AbstractRain events are extremely important for phosphorus (P) dynamics in rivers since large portions of annual river P loads can be transported in particulate forms during only a few major events. Despite their importance, a precise estimation of P contribution in river sediments after rainy seasons has rarely been reported. This study estimated the longitudinal variation in the concentrations of different inorganic P fractions in bed sediments of the South Han River over a rainy season, through using the sequential extraction method. Non-apatite P was the dominant form, representing more than 60% of total inorganic P (TIP) content in sediments. Although no significant variation of TIP contents was observed, the proportion of bioavailable P in TIP pools decreased after the rainy season. The concentrations of individual inorganic P fractions (NH4Cl-P, NH4F-P, NaOH-P, and H2SO4-P) were significantly different across sites and after the rainy season (p < 0.05, two-way ANOVA). NH4F-P and NaOH-P concentrations in sediments increased in a downstream direction. After the rainy season, NH4Cl-P concentrations in sediments decreased whereas NH4F-P and H2SO4-P concentrations increased. The redistribution of individual P fractions in sediments observed after rainy seasons were possibly due to the changing contribution of various sources of runoff and the variation in flow related particle size. Current estimation of P in bed sediments of the South Han River suggests a lower potential of internal P loading from sediments after the rainy season.
1. IntroductionEutrophication in river systems due to widespread phosphorus (P) enrichment has caused considerable problems for environment [1]. Phosphorus bioavailability in rivers is related to the quality of P inputs and to the timing and magnitude of transport fluxes. Within the river ecosystem, different compartments function alternatively as sources or sinks regulating P supply to primary producers along with the succession of seasonal events [2]. The P flux across sediment-water interfaces depends upon P speciation in river bed sediments, the P concentration in water column, and a range of conditions, such as redox potential, pH, and temperature [3–5]. Sequential chemical extraction techniques have been used as a useful tool to assess the processes leading to the fractionation of P, thereby evaluating the potential reactivity of P in river sediments.
Rain events are typically concentrated over short periods of time and runoff P contributes in major proportion to the annual P supply to rivers [6]. During a rainy period, various combinations of P sources, either natural or anthropogenic, runoff into connected water body and may increase the incidence of eutrophication in a following baseflow period. P loading during rainy periods is dominant in particulate form attached to suspended sediments and transiently deposited within the river channel [7–9]. Several studies have shown that the sediment-P content increased with decreasing particle size, which are directly related to changing transport capacity of runoff with the succession of rain events [10]. The composition of metallic elements in sediment particles, such as Fe, Al, and Ca, is considered as the main factor explaining the relation between particle size and the concentrations of different sediment P fractions [11–15].
In addition, land uses are expected to directly influence the amount and bioavailability of runoff P by determining the size of particles that make up the bed load of rivers during rainy periods. Increased impervious areas in urban catchments can result in higher rates of runoff for a given rainfall rate than in non-urbanized catchments [16]. Thus, storm water runoff may transport larger-sized particles which tend to have lower P contents [17]. Cowen and Lee [18] suggested that the particulate P transported by surface runoff from different urban land uses was similar in form if it is derived from a common source, such as eroded soil. Forest catchments, however, generally have buffer strips, which can prevent some large sediment particles from reaching the river. Agricultural catchments often lack riparian vegetation along river banks [19, 20].
In Korea, the annual monsoon period is characterized by intense rainfall between June and September. Since the South Han River catchment is mainly covered by forests and rice paddies (>85% of catchment area), P sources runoff into the South Han River over monsoon periods may include soil particles, fertilizers, and plant debris [21]. The transport of variable combinations of these sources from variable areas of fields and catchments during rain events provide P inputs to rivers with large degree of both spatial and temporal variability, and the variability is partly reflected in changing sediment-P speciation [2].
Phosphorus fractionation in sediments can be considered as in indexing of various biogeochemical processes. Chemical fractionations have been used widely to differentiate the sediment P pools according to solubility and reactivity against various reagents. There has been diverse terminology used to describe P forms and most researchers used terminology based on the extractant used. For example, the P fractionation scheme, summarized by Zhang and Kovar [22], used NH4Cl to extract soluble and loosely bound inorganic P from soil and was often termed as NH4Cl-P fraction. In principle, most of P fractionation procedures have aimed to extract labile, metal bound, reductant, occluded and organic P fractions [23]. However, each fractionation scheme has been likely adapted to specific soil samples by using specific extractants [24, 25].
Although there are several forms of P in sediments, not all of them are easily released in to water and readily usable by organisms. Thus, the use of total P content in sediments to estimate ecological risk of eutrophication is limited. P bound to redox-sensitive iron and manganese or pH-sensitive aluminum oxides is potentially available, whereas P co-precipitated with calcium carbonate bound to recalcitrant organic matter (i.e., humic acids) is almost completely unavailable [26]. The contents of exchangeable P fractions in sediments, therefore, are important in predicting future internal loadings (bed release of P) and potential ecological danger.
The aims of this study were 1) to investigate the variation in longitudinal patterns of different inorganic P fractions in the bed sediments of the South Han River over a rainy season, 2) to estimate P bioavailability in sediments, and 3) to discuss the processes determining the spatiotemporal heterogeneity of inorganic P fractions in sediments.
2. Materials and Methods2.1. Study Sites and SamplingNine sites were selected for sampling along a reach of the South Han River passing through Yeoju in Gyeonggi Province (Table 1 and Fig. 1). The sediments were collected using grab samplers in June and October 2012 (before and after the rainy season, respectively). Five replicate (in June 2012) or three replicate (in October 2012) samples were collected at each site, sealed in plastic bags and transferred to the laboratory.
The South Han River is one of two major branches of the Han River passing through Seoul, the capital city of Korea, before it flows in the Yellow Sea. In the South Han River basin, about 70% of the annual precipitation occurs during the summer monsoon from mid-June to mid-September (Korea Meteorological Administration). Water resource from the South Han River contributes to various purposes including reduction of flood damages in downstream areas, the supply of water for municipal, industrial and irrigation purposes, and electrical supply [21]. Monthly mean rainfall and discharge of year 2012 at Yeoju gauging station (South Han River) was shown in Fig. 2.
