1. Introduction
Algae cultures have primarily been developed as an important source of aquaculture feeds, human food supplements and pharmaceutical [1], and algae have been proposed as a good candidate for fuel production [2]. Algae strains that are robust and highly productive are selected for the conversion of biomass into energy [3], and strains with relatively high lipid contents are highly attractive for biodiesel fuel production [4]. Microalgae have high growth rates and produce lipids for biofuel production, which is essential for increasing biomass production and amount of lipids that decrease the cost of biodiesel production [5]. Microalgae as a source of renewable energy have received considerable interest; however, further optimization of mass culture conditions is necessary for microalgal biofuel production to be economically viable and sustainable [6].
The ability of microalgae to transition from photoautotrophic to mixotrophic growth is a phenomenon that exists in a number of genera and species throughout the major taxonomic divisions [7, 8]. Many algal organisms can use either autotrophic or heterotrophic metabolic processes for growth; therefore, they can photo-synthesize and utilize organic materials [9]. In heterotrophy, algae grow in darkness where cells get energy completely from organic carbon in the media, while in mixotrophy, algae can obtain energy from both organic carbon and light. Such a condition is suitable for algal species that cannot grow in complete darkness but require low light or agitation [10, 11]. Growth rate and biomass production for some algae in mixo- or heterotrophic conditions can be several times higher than those in a photoautotrophic condition alone [11]. Moreover, the synthesis of metabolic products such as lipids and pigments is influenced by the quality and quantity of organic carbon. The use of organic carbon in mixotrophic culture would also reduce the need for carbon dioxide in the culture and facilitate the growth of algal species sensitive to agitation [12]. Bouarab et al. [13] reported that Micractinium pusillum grew in the presence of organic substrates, such as glucose and acetate under both mixotrophic and heterotrophic conditions. It can be concluded from the above that mixotrophism is an ideal nutritional mode for high density cultivation of microalgae for the production of biofuels and functional components. However, even though the biomass and lipid productivities are significantly higher compared with those from authotrophic growth, the cost of the organic carbon sources (usually in the form of glucose or acetate) is high when compared against all other added nutrients. To overcome this high carbon cost, a cheap resource must be found. Crude glycerol, which is derived from biodiesel production process, is capable of providing such a supply. As biodiesel production continues to increase, the market is being flooded with crude glycerol [14]. Crude glycerol prices have dropped from $0.25/lb in 2004 to $0.025–0.05/lb in 2006 [15, 16]. The increase supply and low demand for crude glycerol have pushed biodiesel producers into eagerly seeking ways to dispose this by-product. Therefore, development of sustainable processes for utilizing this organic raw material is imperative.
Recently, a process using crude glycerol as a substrate for the fermentation of the microalga Schizochytrium limacinum was developed. The oleaginous Schizochytrium limacinum can produce significant amounts of total lipids and docosahexaenoic acid, particularly when grown on a variety of carbon sources, such as glucose, glycerol or fructose [17, 18]. The above findings suggest that biodiesel-derived crude glycerol is a potential substrate for the mixotrophic cultivation of oleaginous microalgae to utilize crude glycerol and reduce the production cost of microalgal biodiesel. However, there are few reports examining the effects of crude glycerol, particularly on biomass production and algal cell components under mixotrophic conditions. In this study, the effects of various concentrations of crude glycerol on the biomass growth and oil content of Neochloris oleabundans, Botryococcus Braunii and Dunaliella sp. under mixotrophic conditions were evaluated.
