1. Introduction
Microbial fuel cell (MFC) is a device that uses electrically-catalytic microorganisms to convert organic or inorganic substances into electricity [1, 2]. Increasing interest has been given to the MFC due to its potential of generating alternative energy using organics contained in the wastewater. Therefore, MFC has been introduced as a good candidate for the sustainable and renewable energy production in the future. However, its field application has rarely been reported because of low power generation efficiency and high operating cost [3]. Therefore, a number of previous studies have focused on how to increase the electric power density produced from MFCs.
Microorganisms obtain electrons by oxidizing organic substances and then transfer the electrons eventually to extracellular electron acceptors via their metabolic process [4]. In the MFC system, electrically-catalytic microorganisms transfer electrons to electrode as a final electron acceptor [5]. Various mechanisms that explain the electron transfer from bacteria to the anodic electrode have been suggested: 1) electron transfer via direct contact between cells and electrode through multiheme cytochromes in the outer cell membrane [6]; 2) electron transfer using soluble mediators that function as electron shuttles from cells to electrode [7, 8]; and 3) electron transfer through extracellular appendages referred to as bacterial nanowires or nano-pili [9–11]. Anode plays an important role in transferring electrons within the circuit of MFC, and it serves as a medium on which the electrically-catalytic microorganisms are attached. In recent years, several studies demonstrated that microbial communities have the capability to transfer electrons directly to electrode without mediators (mediator-less MFC) using anode electrode as the sole electron acceptor [12, 13]. Electrogenic bacteria such as Geobacter and Shewanella genera have been shown to form layers of biofilm on the anode surface [6]. Thus, the role of biofilm formation on the anode may be critical to increase the current production in MFCs.
In this study, Desulfovibrio desulfuricans, which is a predominant sulfate-reducing bacteria (SRB) in diverse anoxic environments, was employed to investigate electron transfer function of electrically-catalytic bacteria on the electrode surface. The response of D. desulfuricans biofilm formed on the electrode was evaluated by current production in the MFC system. Quantitative characterization of the biofilm was performed by measuring the biomass concentration at the end of batch operation. The morphology of the bacteria that formed biofilm on the anode surface was examined by scanning electron microscopy (SEM). The results of this study are expected to provide a good understanding about the mechanism of electron transfer through biofilm formed and nano-pili produced by electrically-catalytic microorganisms.
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
D. desulfuricans (ATCC 27774) was obtained from the American Type Culture Collection. D. desulfuricans was grown in a medium containing 0.5 g/L of K2HPO4, 1.0 g/L of NH4Cl, 1.0 g/L of Na2SO4, 0.1 g/L of CaCl2·H2O, 2.0 g/L of MgSO4·7H2O, 2.0 g/L of sodium lactate, 1.0 g/L of yeast extract, 1.0 mg/L of resazurin, 0.5 g/L of FeSO4·7H2O, 0.1 g/L of sodium thioglycolate, and 0.1 g/L of ascorbic acid as described previously [14]. The medium was adjusted to pH 7.8 with 0.1 M of NaOH prior to sterilization by autoclaving at 121°C for 15 min. Cells were grown anaerobically and incubated at 37°C. After 24 hr, the cells were washed with phosphate buffer solution (pH 7.0) then inoculated into the anode chamber of MFC.
2.2. Microbial Fuel Cell Operation
A dual-chamber MFC reactor made of acrylic with a working volume of 150 mL was employed in this study. The anode and cathode compartments were separated by a Nafion 117 proton exchange membrane (PEM; DuPont, Wilmington, DE, USA). Prior to use, PEM was pre-treated by boiling in H2O2 (30%, v/v) and deionized water, followed by soaking in 0.5-M H2SO4 and then deionized water, each for 1 hr as described previously [15, 16]. The cathode chamber was filled with an electrolyte solution containing 30-mM Tris buffer solution (pH 7.0) [17, 18], which was continuously purged with water-saturated air. The pure culture of D. desulfuricans was inoculated into the anode chamber and lactate (10 mM) was supplemented as an electron donor and neither an electron acceptor nor an electron shuttling mediator, except for the electrode, was provided. The medium of the anode chamber was purged with N2/CO2 (8:2, v/v) to maintain anaerobic condition. The medium in the anode chamber was replaced with a fresh medium of lactate when the current dropped below 0.03 mA. Graphite felt (surface area = 35 cm2) served as cathode and anode (GF series; Electrosynthesis, Lancaster, NY, USA), and both electrodes were connected with a platinum wire through an external resistance of 100 Ω. Voltage was monitored using a digital multimeter (Model 2700, Keithley Instruments, Cleveland, OH, USA). Data were recorded hourly on a spreadsheet using ExceLINX (Keithley Instruments) via an interface card (GPIB Interface Boards, Keithley Instruments) linked to a personal computer.