2.2. Long-Term Patterns of Rainfall, Discharge, and River Water QualityTrends of local climate condition (rainfall), hydrological regime (discharge), and water quality were investigated over a 5-years period (2007–2011). The data were acquired from Yeoju gauging station representing the water quality of the studying sites. Water quality are described by temperature, conductivity, pH, dissolved oxygen (DO), chemical oxygen demand (COD), total suspended solid (TSS), total nitrogen (TN), ammonia (NH4+), nitrate (NO3−), total phosphorus (TP), phosphate (PO43−). Monthly mean data were used to conduct statistical analysis. The data originates from Korea Ministry of Land, Infrastructure and Transport (for rainfall and discharge) and Korea Ministry of Environment (for water quality) (see Appendix 1).
2.3. Land Use PatternsFor better understanding the processes determining the spatiotemporal variation of sediment P in the South Han River, land use patterns were classified from Landsat TM 5 image of target area. The image was acquired in October 2012 and extracted the information by using unsupervised classification method (ISODATA). The proportional areas of different land uses in a 1-km radial buffer of the sampling site were determined using buffer analysis function in ArcGIS 9.3 software. Based on the dominant land uses, the sites were then classified into groups. All land uses which covered at least 10% of total land area of the defined buffer zone were considered. The major land use was defined as the one covered more than 30% of total land area.
2.4. Chemical AnalysisThe half of samples were air-dried, passed through a 2-mm sieve and ground for metal analysis (total Fe, total Al, and total Ca) conducted in National Instrumentation Center for Environmental Management, Seoul National University. Remaining samples were stored at 4°C for further analysis. Sediment pH was determined in a solution of 1:2.5 soil/water using a glass electrode (Orion 3-Star Plus Benchtop pH meter). Sediment organic matter content (SOM) (% of wet soil) were measured based on the weight loss in 2.5 g of soil burned at 550°C for 24 hr, following an oven drying at 105°C for 24 hr.
The non-calcareous wet sediments were subjected to sequential inorganic P fractionation using a modified fractionation procedure of inorganic P according to Zhang and Kovar [22] (Fig. 3). This procedure removes NH4Cl extractable P, called labile-P, which is the most available P to biota. The residue from NH4Cl extraction is continuously extracted with NH4F to separate Al bound-P from Fe bound-P in the next extraction with NaOH. The reductant-soluble P fraction is extracted with sodium citrate–sodium dithionite–sodium bicarbonate (CDB) extraction. The total amount of P precipitated with metal ions (Fe and Al) and reductant-soluble P is called non-apatite P (NAIP). NAIP is less available, but can be released into water column by altering pH and redox potential. The total amount of labile-P and NAIP can represent the bioavailable P (BAP) in sediments. Finally, the apatite P (AIP), calcium precipitated P, is removed with H2SO4. The amount of P in all extractions was determined using colorimetric ascorbic acid method [27].
2.5. Statistical AnalysisThe data of rainfall, discharge and water quality were analyzed for trends using seasonal Kendall tests at the 95% confidence level. Before the trend analysis, Principal Component Analysis (PCA) was conducted to create surrogate variables (principal components) for individual water quality constituents. Correlation analysis was used to examine the relationships among the contents of P fractions and environmental factors in sediments. The influence of land use patterns on the distribution of P fractions in sediments was examined using independent sample t-tests. Two-way ANOVA was used to analyze the differences in P fractions between the sites (Site) and sampling times (Time). Except for the seasonal Kendall tests running by XLSTAT 2014 (Addinsoft, Paris, France), other statistical analyses were conducted by using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
3. Results3.1. Land Use PatternsAgricultural land was the major land use in nearly all sampling sites (Table 1). The proportional areas of urban land were considerably higher at site S7, S8, and S9. The upstream sites, such as S1, S2, and S3, have higher proportional areas of forests compared to the downstream sites. The total area of agricultural, forest and urban land covered 63%–74% of the overall land area in a 1-km radial buffer of the sampling sites (Table 1). Based on the dominant land use patterns, the sites were classified into four groups: S6 represents for the agricultural land (A); S4 represents for the mixed urban and agricultural land (AU); S1, S2, S3, and S5 represent the mixed agricultural and forest land (AF); S7, S8, and S9 represent the mixed agricultural, forest and urban land (AFU) (Table 1).
3.2. Long-Term Trends of Rainfall, Discharge, and Water Quality in the South Han RiverPrincipal component analysis of water quality parameters could extract three main factors (PC1, PC2, and PC3) describing 37.95%, 19.85%, and 12.66% of the variance, respectively. PC1 were dominated by TSS (0.242), COD (0.267), TP (0.276), PO43− (0.218), and NH4+ (0.288). PC2 was dominated by temperature (−0.408), EC (0.227), and DO (0.359). PC3 was dominated by pH (−0.301), TN (0.396), and NO3− (0.501). Seasonal Kendall tests showed that rainfall and discharge did not exhibit significant trends over time (p=0.116 and 0.832, respectively). For water quality, PC1, PC2, and PC3 did not exhibit significant trend (p=0.724, 0.525, and 0.724, respectively). These results indicated the general conditions of local climate and water quality in the river over time.
3.3. Longitudinal Patterns of P Fraction Concentrations and Sediment Chemical Properties over the Rainy SeasonTwo-way ANOVA analysis showed that metal concentrations (Fe, Al, and Ca) were significantly different across the sites and over the rainy season (Table 2). After the rainy season, Fe, Al, and Ca concentrations in sediments decreased, whereas pH and SOM increased (Fig. 4). pH was remained across the sites (Fig. 4(a)). The longitudinal patterns of Fe, Al, and Ca concentrations, however, varied depending on the sampling time (Fig. 4(b)–(d)). Before the rainy season, Fe concentrations varied inconsistently across the sites. The highest concentrations of Fe were recorded at site S1, S2, S5, and S8 before the rainy season. After the rainy season, Fe concentrations over the first three sites (S1, S2, and S3) and substantially increased across the remaining sites (Fig. 4(b)). Similar longitudinal patterns of Al concentrations were observed before and after the rainy season (Fig. 4(c)). Ca concentrations were equally distributed across the sites regardless of the sampling time (Fig. 4(d)). SOM varied substantially from site to site, however, in overall, no significant change was observed in the downstream direction at both sampling times (Fig. 4(e)).
