013;78:67–73.
19. Chung J, Kim J, Kim Y, Hwang Y. Assessment and selection of best available technology (BAT) for wastewater facilities in the leather tanning and finishing industry. Resour Conserv Recy. 2013;70:32–37.
20. Kim JK, Park KJ, Cho KS, Nam SW, Park TJ, Bajpai R. Aerobic nitrification–denitrification by heterotrophic Bacillus strains. Bioresour Technol. 2005;96:1897–1906.
21. Kim JB, Cho KS, Jeong SK, Nam SW, Jeong HD, Kim JK. Identification and characterization of a pigment-producing denitrifying bacterium. Biotechnol Bioprocess Eng. 2008;13:217–223.
22. Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402.
23. APHA, AWWA. Standard methods for the examination of water and wastewater. 19th edAmerican Water Works Association, Washington: American Public Health Association; 1995.
24. Nielsen J, Villadsen J. Bioreaction engineering principles. New York: Plenum Press; 1994.
25. Li B, Irvin S. The comparison of alkalinity and ORP as indicators for nitrification and denitrification in a sequencing batch reactor (SBR). Biochem Eng J. 2007;34:248–255.
26. Ruiz G, Jeison D, Chamy R. Development of denitrifying and methanogenic activities in USB reactors for the treatment of wastewater: Effect of COD/N ratio. Process Biochem. 2006;41:1338–1342.
27. Kim M, Jeong SY, Yoon SJ, et al. Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J Biosci Bioeng. 2008;106:498–502.
28. Pan Y, Ye L, Ni BJ, Yuan Z. Effect of pH on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers. Water Res. 2012;46:4832–4840.
29. Shoda M, Ishikawa Y. Heterotrophic nitrification and aerobic denitrification of high-strength ammonium in anaerobically digested sludge by Alcaligenes faecalis strain No. 4. J Biosci Bioeng. 2014;117:737–741.
30. Yubo CUI, Tieheng SUN, Lihui ZHAO, Jiang T, Zhang L. Performance of wastewater sludge ecological stabilization. J Environ Sci. 2008;20:385–389.
Fig. 1Process flow diagram of the LIW treatment plant. Arrows indicate the flow of wastewater (→), the flow of sludge (⇢) and the flow of backwash water (→), respectively. 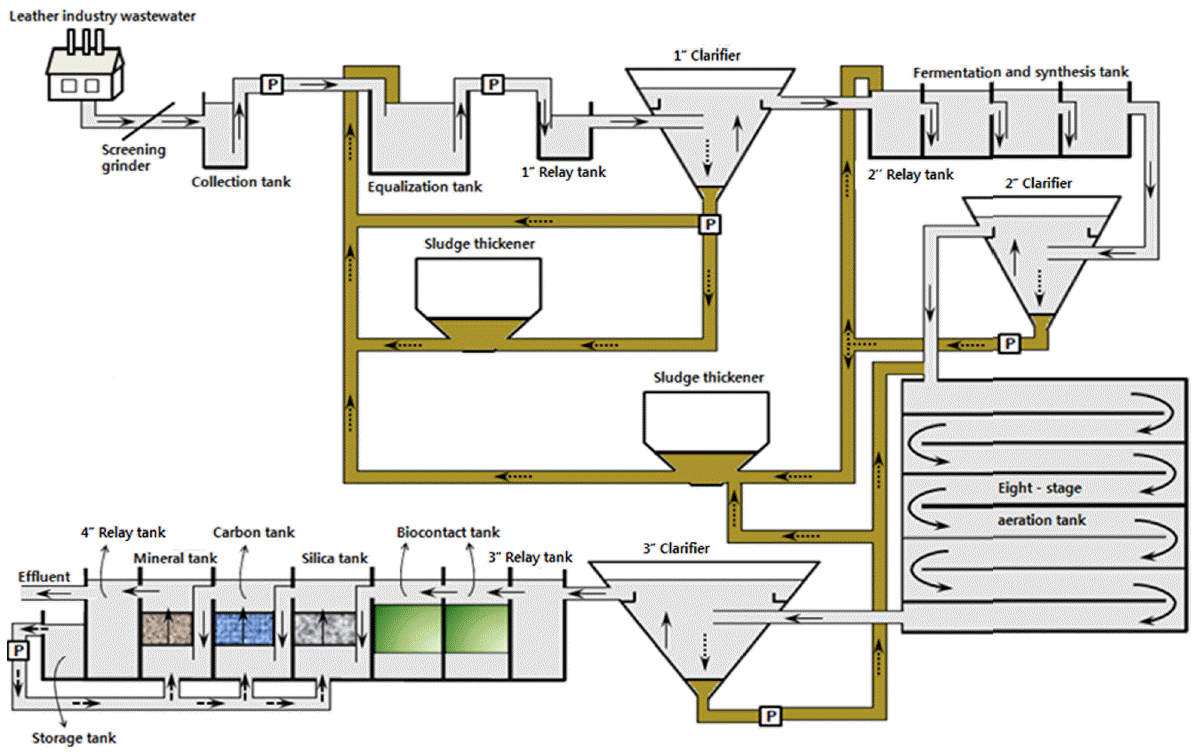
Fig. 2Phylogenetic tree based on a partial 16S rRNA gene of the strain KH8 and other related species. 