2. Materials and Methods
2.1. Microalgae Cultures and Medium
The investigated microalgae isolated from KMCC (Korea Marine Microalgae Culture Center). The seed cultures of Neochloris oleabundans (N. Oleabundans), Botryococcus Braunii (B. Braunii) and Dunaliella sp. were cultivated in Jaworski’s medium (JM) under light emitting diode (LED) lamps at ambient temperature. JM comprises 4.0 g Ca(NO3)2·H2O, 2.48 g KH2PO4, 10.0 g MgSO4·7H2O, 3.18 g NaHCO3, 0.45 g EDTAFeNa, 0.45 g EDTANa2, 0.496 g H3BO3, 0.278 g MnCl2·4H2O, 0.20 g (NH4)6Mo7O24·4H2O, 0.008 g cyanocobalamin, 0.008 g thiamine HCl, 0.008 g biotin, 16.0 g NaNO3 and 7.2 g Na2HPO4·12H2O in 200 mL deionized water. The microalgae were cultured in 200 mL conical flask containing 100 mL of JM pH (7.2 ± 0.3), and then 10 mL N. Oleabundans, Botryococcus Braunii and Scenedesmus sp. were added. The cultures were maintained in a dark and light cycle of 8 and 16 hr, respectively.
2.2. Experimental Design
2.2.1. Optical panel photobioreactor (OPPBR) construction and operation
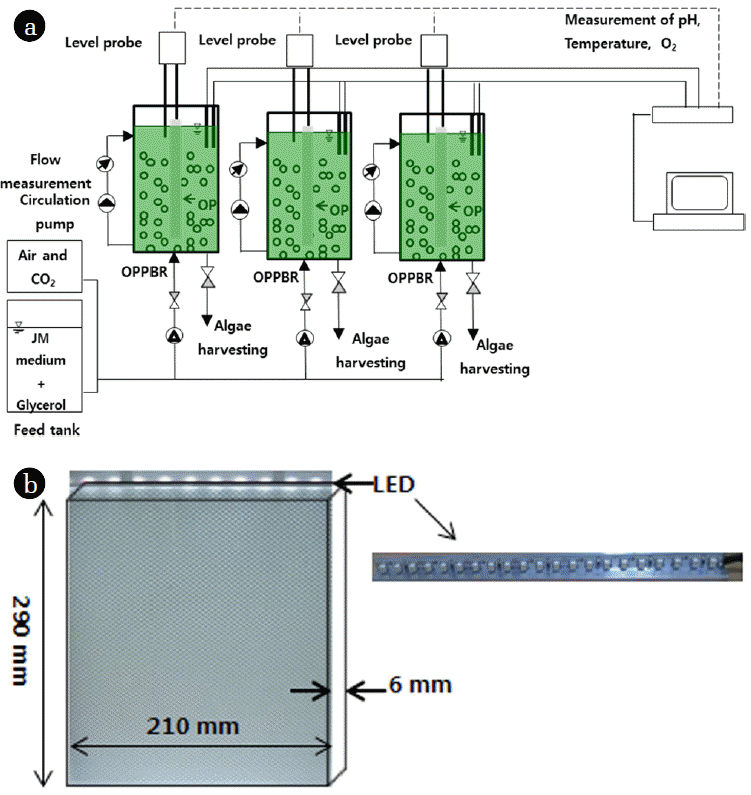
A schematic diagram of the OPPBR is shown in Fig. 1(a). The OPPBR was operated at a 15-L working volume and was equipped with an OP in each reactor. The initial concentrations of the inoculated microalgae were N. Oleabundans, 0.357 ± 0.7 g/L, B. Braunii, 0.342 ± 0.7 g/L and Dunaliella sp., 0.367 ± 0.6 g/L. The experiments were conducted at neutral pH (7.2 ± 0.3) under dark and light cycles of 8 and 16 hr, respectively. The temperature was maintained at 23 ± 1°C using LEDs for 20 days. The OPPBRs were aerated continuously at an aeration rate of 0.5 L/min. CO2 at the equivalent aeration rate of 0.02 vvm (ca. 2%) was used for cultivation. The OPPBR was designed such that the light source (22 LEDs), an LED panel (bar type), was placed in the OPPBR. A v-grooved optical panel (OP) was inserted underneath this in the photobioreactor (PBR). The thickness of the OP was 6 mm (cf. Fig. 1(b)). The light incident was uniformly distributed across both sides of the OP in the reactor and provides greater functionality. The LED light source was used because it was efficient and provided the required wavelength light from 430 nm to 670 nm, which is selective for microalgal growth. Moreover, the light intensity, which represents the amount of light used for photosynthesis, was ~250 μmol photons/(m2·s). The mixotrophic conditions for algae cultivation were achieved with crude glycerol purchased as a byproduct of biodiesel production. The corresponding amount of crude glycerol was added to the JM growth medium to achieve to the desired mixotrophic medium.