2.3. Analytical Procedures
Quantity of microbial biofilm was measured by its protein concentration at the end of batch operation. The protein extraction procedure was described in a previous study [6]. Total protein concentration was determined by Bradford analysis with bovine serum albumin as a standard (Quick Start Bradford protein assay; Bio-Rad Company, Hercules, CA, USA). Aqueous samples were collected from the anode chamber and their organic levels were analyzed as chemical oxygen demand (COD) as described by Standard Methods [19]. The recorded voltage was converted into current density using Ohm’s law (I = V/R·A), where I is current (ampere), V is voltage (V), R is resistance (Ω), and A is electrode surface area (m2). The Coulombic efficiency (CE) was calculated by CE = CP/CT × 100%, where CP is the total number of coulombs calculated by integrating the current over time, and CT is the theoretical number of coulombs that can be produced from the substrate used expressed as COD [20]. At the end of batch operation, the electrode was removed from the anode chamber of MFC reactor and rinsed with distilled water. The electrode was fixed with 2% glutaraldehyde at 4°C for 2 hr and rinsed 3 times with 0.05-M sodium cacodylate buffer (pH 7.2) at 4°C for 10 min, and then fixed with 1% osmium tetroxide in 0.05-M sodium cacodylate buffer (pH 7.2) at 4°C for 2 hr, then rinsed twice with distilled water at room temperature. After this fixing step, the electrode was then dehydrated by ethanol (30, 50, 70, 80, 90, and 100%) several times sequentially at room temperature, each for 10 min. It was then gently washed twice with 100% hexamethyldisilazane at room temperature for 15 min and air-dried overnight. The electrode was then coated with platinum and analyzed by a SEM (JSM-5410LV; JEOL Ltd., Tokyo, Japan).
3. Results and Discussion
3.1. Electricity Generation
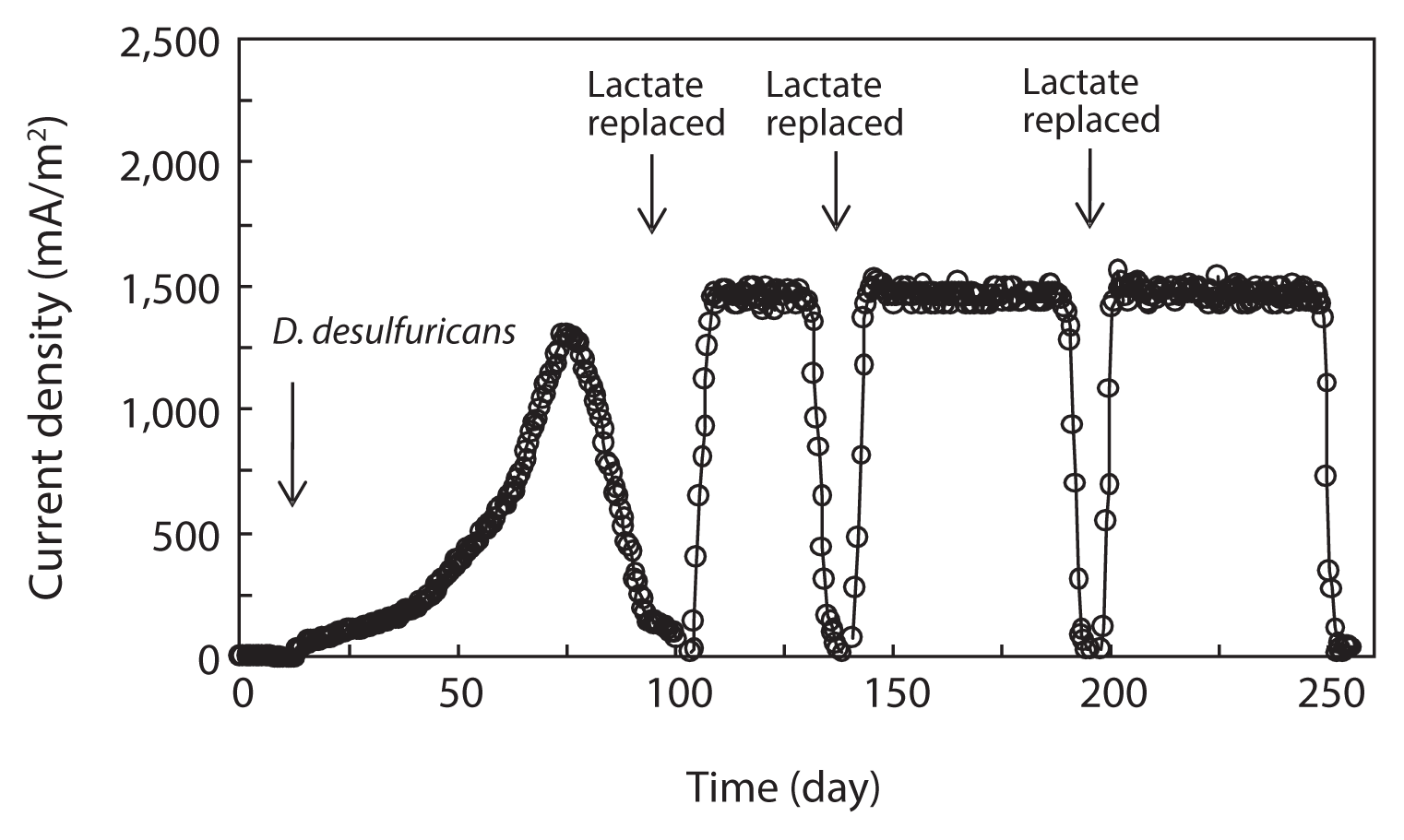