P fractionation showed that NAIP was the dominant form of inorganic P in sediments, representing more than 60% of total inorganic phosphorus (TIP) pools in nearly all sites (Table 3). The labile-P accounted for less than 10% of TIP pools. The AIP, or the mostly unavailable P, represented 30%–40% of TIP pools. TIP concentrations did not vary significantly across the sites and after the rainy season. The mean concentration of TIP in sediments was 0.39±0.05 mg g−1 dry sediment (Fig. 5(a)). However, the concentrations of individual P fractions (NH4Cl-P, NH4F-P, NaOH-P, and H2SO4-P) and the relative contributions of each fraction to the TIP pool were significantly different across the sites and after the rainy season (Table 2, Figs. 5 and 6). After the rainy season, NH4Cl-P concentrations decreased (Fig. 5(b) and (d)), whereas NH4F-P and H2SO4-P concentrations increased (Fig. 5(c) and (f)). After the rainy season, NH4Cl-P:TIP and NaOH-P:TIP ratios decreased but NH4F-P:TIP and H2SO4-P:TIP ratios increased (Fig. 6). Interestingly, after the rainy season, the proportions of BAP in the TIP pools decreased significantly (Table 3). NH4F-P and NaOH-P concentrations varied substantially over the first three sites (S1, S2, and S3) and consistently increased across the downstream sites (Fig. 5(c) and (d)). No significant variation of NH4Cl-P, CDB-P, and H2SO4-P concentrations across the sites was observed (Fig. 5(b), (e), and (f)).
3.4. Factors Controlling the Distribution of P Fractions in Sediments over Rainy Seasons
Table 4 showed the correlations between P fraction concentrations and chemical properties of sediments. pH was negatively correlated with Fe, Al, Ca, and NH4Cl-P concentrations. SOM was positively correlated to NH4F-P, NaOH-P, and H2SO4-P concentrations. NH4Cl-P concentrations were positively correlated to Fe, Al, and Ca concentrations. Labile-P:TIP ratios were significantly correlated with SOM and Ca concentrations. AIP:TIP and NAIP:TIP ratios were significantly correlated with SOM, Al, and Fe concentrations.
The concentrations of NH4F-P, NaOH-P, and H2SO4-P fractions in sediments were also different across different land use patterns (Fig. 7). NH4F-P concentrations were significantly greater in sediments of the agricultural site (S6) compared to the mixed urban and agricultural site (S4) (Fig. 7(b)). NaOH-P concentrations in river sediments were significantly increased with the increase of urban cover around the mixed agricultural and forest sites (i.e., S7, S8, and S9 compared to S1, S2, S3, and S5) (Fig. 7(c)). H2SO4-P concentrations were also significantly increased with the increase of forest areas around the agricultural sites (i.e., S1, S2, S3, and S4 compared to S6) (Fig. 7(e)).
4. DiscussionWe hypothesized that the distribution of inorganic P fractions in river sediment would vary after elevated discharges during the rainy season. Our measurement of P fractionation in river sediments at nine sites along the South Han River indicated that the concentrations of specific P fractions altered after the rainy season, whereas total concentrations of inorganic P in sediments remained. Particularly, after the rainy period, labile-P and Fe-bound P concentrations decreased while Al and Ca-bound P concentrations increased. The variation of inorganic P fractions in river sediments over the rainy season could relate to the fluctuation of sediment-bound metal concentrations (Fe, Al, and Ca) and land use patterns. No significant trends of rainfall, river discharge and water quality over the previous years (2007–2011) implied that the survey conducting in 2012 can represent the river condition in general.
Previous studies reported that changes in the sediment-P contents were related to elemental composition of sediments [3, 11, 15]. Metal hydroxides of Fe, Al, and Ca in sediments are well-known to fix phosphorus into sediments mainly by providing ligand-exchange sites (Me-OH2+ and MeOH). The stability of phosphate surface complexes, thus, determines the amount of individual P fractions in sediments [28]. In this study, we found that Fe-bound P concentrations decreased whereas Al-bound P and Ca-bound P concentrations increased after the rainy season. This result is consistent with the report by Pacini and Gächter [3]. They suggested that decreased contribution of Fe-bound P with the progression of storms was due to the flushing effect on fine particles enriched with Fe-bound P fractions, which were deposited transiently within the channel bed. During base flows and during the very beginning of rain events, the ‘iron curtain’ mechanism, which is firstly observed by Chambers and Odum [29], attribute to the trapping of soluble reactive P (SRP) in water column on fine particles within the channel bed. With the progression of storms, Fe-bound P fractions previously concentrated near the sediment surface would be flushed downstream. The coupling increase of Fe and Fe-bound P concentrations observed across the sites after the rainy season could confirm the flushing effect occurred during rain events. The measurement of particle size distribution in sediments of the South Han River also confirmed that the clay and silt contents of sediments decreased after the rainy period, especially at the upstream sites (see Appendix 2).
On the other hand, large particles eroded from river banks and from outside the stream channel could reach the river at higher discharge and hence more particulate P [3, 30]. Since the particles eroded from river banks and from outside the river channel are likely to bear a higher content of organic matter, the weakly increase of SOM after the rainy season and its strong relations to the increases of Al-bound P and Ca-bound P can partly prove the substantial effects of external particle sources on the variation of P fractions in sediments. Tracing particle sources by their geochemical composition at high discharges can clarify this hypothesis [31].
Overland flows and sediment transport are important in controlling the P loading to streams in agricultural landscapes [32–34]. Rainfall falling on a site could reach receiving waters through several ways including surface runoff. High intensity of rainfall events tends to favor surface runoff, through rapid saturation of surface soils [35]. The contribution of P to receiving waters via surface runoff will depend on geomorphological characteristics and the intensity of land management. Schulte et al. [36] found that P loss were mainly governed by the occurrence, frequency and timing of intense overland-flow events that followed intense rainstorm events. Within individual overland flow events, higher rates of flow could be accompanied by increased P concentrations [37, 38].
Land use management is directly related to runoff and is considered as a main factor influencing the amount of non-point source P transported to rivers [8, 39]. Although the effect of land uses on non-point source P export is evident, a precise estimation of the P contribution from specific land use practices is very difficult and has rarely been reported. Interestingly, our results suggested that runoff from urban areas to water systems possibly carried more Fe-bound P while agricultural areas contributed more Al-bound P to water systems. On the other hand, forests could also contribute more Ca-bound P to water system. These results, however, may only represent the effect of land use on the P distribution in river sediments at the buffer scale (1-km radial buffer zones of sampling sites). In addition, further investigation is required to clarify the dominant form of P delivered to water system from different land uses since several land use patterns have been defined in this study with only one site as the sample—e.g., only site S6 (or S4) represents for agricultural (or mixed urban and agricultural) land.