2.2.2. Optical panel in photobioreactor
The characteristics of the OP are listed in Table 1. The OP exhibited 93% transmittance and 1.19 g/cm2 specific gravity. The OP dimensions were 210 mm (L) × 290 mm (H) × 6 mm (W) as shown in Fig. 1(b) and were constructed from a transparent panel of pure PMMA (poly-methylmethacrylate). This material has good transparency and its light absorption in the visible region is negligible [19].
In this study, a v-cut OP (Fig. 1(b)) was designed. This was used to evaluate and assess the quantitative effects of the illumination area and OP arrangement on cell growth and biomass productivity. With the v-cut technology, the light was guided into the v-grooves that have x-, y- and z- direction dimensions, such as enlarged horizontal and vertical grooves. The vertical v-grooves are widely spaced when they are close to the light source and narrow when distant from the light source. The enlarged horizontal v-grooves are arranged in straight lines along the x-direction from the end edge of the OP and have maximum enlarged portions located on the other edge of the OP. In addition, the v-cut is varied to provide a uniform distribution of light in the PBR.
2.3. Analytical Methods
2.3.1. Measurement of cell weight and specific growth rate
The effect of the crude glycerol concentrations (0, 2, 5 and 10 g/L) added during the initial growth phase was evaluated in relation to the growth of the algae biomass and lipid accumulation. Previous study was reported that the high concentration of glycerol (10%) showed an inhibitory effect on the growth of microalgae [15, 20]. To determine the biomass concentration, a sample of microalgae in growth medium was centrifuged for 10 min at 628 g, washed with distilled water and then dried in an oven at 105°C for 24 hr to a constant weight. The biomass productivity P (g/(L·day)) was calculated from the variation in biomass concentration (g/L) within a cultivation time (in days) according to the following equation:
The specific growth rate μ (in days) was calculated using Eq. (2)
where X1 and X0 are the biomass concentration (g/L) on days t1 and t0, respectively.
2.3.2. Extraction of lipids
The algal biomass for lipid extraction was prepared by centrifugation and drying. After oven drying, the algae were pulverized and subjected to Soxhlet extraction. All Soxhlet extractions were performed for 72 hr using 500 mL solvent for 1 g of pulverized dry algae with a cycle time of 10–15 min. The Soxhlet extraction with hexane was selected because the Bligh and Dyer [21] extraction method is suitable for the extraction of all lipids, including triglycerides, phospholipids and other pigments [22]. The lipid content does not reflect the exact amount of triacylglycerols (TAG; consisting of a glycerol moiety with each hydroxyl group esterified to a fatty acid) because only triglycerides are used in the synthesis of biodiesel and other components are undesirable. The excess hexane was evaporated by rotary evaporation until the total volume reached 30–40 mL. The solutions were diluted to 50 mL and used to determine the TAG content. The amount of TAG was determined using a Fourier transform infrared (FTIR) spectrometer Spectrum RX 1 (Perkin Elmer) according to the carbonyl stretching absorption at 1740 1/cm [23]. The amount of TAG in the extract solutions was determined using a standard graph, and the amount of TAG was calculated in the dry algae (%, w/w). The experiments were performed five times, and mean values and the standard deviation was calculated.
2.3.3. Measurement of fatty acids composition
The fatty acid composition of the algae oil was determined using the standards EN ISO 5508 and EN ISO 5509. The analysis was performed with a Clarus 500 (Perkin Elmer) gas chromatograph. The conditions for analysis were as follows: capillary column Alltech AT-FAME (30 m – 0.25 mm – 0.25 μm), initial oven temperature of 210°C held for 5 min then increased at 20°C/min from 210°C to 230°C and held at 230°C for 12 min. Nitrogen was used as the carrier gas. The injector temperature was 250°C. The fatty acids were identified by comparing their retention times with the standards. The experiments were performed five times and the mean values and standard deviations were calculated.