D. desulfuricans was inoculated into the anode chamber which was pre-sterilized to initiate its pure culture type growth on graphite felt electrode. Lactate was supplied as an electron donor and no electron acceptor except the anodic electrode was provided. Current production began after D. desulfuricans was inoculated into the anode chamber. Current was produced continuously, reaching a maximum current density of 1,310.0 ± 22.3 mA/m2 (mean ± standard deviation, n = 3), and then the current density gradually decreased to 29.4 ± 5.2 mA/m2 at the end of MFC operation (Table 1 and Fig. 1). As observed in a number of previous studies, there was a lag period for the current production in the initial period of the system operation, which appeared to be related to the slow initiation of bacterial growth on the anode [21]. When the current decreased to a level as low as 0.03 mA, the medium in the anode chamber was replaced with a fresh lactate medium. Then, current production rapidly returned to a maximum level and the maximum current production was sustained for a couple of days. Note that as the replacement of medium was repeated, the time to reach the maximum current production became shorter and the level of maximum current production was increased. The levels of maximum current production in the 2nd, 3rd, and 4th cycles were 1,506.9 ± 19.4, 1,523.2 ± 30.1, and 1,539.4 ± 25.8 mA/m2 (n = 3), respectively (Table 1). The pattern of immediate resumption in current production was very similar in 4 times of exchange of anodic medium. This indicated that microorganisms attached to the anode were primarily responsible for the current production [6, 12, 22]. A more detailed discussion is provided in the following sections with the results obtained from the monitoring of the MFC operation parameters, including the concentration of biomass attached on the anode surface, electron recovery by lactate oxidation, and COD removal efficiency.