In agricultural watersheds, various sources from sedimentary processes and fertilizer P runoff can contribute to the Ca-bound P in river sediments [30]. In addition, forests and urban areas are land uses that potentially contribute to increases in sedimentation, thus particulate P, in water systems [40]. By increasing impervious areas, urban land use increases runoff volumes and storm flow rates, in turn affects water quality [41]. On the other hand, the quantity of sediments from forests transported by overland flows during rainy seasons depends on many factors, such as the disruption of soil surface, the weathering of parent material and the transportation of loose or decaying organic particles [42]. Ellison and Brett [10] demonstrated that the concentration of particulate P in stream waters increased during storms compared to baseflow. They showed that highest concentrations of particulate P during storms were observed in urban streams followed by agricultural, forested, and mixed land cover streams.
The substantial variations of NH4F-P, NaOH-P, and H2SO4-P across the sites were possibly due the fluctuation of flow velocity over flood-controlling weirs installed along the stream (i.e., the presence of Kangchun weir between S2 and S3, of Yuju weir between S5 and S6, and of Ipo weir between S8 and S9). Among the weirs, Kangchun weir is the biggest one which could lead to the largest discrepancy in P fraction concentrations at site S2 compared to others. Manipulation of hydraulic residence time by artificial structures strongly affects in-stream P retention, which accounts for a large legacy of P in watersheds [43]. Reduced hydraulic energy upstream of weirs can promote siltation with large stores of P [44–46]. This resulted in the substantial reduction of NH4F-P, NaOH-P, and H2SO4-P concentrations in sediments at the sites downstream of weirs (i.e., site S3, S6, and S9).
The decrease of BAP concentrations in sediments after the rainy season suggests a lower availability of P in bed sediments, and hence a lower potential of P release into the water column. Previous studies showed that P exchange processes at the sediment-water interface via precipitation, dissolution and sorption may considerably occur under effects of pH and redox condition [47–50]. Anoxic conditions prevailing the sediment-water interface, which is a function of flow velocity and the degree of water turbulence, can cause a release of NAIP from sediments for the growth of phytoplankton [51]. The P-binding capacity of Fe (and Al) oxides decreased under alkaline pH, primarily due to the replacement between OH- and PO43- in ligand exchange reactions, can lead to P releases from sediments [51].
5. ConclusionsRedistribution of individual inorganic P fractions in the bed sediments of rivers could occur after rainy seasons. Moreover, the amount of bioavailable P in the bed sediments decreased after the elevated flows of a rainy period. This implied that the internal P loading from bed sediments would progressively loose the importance in determining P availability, thus eutrophication, in rivers after rainy seasons. Further studies need to be accomplished to trace the sediment sources contributing to the variation of P pools within a river and to evaluate the dynamics of P in river bed sediments.
AcknowledgmentsThis study was supported by the Center for Aquatic Ecosystem Restoration of the Eco-STAR project from the Ministry of Environment (No. EW33-08-11), National Research Foundation of Korea (No. 2013056833) and S/ERC (No. 2013067218).
References1. Welch EB, Lindell T. Ecological effects of wastewater: applied limnology and pollutant effects. Boca Raton: CRC Press; 1992.
2. Withers PJ, Jarvie HP. Delivery and cycling of phosphorus in rivers: a review. Sci. Total Environ. 2008;400:379–395.
3. Pacini N, Gächter R. Speciation of riverine particulate phosphorus during rain events. Biogeochemistry. 1999;47:87–109.
4. Rickard C, Day R, Purseglove J. River weirs: good practice guide. Bristol: Environment Agency; 2003.
5. Mortimer CH. Chemical exchanges between sediments and water in the Great Lakes-speculations on probable regulatory mechanisms. Limnol. Oceanogr. 1971;16:387–404.
6. Rose P. Long-term sustainability in the management of acid mine drainage wastewaters-development of the Rhodes BioSURE Process. Water SA. 2013;39:583–592.
7. Douglas RW, Menary W, Jordan P. Phosphorus and sediment transfers in a grassland river catchment. Nutr. Cycl. Agroecosyst. 2007;77:199–212.
8. Haygarth PM, Hepworth L, Jarvis SC. Forms of phosphorus transfer in hydrological pathways from soil under grazed grassland. Eur. J. Soil Sci. 1998;49:65–72.
9. Hodgkinson RA, Withers PJ. Sourcing, transport and control of phosphorus loss in two English headwater catchments. Soil Use Manag. 2007;23(s1)92–103.
10. Ellison ME, Brett MT. Particulate phosphorus bioavailability as a function of stream flow and land cover. Water Res. 2006;40:1258–1268.
11. Stone M, English MC. Geochemical composition, phosphorus speciation and mass transport of fine-grained sediment in two Lake Erie tributaries. Hydrobiologia. 1993;253:17–29.
12. House WA, Denison FH. Exchange of inorganic phosphate between river waters and bed-sediments. Environ. Sci. Technol. 2002;36:4295–4301.
13. Zhang JZ, Huang XL. Relative importance of solid-phase phosphorus and iron on the sorption behavior of sediments. Environ. Sci. Technol. 2007;41:2789–2795.
14. Zhou A, Tang H, Wang D. Phosphorus adsorption on natural sediments: modeling and effects of pH and sediment composition. Water Res. 2005;39:1245–1254.
15. Jalali M. Phosphorus fractionation in river sediments, Hamadan, Western Iran. Soil Sediment Contam. 2010;19:560–572.
16. Booth DB. Urbanization and the natural drainage system--impacts, solutions, and prognoses. Northwest Environ. J. 1991;7:93–118.
17. Sharpley AN, Smith SJ, Jones OR, Berg WA, Coleman GA. The transport of bioavailable phosphorus in agricultural runoff. J. Environ. Qual. 1992;21:30–35.
18. Cowen WF, Lee GF. Phosphorus availability in particulate materials transported by urban runoff. J. Water Pollut. Control Fed. 1976;48:580–591.
19. May CW, Horner RR, Karr JR, Mar BW, Welch EB. Effects of urbanization on small streams in the Puget Sound ecoregion. Watershed Prot. Tech. 1997;2:483–494.