3. Results and Discussion
3.1. Effect of Glycerol Concentration on the Growth of Algal Species
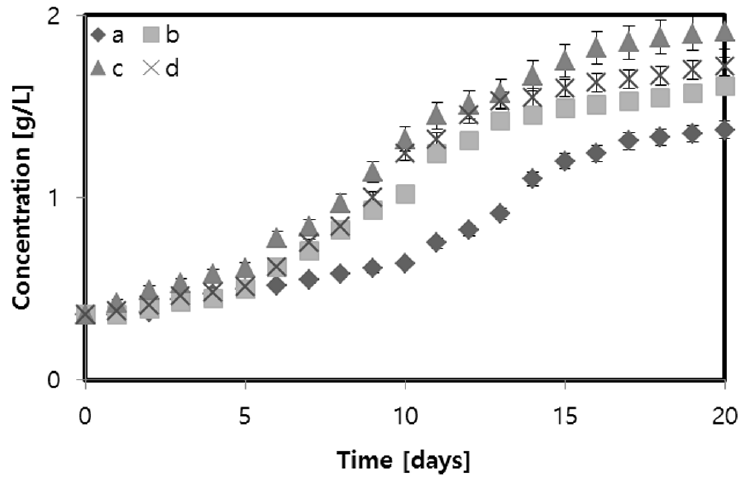
Fig. 2 shows the effect of different glycerol concentrations on the growth of N. Oleabundans compared to growth on JM medium. During the first five days, the microalgae grew similarly in all growth media with different amounts of glycerol. A slight difference in biomass is characteristic of a growth medium with initial concentrations of 2 g/L and 10 g/L. The maximum biomass concentrations of 1.61, 1.91 and 1.72 g/L were obtained in the medium containing 2, 5 and 10 g/L, respectively. By comparison, the highest biomass concentration with 5 g/L glycerol was 39.42% higher than the concentration achieved with N. Oleabundans in autotrophic medium, which was 1.37 g/L. All media with glycerol yielded a higher biomass than the autotrophic condition.
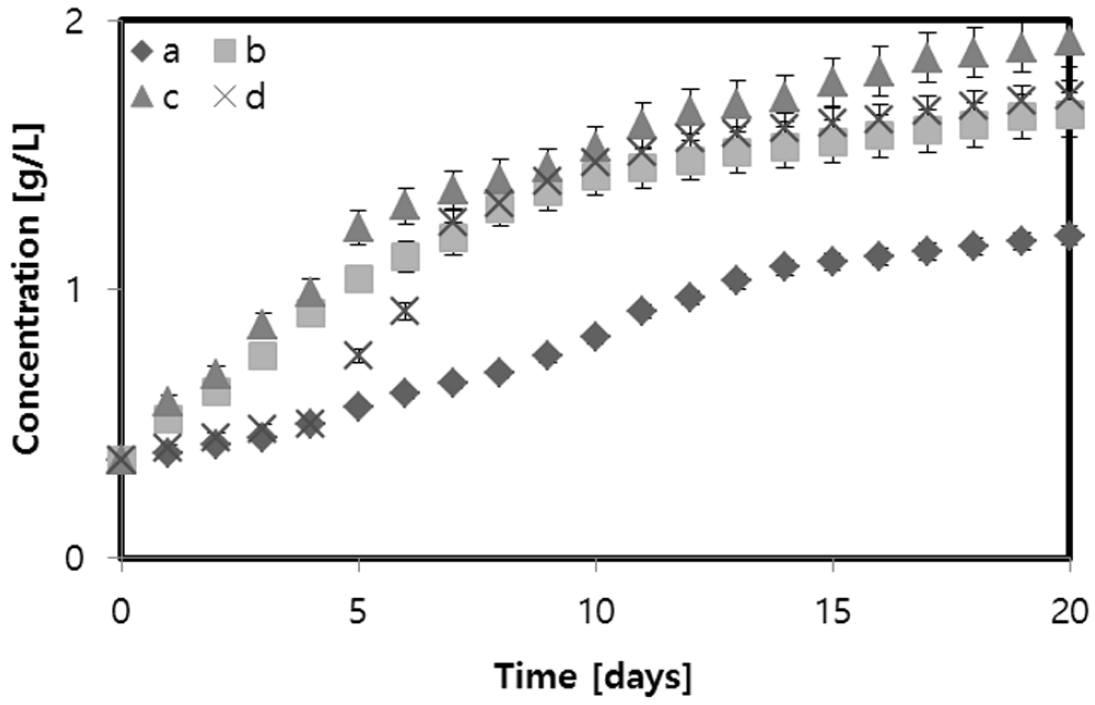
The growth process was observed for Dunaliella sp. in JM medium with different glycerol concentrations (Fig. 3). During the first 4 days, the microalgae grew similarly in the autotrophic condition and 10 g/L glycerol. Additionally, for the media with 2 g/L and 5 g/L glycerol, the growth was similar