Fig. 3Changes in reaction parameters during aerobic denitrifications carried out in 1-L 5-neck flasks at different compositions of the seeded cells. The combination ratios of the isolate KH8 to other seven isolates in the seeded cells were 1:7 (a) and 10:1 (b), respectively. 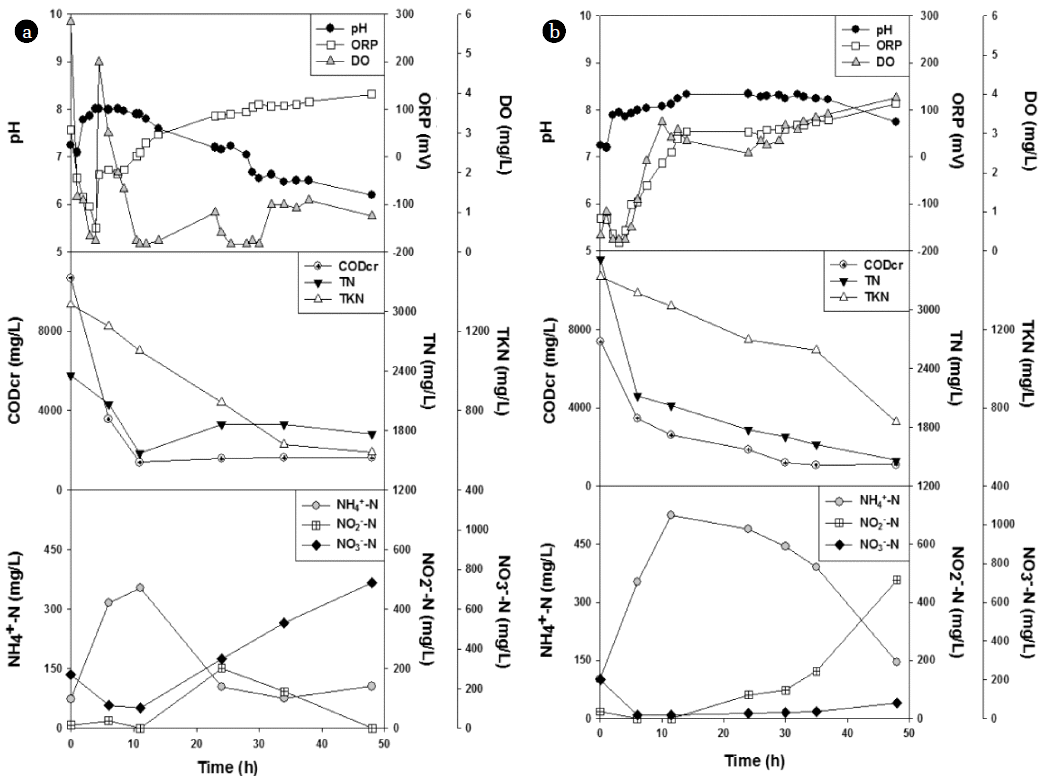
Fig. 4Changes in pH (■), COD (●) and TN (▲) values of plant-scale operation under different types of treatments. (a) Control, (b) Treatment 1, and (c) Treatment 2. Open and closed symbols represent influent and effluent in the aeration tank, respectively. 
Fig. 5Change in NH4+–N(▼), NO2−–N (◆) and NO3−–N (★) values of plant-scale operation under different types of treatments. (a) Control, (b) Treatment 1, and (c) Treatment 2. Open and closed symbols represent influent and effluent in the aeration tank, respectively. 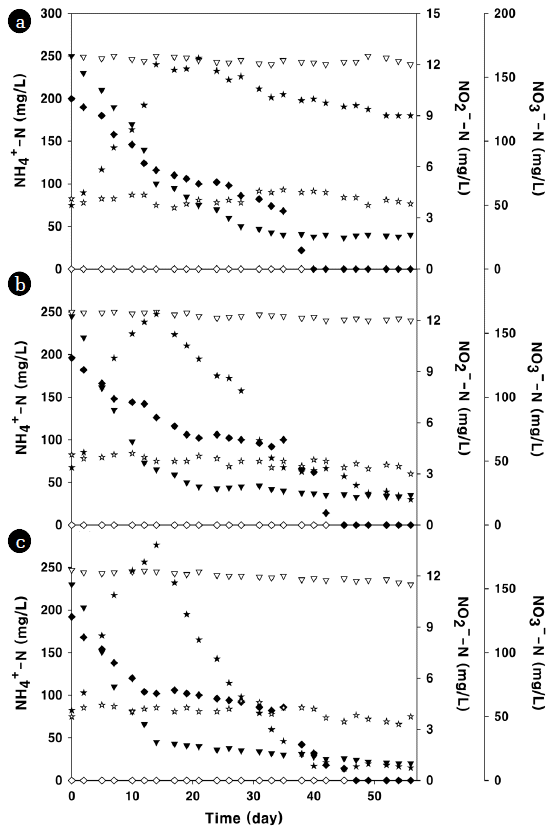
Table 1Characteristics of Each Isolated Strain
Table 2Comparison of Characteristics of Denitrification Caused by the Isolate KH8 Between Aerobic and Anaerobic Conditions
Table 3Nitrogen Balances for Aerobic Denitrifications at Different Compositions of Seeded Cells
Table 4Average Values of Reaction Parameters Indicating Water Quality of Influent at day 0 and Effluent at day 56 in the Aeration tank Under Different Types of Treatments
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||