20. Osborne LL, Kovacic DA. Riparian vegetated buffer strips in water-quality restoration and stream management. Freshw. Biol. 1993;29:243–258.
21. Pawitan H, Jayawardena AW, Takeuchi K, Lee S. Catalogue of rivers for South East Asia and the Pacific. III:Paris: The UNESCO-IHP Regional Steering Committee for Southeast Asia and the Pacific; 2000.
22. Zhang H, Kovar JL. Fractionation of soil phosphorus. Methods of phosphorus analysis for soils, sediments, residual, and waters. 2nd edBlacksburg: Virginia Tech University; 2009. p. 50–60.
23. Katsaounos CZ, Giokas DL, Leonardos ID, Karayannis MI. Speciation of phosphorus fractionation in river sediments by explanatory data analysis. Water Res. 2007;41:406–418.
25. Williams JD, Syers JK, Walker TW. Fractionation of soil inorganic phosphate by a modification of Chang and Jackson’s procedure. Soil Sci. Soc. Am. J. 1967;31:736–739.
26. Reynolds CS, Davies PS. Sources and bioavailability of phosphorus fractions in freshwaters: a British perspective. Biol. Rev. Camb. Philos. Soc. 2001;76:27–64.
27. Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim Acta. 1962;27:31–36.
28. Nemeth Z, Gáncs L, Gemes G, Kolics A. pH dependence of phosphate sorption on aluminum. Corros. Sci. 1998;40:2023–2027.
29. Chambers RM, Odum WE. Porewater oxidation, dissolved phosphate and the iron curtain. Biogeochemistry. 1990;10:37–52.
30. Liu Q, Liu S, Zhao H, et al. Longitudinal variability of phosphorus fractions in sediments of a canyon reservoir due to cascade dam construction: a case study in Lancang River, China. PLoS One. 2013;8:e83329.
31. Foster ID. Sediment yields and sediment delivery in the catchments of Slapton Lower Ley, South Devon, UK. Field Stud. 1996;8:629–661.
32. Walling DE. Suspended sediment transport by rivers: a geomorphological and hydrological perspective. Adv. Limnol. 1996;47:1–27.
33. Sharpley AN, Chapra SC, Wedepohl R, Sims JT, Daniel TC, Reddy KR. Managing agricultural phosphorus for protection of surface waters: issues and options. J. Environ. Qual. 1994;23:437–451.
34. Zaimes GN, Schultz RC. Phosphorus in agricultural watersheds: a literature review. Ames: Department of Forestry, Iowa State University; 2002.
35. Nihlgard BJ, Swank WT, Mitchell MJ. Biological processes and catchment studies. Biogeochemistry of small catchments: a tool for environmental research. New York: John Wiley & Sons; 1994. p. 133–161.
36. Schulte RP, Richards K, Daly K, Kurz I, McDonald EJ, Holden NM. Agriculture, meteorology and water quality in Ireland: a regional evaluation of pressures and pathways of nutrient loss to water. Biol Environ. Proc. R. Ir. Acad. B. 2006;106:117–133.
37. Heathwaite AL, Dils RM. Characterising phosphorus loss in surface and subsurface hydrological pathways. Sci Total Environ. 2000;251–252:523–538.
38. Kurz I, Coxon C, Tunney H, Ryan D. Effects of grassland management practices and environmental conditions on nutrient concentrations in overland flow. J. Hydrol. 2005;304:35–50.
39. Dorioz JM, Cassell EA, Orand A, Eisenman KG. Phosphorus storage, transport and export dynamics in the Foron River watershed. Hydrol. Process. 1998;12:285–309.
40. Giller PS, Johnson M, O’Halloran J. Managing the impacts of forest clearfelling on stream environments. Dublin: COFORD; 2002.
41. Sun G, Lockaby BG. Water quantity and quality at the urban-rural interface. urban-rural interfaces: linking people and nature. Washington: American Society of Agronomy; 2012. p. 29–48.
42. Binkley D, Brown TC. Forest practices as nonpoint sources of pollution in North America. J. Am. Water Resour. Assoc. 1993;29:729–740.
43. Sharpley A, Jarvie HP, Buda A, May L, Spears B, Kleinman P. Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013;42:1308–1326.
44. Bosch NS, Allan JD. The influence of impoundments on nutrient budgets in two catchments of Southeastern Michigan. Biogeochemistry. 2008;87:325–338.
45. Bosch NS. The influence of impoundments on riverine nutrient transport: an evaluation using the Soil and Water Assessment Tool. J. Hydrol. 2008;355:131–147.
46. Kunz MJ, Anselmetti FS, Wüest A, et al. Sediment accumulation and carbon, nitrogen, and phosphorus deposition in the large tropical reservoir Lake Kariba (Zambia/Zimbabwe). J. Geophys. Res. Biogeosci. 2011;116:G03003.
47. Lijklema L. Interaction of orthophosphate with iron(III) and aluminum hydroxides. Environ. Sci. Technol. 1980;14:537–541.