3.2. Electron Recovery
The concentration of COD was monitored over the course of MFC operation. In the anode chamber, the COD removal efficiency was increased from the first cycle (78.0% ± 3%, n = 3) to the final cycle (92.2% ± 3%) (Table 1). The COD removal efficiency clearly demonstrated the feasibility of removing organic carbon using MFC technologies. Assuming that lactate are completely oxidized to carbon dioxide, the recovery of electrons from the substrate oxidation was calculated by comparing the total charge or number of coulombs through the MFC circuit (Fig. 1) with the theoretical value from substrate oxidation. The total electron recovery is a function of the substrate concentration for lactate-fed MFC. Values of CE or electron recovery for the lactate oxidation were 89.4% ± 0.2%, 97.2% ± 0.6%, 98.6% ± 0.4%, and 98.9% ± 0.5% (n = 3) for the 1st, 2nd, 3rd, and 4th cycles of MFC run, respectively (Table 1). By stoichiometry, oxidation of lactate to carbon dioxide yields 12 electrons:
The CE in our study was relatively higher than those obtained in previous studies [12, 20, 23]. Given that current was produced in the mediator-less MFC, the generated electrons were transferred directly to electrode, not to soluble electron mediators such as sulfate [20]. Based on these results, it is clear that D. desulfuricans effectively transferred electrons derived from lactate oxidation to the solid electrode [12]. This suggests that the transfer of electrons recovered from lactate oxidation to electrode was effective in a pure culture study of D. desulfuricans.
Lactate was consumed at the expense of current production and cell growth, indicated by the increase in cell biomass over time in the anode chamber (Table 1). Cells continuously formed dense biofilm on the anode surface and the biomass reached 0.028 ± 0.004 mg protein/cm2 anode surface (n = 3) at the beginning of MFC operation. It increased to 0.064 ± 0.005 mg protein/cm2 anode surface at the end of the MFC operation, which was higher than the values observed in prior studies [6, 12]. Rate of current production calculated based on the attached bacterial populations on the anode surface was increased from the first (2.30 ± 0.04 μmol of electrons/mg of protein·min, n = 3) to final (2.92 ± 0.05 μmol of electrons/mg of protein·min) cycles of MFC run (Table 1), indicating that electron transfer to electrode was increased. In this study, the rate of electron transfer to electrode was higher than those reported by Liu et al. [21]. They reported that the electron transfer rates using flat gold and carbon cloth as electrodes were 2.2 and 2.4 μmol of electrons/mg of protein·min, respectively. Thus, it can be concluded in this study that the current production efficiency was related to the biomass of biofilm formed on the electrode, which was increased as the MFC run was repeated.
3.3. Biofilm Formation on Anode
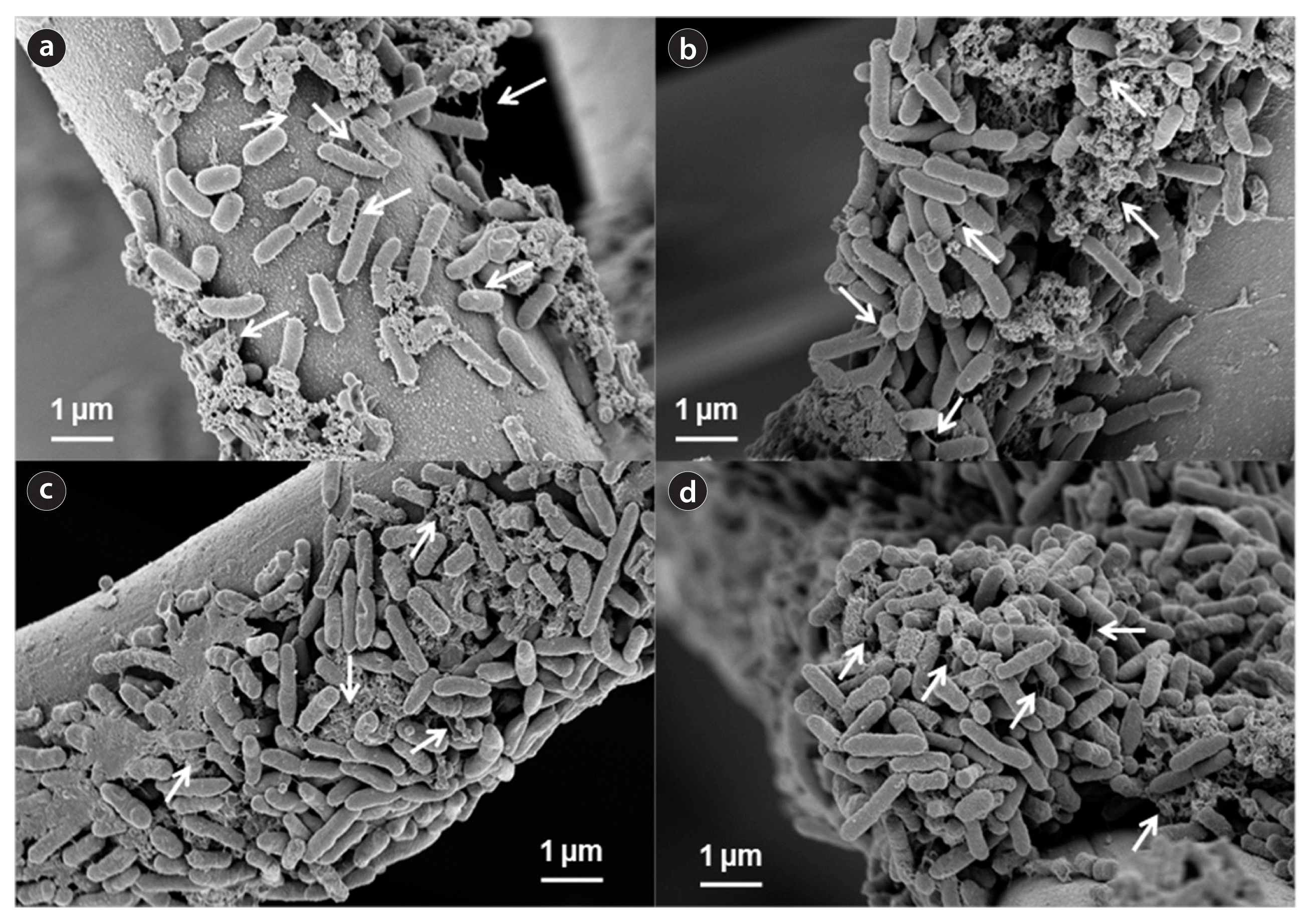