for 4 days. Faster growth was observed for the medium with 5 g/L glycerol. The highest biomass concentration (1.92 g/L) was obtained during the stationary growth phase. This concentration is 60.00% higher than the microalgae concentration in the autotrophic medium, which was 1.20 g/L. These data indicate that Dunaliella sp. grew better under mixotrophic conditions using crude glycerol compared to the autotrophic condition.
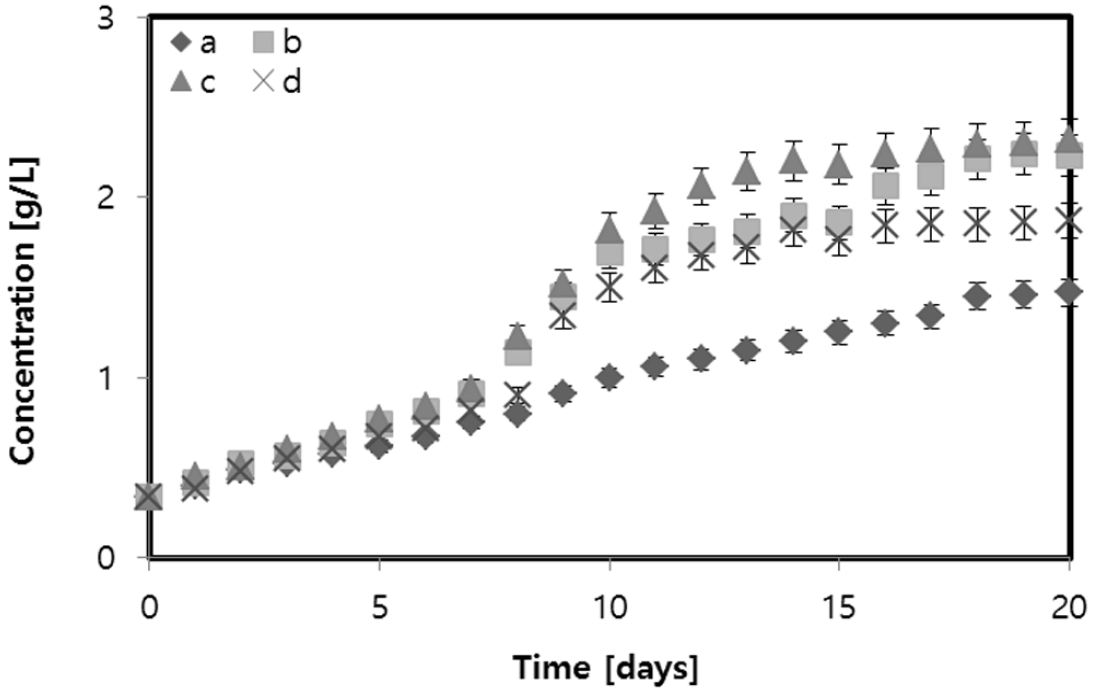
Biomass growth during all phases was characteristic of B. Braunii cultures as shown in Fig. 4. During the first 5 days, the microalgae grew similarly in all growth media with different amounts of glycerol. The biomass concentrations for 2 g/L and 5 g/L glycerol were similar. The highest achieved biomass concentrations (2.23 g/L and 2.32 g/L) were similar for the same concentrations of glycerol (2 g/L and 5 g/L) and were 51.70% and 57.82% higher than the corresponding biomass concentrations obtained without glycerol (the highest concentration was 1.47 g/L). Therefore, glycerol has a large impact on the growth of B. Braunii compared to autotrophic growth in JM medium. A slight decrease in the biomass of 1.87 g/L was obtained with 10 g/L glycerol.