48. Moore A, Reddy KR. Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida. J. Environ. Qual. 1994;23:955–964.
Fig. 2Monthly mean rainfall and discharge in the south han river in 2012 (data source: Korea Ministry of Land, Infrastructure and Transport). 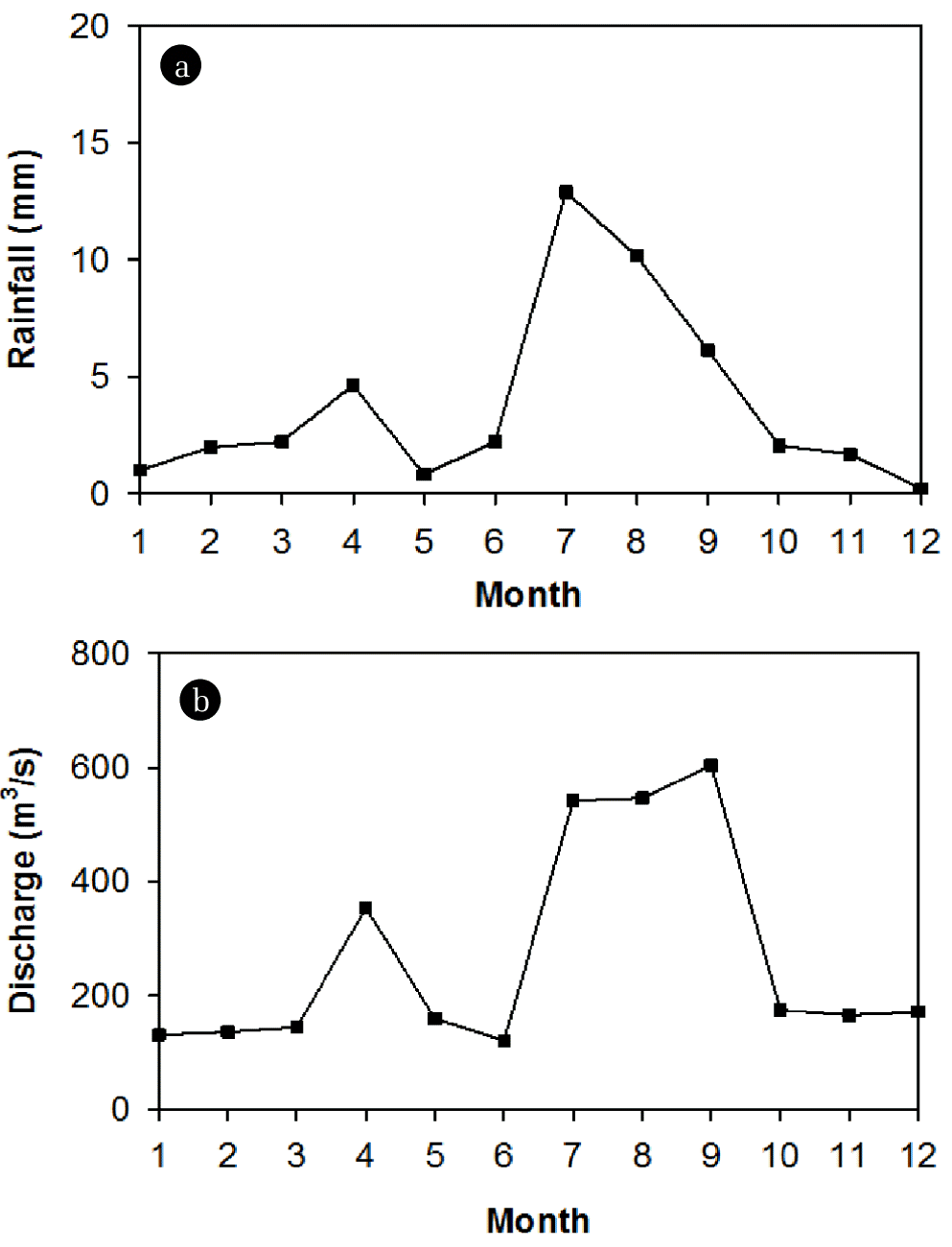
Fig. 4Spatiotemporal patterns of (a) pH, (b) Fe Concentrations, (c) Al Concentrations, (d) ca Concentrations, and (e) sediment organic matter (SOM) in Sediments. The values shown are means±SE. 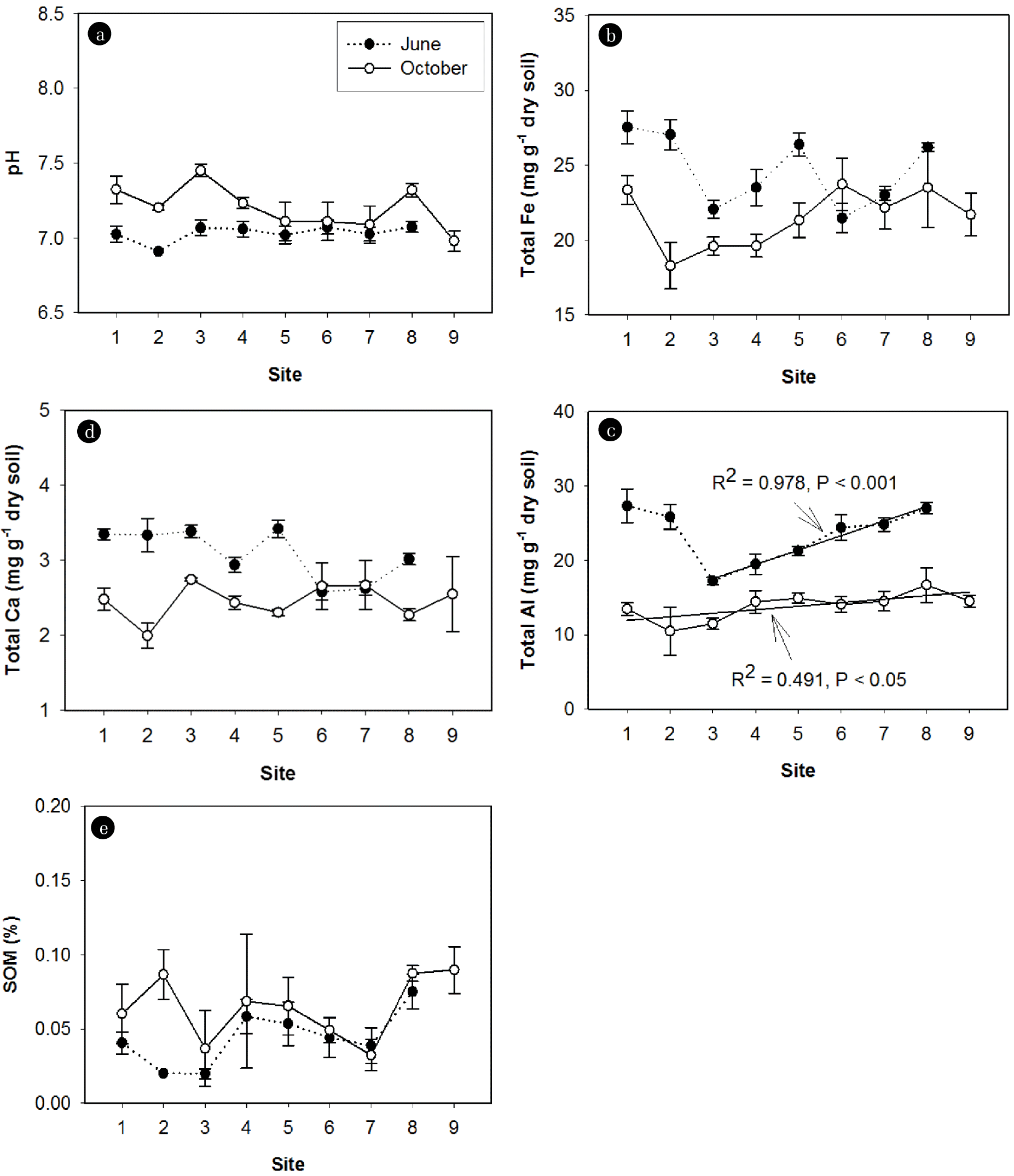
Fig. 5Spatiotemporal patterns of (a) total inorganic phosphorus (TIP) concentrations, (b) NH4Cl-P concentrations, (c) NH4F-P concentrations, (d) NaOH-P concentrations, (e) CDB-P concentrations, and (f) H2SO4-P concentrations in sediments. The values shown are means±SE. 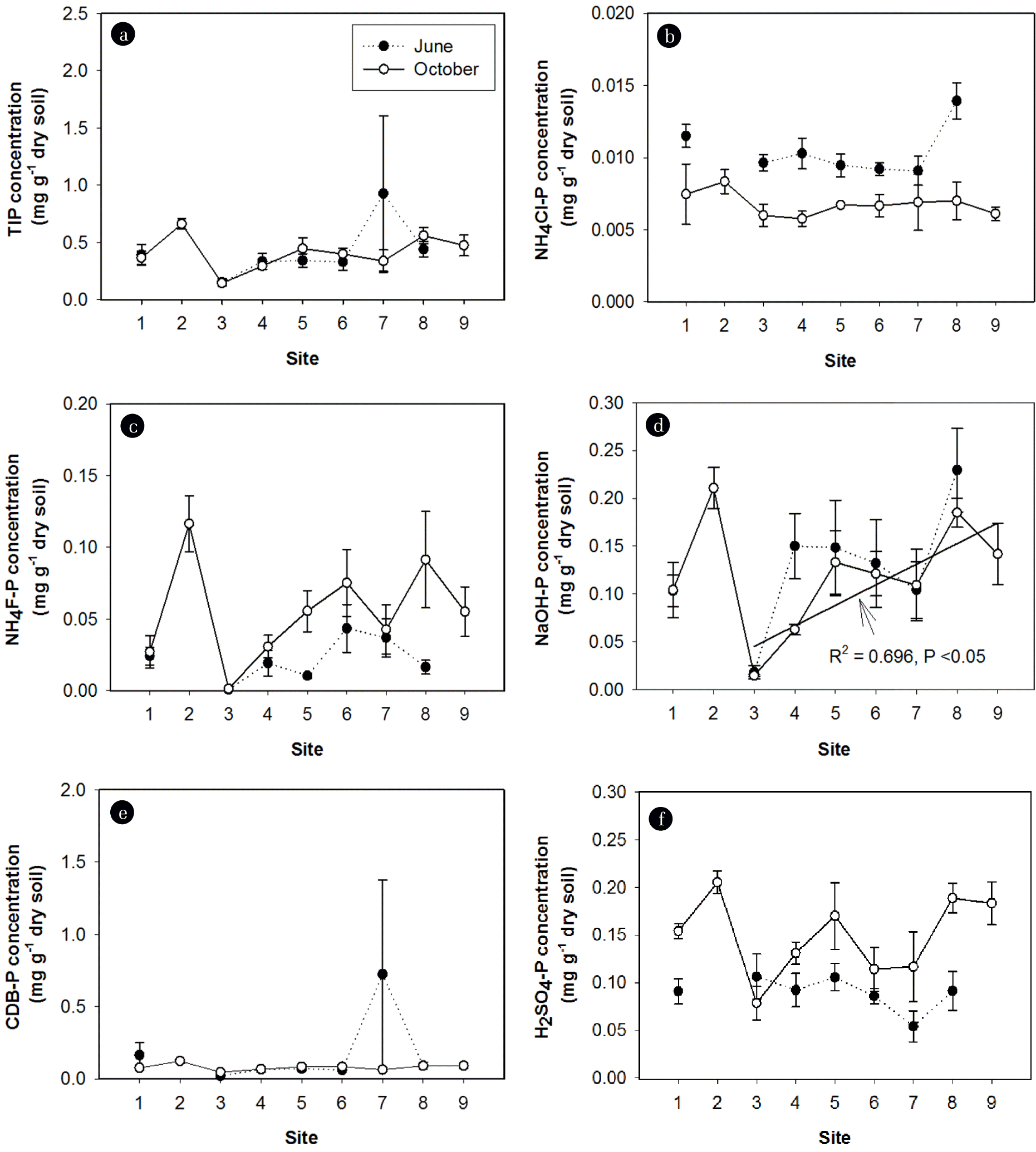