At the end of batch operation, the electrode was collected from the anode chamber. Biofilm formed on the electrode surface in each cycle of MFC operation was examined by SEM (Fig. 2). The morphology of bacteria was rod-shaped. Biofilm was formed on the electrode surface in the first cycle of MFC run, which was referred to as the primary biofilm in Fig. 2(a). During further batch operation, significantly thick and dense biofilm was formed on the electrode, referred to as the secondary, tertiary, and quaternary biofilm in Fig. 2(b)–(d). The formation of the primary biofilm resulted in a gradual increase in current production. When further biofilm was formed, the current production was more rapid and the maximum current production levels were higher than that obtained from the first cycle. These results indicated that D. desulfuricans can form biofilm on the anode surface and the electron transfer to the electrode can occur effectively through the biofilm network of D. desulfuricans attached on the anode surface. Thus, we suggest that the current production tends to increase with the number of cycles of MFC run because of the accumulation of biofilm [24]. In addition, the bacteria that colonized on the electrode were found to produce filaments or nano-pili. These nano-pili were effective for the attachment of cells on the surface electrode. It was reported that the nano-pili produced by Shewanella and Geobacter genera were electrically-conductive and the electron transfer can be facilitated through these conductive nano-pili [9–11]. Likewise, the electron transfer may have been mediated effectively by the nano-pili in our study. In addition, the thick biofilm can transfer electrons from cells to cells and from cells to electrode via the nano-pili network structure. The nano-pili appeared to be conductive material that connect the cells, allow the development of thicker electroactive biofilm, and enhance the anode performances [9].
4. Conclusions
In this study, we found that D. desulfuricans was able to attach onto the anode of MFC, resulting in the formation of biofilm on the electrode surface. The primary biofilm was formed in the first cycle of reactor run and further layers of biofilms were formed continuously throughout the MFC runs. Biomass of biofilm was found to be related to the current production efficiency, supporting the idea that electron transfer from bacteria to the electrode can be enhanced by biofilm formation on the electrode. Moreover, D. desulfuricans was found to produce nano-pili, which was effective to attach the cells on the electrode surface and to facilitate electron transfer from cell-to-cell and to the electrode. The maximum current production increased from 1,310.0 ± 22.3 mA/m2 (n = 3, first cycle) to 1,539.4 ± 25.8 mA/m2 (final cycle). The COD removal efficiency also increased from 78.0% ± 3% (n = 3, first cycle) to 92.2% ± 3% (final cycle). It was confirmed from this study that the enhancement of current production in the mediator-less MFC system was attributed to the biofilm and nano-pili produced by electro-catalytic SRB.