As shown in Table 2, all algae species showed maximum biomass productivity and maximum specific growth rates with glycerol concentrations ranging from 0 to 10 g/L. N. Oleabundans achieved 0.227 g/(L·day) maximum biomass productivity and 0.342 1/day maximum specific grow rate with 5 g/L glycerol. The maximum biomass productivity (0.231 g/(L·day)) and maximum specific growth rate (0.306 1/day) were achieved with 5 g/L glycerol for Dunaliella sp. B. Braunii showed no significant differences in the highest values of the maximum biomass productivity (0.258 and 0.271 g/(L·day)) and maximum specific growth rate (0.228 and 0.301 1/day) with 2 g/L and 5 g/L glycerol. However, the maximum biomass productivity and maximum specific growth rate for the autotrophic condition was lower compared to the mixotrophic conditions.
There are three metabolic possibilities for the cultivation of microalgae, autotrophic, heterotrophic and mixotrophic growth. During mixotrophic growth, there are two distinctive processes, photosynthesis and aerobic respiration. The former is influenced by light intensity, and the latter is related to the organic substrate (glycerol or glucose) concentration [10, 20]. The highest biomass concentration of the three investigated microalgae species under mixotrophic conditions was achieved with 5 g/L crude glycerol in the JM medium. Similar concentrations of microalgae biomass were obtained by other scientists who investigated the mixotrophic growth of microalgae with glycerol. Andruleviciute et al. [24] reported 1.92, 2.15, 1.87 and 2.25 g/L as the highest biomass concentrations for Chlorella sp., Scenedesmus sp., Haematococcus sp. and Nannochloris sp., respectively, in growth medium containing 5 g/L glycerol. Cerón Garcia et al. [25] investigated the cultivation of Chlorella vulgaris in growth medium containing 1, 5 and 10 g/L glycerol. In the growth medium with 5 g/L glycerol, the concentration of microalgae biomass increased to 2.13 g/L (in our study 1.91 g/L). Liang et al. [26] concluded that only the highest amount (10 g/L) of glycerol had an inhibitory effect and that for lower concentrations of glycerol, the biomass concentration was increased compared to the autotrophic conditions (from 0.25 g/L under autotrophic conditions to 0.722 g/L under mixotrophic conditions with 1 g/L glycerol).
In the present study, the glycerol concentration in mixotrophic systems influences the biomass concentration and growth rate of N. Oleabundans, B. Braunii and Dunaliella sp. The highest concentration was obtained 39.42%, 60.00% and 57.82% for N. Oleabundans, Dunaliella sp. and B. Braunii in the mixotrophic conditions with 5 g/L glycerol, respectively. For increased biomass concentration, Dunaliella sp. is the best algae species for growth in mixotrophic medium enriched with glycerol. The results of this study suggest that the investigated algae species may be excellent biofuel producers because organic materials stimulate the growth rate of these strains.
3.2. Total Fatty Acids in Algae Species
The total TAG content using the different glycerol concentrations are represented in Table 3. The highest TAG content was 15.91%, 16.24% and 16.41% for N. Oleabundans, Dunaliella sp. and B. Braunii, respectively, with 5 g/L glycerol. The 5 g/L glycerol medium was determined 12.20%, 13.11% and 9.30% for N. Oleabundans, Dunaliella sp. and B. Braunii, respectively. All microalgal species under mixotrophic condition had 2–13% higher lipid content than the autotrophic condition. The 2 and 10 g/L glycerol media yielded similar TAG contents for N. Oleabundans and Dunaliella sp. However, for B. Braunii,, 2 g/L glycerol was approximately 6% higher than the 10 g/L glycerol. The highest lipid content was 16.41% for Botryococcus braunii with 5 g/L glycerol.