Fig. 6Relative Contributions of individual phosphorus (P) fractions to total inorganic P (TIP) content in sediments (a) before the rainy season (June) and (b) after the rainy season (October). The values shown are the means. ND: no data. 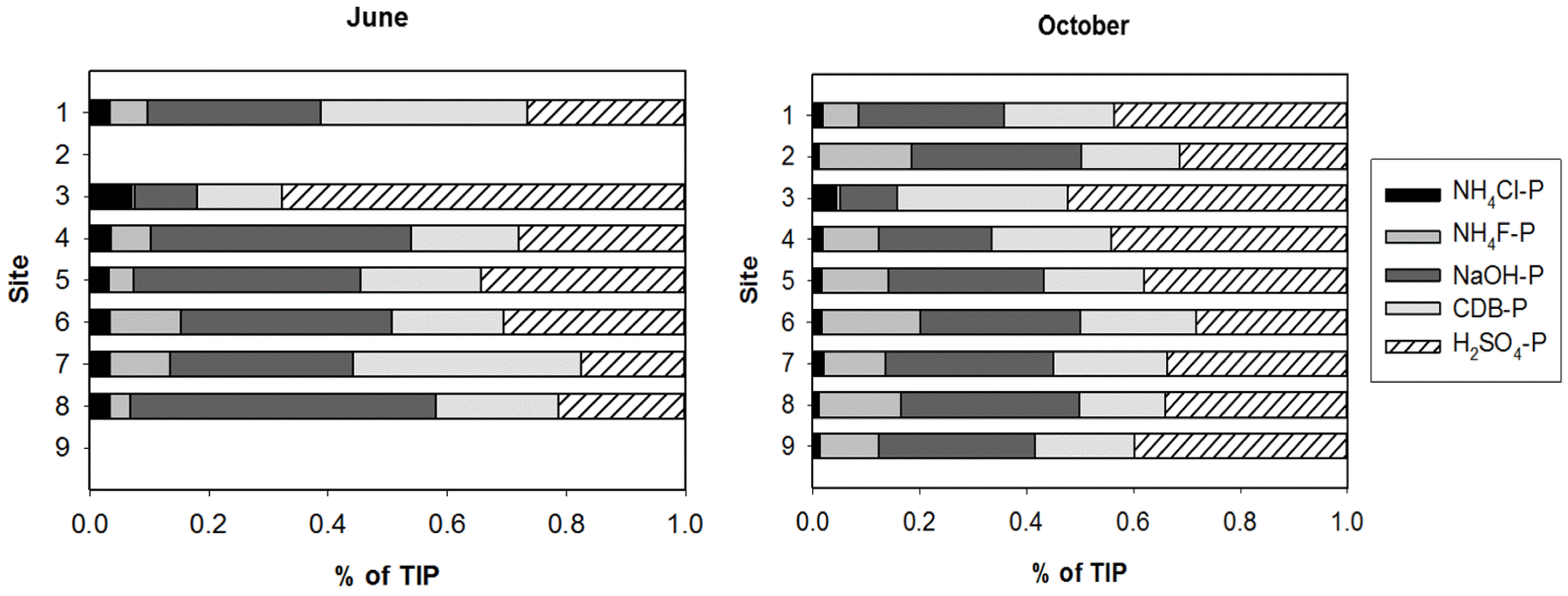
Fig. 7Mean concentrations of different phosphorus (P) fractions in sediments with different land use patterns. p-values indicated the significant difference between different land use patterns (t-tests). TIP: total inorganic P, AU: mixed agriculture and urban, A: agriculture, AF: mixed agriculture and forest, AFU: mixed agriculture, forest and urban. 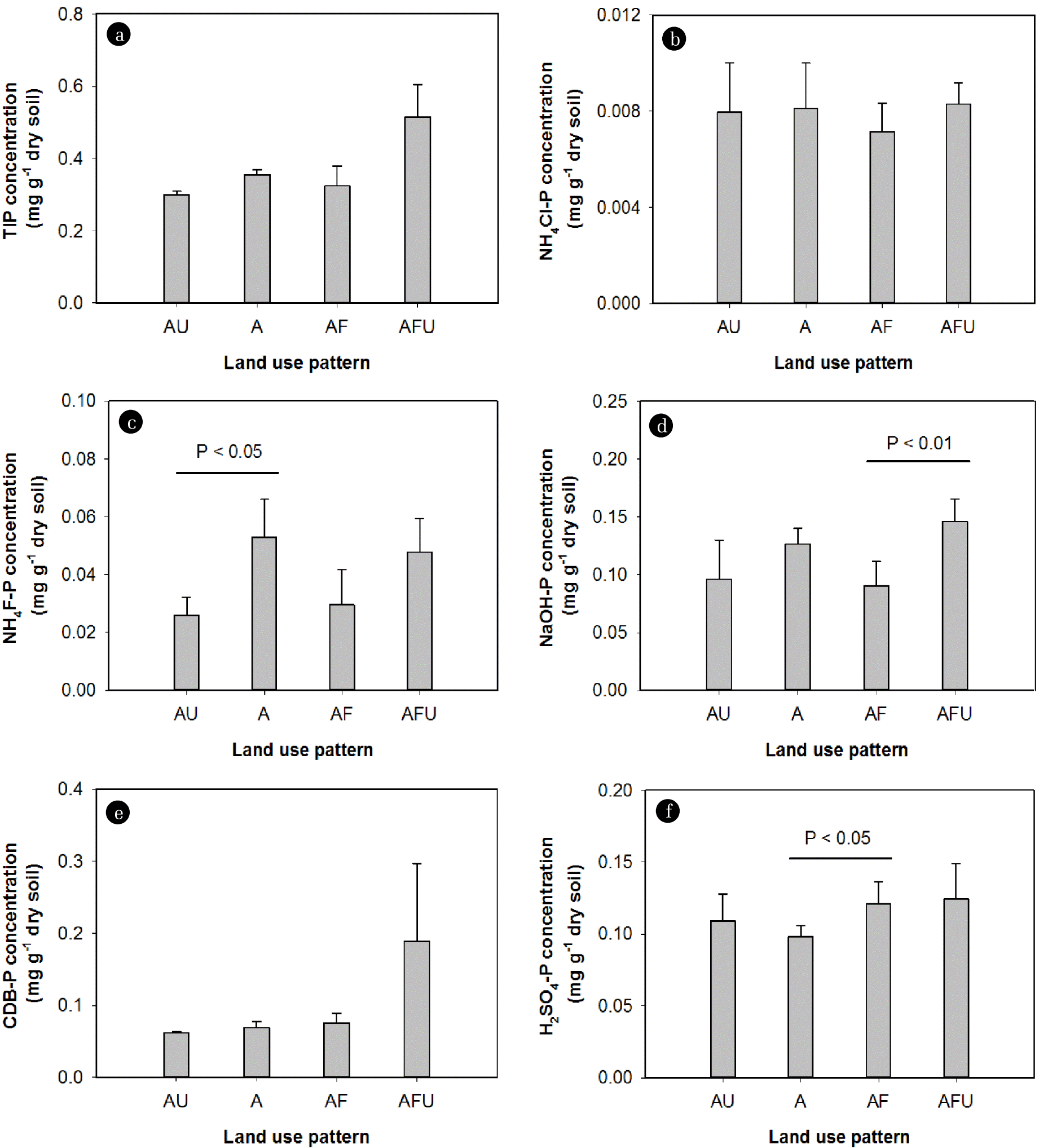
Table 1Location and Land Use Pattern of the Sampling Sites Table 2The Two-Way ANOVA Analysis of Sediment Parameters Table 3Relative Contribution of Individual P Fractions to Total Inorganic P (mean ± SE) Table 4Pearson Correlation Analysis Between P Fraction Concentrations and Other Parameters. Appendix 1Statistical summary of monthly means of rainfall, discharge and water quality parameters during 2007 – 2011at Yeoju gauging station (South Han River) (data source: Korea Ministry of Environment)
Appendix 2Particle Size Distribution of Sediments in the South Han River Before (June) and After (October) the Rainy Season of Year 2012 (data provided by Kang et al. 2012, unpublished). |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||