Compared to the autotrophic conditions, using glycerol for the cultivation of microalgae increased the lipid content of all algae species. The TAG content in the microalgae cells increases with 5 g/L glycerol for the algae species. However, 10 g/L glycerol decreased the lipid oil content for the algae species. Liang et al. [27] observed an increase in lipid content with increasing concentrations of glycerol. The lipid content increased from 22% with 1 g/L glycerol to 32% with 2 g/L glycerol. However, the highest amount (10 g/L) of glycerol had an inhibitory effect for growth of algae and TAG content. Too many glycerols in the media were causing death of microalgae.
The sensitivity analysis showed that the initial glycerol concentration was the most significant factor for algal growth and lipid production [28]. Chen and Walker [29] reported that in batch mode, the biomass and lipid concentration in Chlorella protothecoides cultivated in crude glycerol medium were 23.5 and 14.6 g/L, respectively, during a 6 day cultivation. This study demonstrated the feasibility of crude biodiesel glycerol as an alternative carbon substrate to glucose for microalgae cultivation, and a cost reduction of the carbon substrate feed for microalgal lipid production is expected. Similar results were observed in the current study. In mixotrophic cultures, the lipid contents were 4.50% to 12.20%, 3.19% to 13.11% and 2.22% to 9.30% for N. Oleabundans, Dunaliella sp. and B. Braunii, respectively, which were higher than the autotrophic culture.
The lipid content and effectiveness of microalgae growth for biodiesel production are important. Improved accumulation of oil but slower growth of microalgae may result in lower oil yields compared to faster growing microalgae with less oil accumulation. The results of this study showed that B. Braunii grown with 5 g/L glycerol accumulated the highest concentration of TAG and that growth in this medium was also higher in other glycerol containing medium. B. Braunii grown in 2 g/L glycerol had a lower TAG content compared to N. Oleabundans and Dunaliella sp. grown in 5 g/L glycerol, which had low biomass concentrations. N. Oleabundans, Dunaliella sp. grown in 5 g/L glycerol accumulated higher contents of TAG than in other glycerol concentrations. Therefore, to obtain high TAG content, the recommended mixotrophic condition for the microalgae species is 5 g/L glycerol.
3.3. Composition of Total Fatty Acids
The fatty acid profiles of the algae oil are shown in Table 4. For this experiment, we selected the samples with the highest oil contents, N. Oleabundans, Dunaliella sp. and B. Braunii using 5 g/L glycerol. For comparison, the profiles of the fatty acids of the autotrophic cultures and rapeseed oil, commonly used for biodiesel fuel production, are also provided. The contents of the saturated fatty acids of N. Oleabundans, Dunaliella sp. and B. Braunii were 34.94%, 20.23% and 21.39%, respectively, and the amount of unsaturated fatty acids was 65.06%, 79.77% and 78.61%, respectively, under mixotrophic conditions. The largest impact of glycerol was on the fatty acid profiles of N. Oleabundans. The saturated fatty acid content changed from 16.91% to 34.94% for the autotrophic condition and 5 g/L glycerol, respectively.
The other species did not significantly change. Dunaliella sp. and B. Braunii had changes in the saturated fatty acids content from 13.27% to 20.23% and from 16.28% to 21.39%, respectively. The unsaturated fatty acid content was 65.06%, 79.77% and 78.61% for N. Oleabundans, Dunaliella sp. and B. Braunii, respectively.
The most common source for the synthesis of biofuel is rapeseed oil. The quality of biofuels produced from rapeseed oil is specified by the requirements of the European Standard EN 14214. The quality parameters of biodiesel are influenced by the fatty acid composition of the oil [24]. The results of this study showed that the content of the saturated fatty acids in algal oil is higher than in rapeseed oil (5.40%) and that the content of the unsaturated fatty acids is lower (94.60% in rapeseed oil). A small amount of saturated fatty acids (34.94%) and a large amount of unsaturated fatty acids (65.06%) was characteristic for N. Oleabundans oil, while the other species had greater amounts of unsaturated fatty acids compared with saturated fatty acids.
A high proportion of polyunsaturated fatty acids for biodiesel are not wanted because they adversely impact the stability of the biodiesel [22]. For user acceptance, microalgae biodiesel will need to comply with existing standards. Microalgae oils differ from most vegetable oils in being quite rich in polyunsaturated fatty acids with four or more double bonds. For example, eicosapentaenoic acid (EPA, C20:5n-3; five double bonds) and docosahexaenoic acid (DHA, C22:6n-3; six double bonds) occur commonly in algal oils [30]. Fatty acids and fatty acid methyl ester (FAME) with 4 and more double bonds are susceptible to oxidation during storage and this reduces their acceptability for use in biodiesel. Some vegetable oils (Soybean and sunflower etc.) also face this problem. Although these fatty acids have much higher oxidative stability compared with DHA and EPA, the European Standard EN 14214 limits linolenic acid methyl ester content in biodiesel for vehicle use to 12% (mol). No such limitation exists for biodiesel intended for use as heating oil, but acceptable biodiesel must meet other criteria relating to the extent of total unsaturation of the oil [14, 24, 30]. Total unsaturation of oil is indicated by its iodine value. Standards EN 14214 and EN 14213 require the iodine value of biodiesel to not exceed 120 and 130 g iodine/100 g biodiesel, respectively. Furthermore, both the European biodiesel standards limit the contents of FAME with four and more double bonds, to a maximum of 1% mol. In view of the composition of many microalgae oils, most of them are unlikely to comply with the European biodiesel standards, but this need not be a significant limitation [14–16]. The extent of unsaturation of microalgae oil and its content of fatty acids with more than 4 double bonds can be reduced easily by partial catalytic hydrogenation of the oil [31]. Compared to rapeseed oil (59.1–66.3) and corn oil (22.3–25.2), the algal oil (9.42–20.99) is not as rich in polyunsaturated fatty acids. Rapeseed biodiesel presented a cetane number near to palm biodiesel. However higholeic sunflower and corn biodiesels, those which were richer in unsaturated ester of linoleic acid (C18:2), presents a cetane number in medium range [30].
Furthermore, the content of linolenic acid in the algae species corresponds to the requirements of the Standards EN 14214, which states that the content of linolenic acid methyl ester in biodiesel fuel should not exceed 12%. Therefore, the biodiesel fuel produced from algae oil will likely meet the requirements for the linolenic acid methyl ester content.
4. Conclusions
The growth of the algae strains N. Oleabundans, Dunaliella sp. and B. Braunii under mixotrophic conditions in the presence of different concentrations of crude glycerol was investigated with the objective of increasing the biomass growth and algae oil content. The highest biomass concentration was obtained in medium containing 5 g/L glycerol for N. Oleabundans, Dunaliella sp. and B. Braunii and was 39.42%, 60.00% and 57.82% higher, respectively, than for the autotrophic growth of these species. The maximum biomass productivity with 5 g/L glycerol was 0.227, 0.231 and 0.271 g/(L·day) and maximum specific growth rate 0.342, 0.306 and 0.301 1/day for N. Oleabundans, Dunaliella sp. and B. Braunii, respectively. The content of TAG under mixotrophic conditions was higher than under the autotrophic conditions. In particular, the TAG contents for 5 g/L glycerol medium were 12.20%, 13.11% and 9.30% higher for the respective autotrophic conditions. The content of the saturated fatty acids for N. Oleabundans, Dunaliella sp. and B. Braunii was 34.94%, 20.23% and 21.39%, and the unsaturated fatty acids was 65.06%, 79.77% and 78.61% under mixotrophic conditions, respectively. Based on the results of this experiment, Dunaliella sp. in the mixotrophic condition was shown to provide an effective for lipid content. However, the all investigated algal strains grown on glycerol produced higher biomass concentrations and lipid contents compared to the autotrophic growth. The fatty acid content of the oils from these species suggests their potential use as biodiesel feedstock.